Abstract
Primary gastric melanoma is an extremely rare clinical entity. The clinical manifestation is not specific and usually similar with other common malignancies at this site, such as gastric cancer and lymphoma. And there are no specific radiological features either. Preoperative diagnosis via biopsy is usually difficult, since melanoma pigment could be absent in the biopsy tissue. Here, we report a case of a 50-year-old woman with a mass in the stomach found by gastroscopy. Biopsy was taken twice preoperatively under gastroscope and it was diagnosed as gastric carcinoma and neuroendocrine tumor respectively. Radical surgery was performed with gastrectomy and D2 lymph node dissection. Postoperative pathological examination finally made a definite diagnosis of gastric melanoma by immunohistochemistry. We summarize the reasons for preoperative misdiagnosis and discuss the difficulty in diagnosing gastric melanoma according to literature.
Keywords: Primary gastric malignant melanoma, extracutaneous melanoma, preoperative diagnosis
Introduction
Extracutaneous melanoma (ECM) is rare in clinic practice. Epidemiological data from Western countries indicate that the incidence of ECM is less than 1/100,000. Primary gastric melanoma is extremely rare and only accounts for 1% of all ECM. Similar with ECMs at other sites, primary gastric melanoma has been reported to occur in older patients, often at an advanced stage, and to have worse prognosis. No gender predilection has been found [1]. Preoperative diagnosis is difficult, especially when the tumor tissue taken by biopsy contains no melanin pigmentation. Moreover, due to the extremely low incidence, lack of awareness of this disease may also contribute to the misdiagnosis by physicians and pathologists. Herein, we report a rare case of primary gastric malignant melanoma, which was misdiagnosed as gastric carcinoma and neuroendocrine tumor preoperatively via biopsy.
Clinical data
A 50-year-old woman who complained of epigastric discomfort and occasional vomiting for 2 months was admitted to a local hospital. Gastroscopy revealed a tumor at the upper stomach and biopsy was taken. The patient was then referred to the First Affiliated Hospital of Sun Yat-Sen University for further treatment. Her past medical history was not remarkable. No specific sign was found via physical examination except moderate anemia. Laboratory tests found significantly decreased hemoglobulin (7.1 g/dl) and Hct (25.5%). Serum tumor markers, including CEA, CA-125 and CA-199, were all in the normal range. Fecal occult blood testing was positive.
Contrast-enhanced CT (CE-CT) (Figure 1) scan of the abdomen revealed a vascular-rich polypoid tumor located at the lesser curvature of the stomach protruding to the gastric lumen. The gastric wall was slightly thickened at the tumor region. Multiple enlarged lymph nodes were found in the perigastric region. No liver metastasis or other visceral organ invasion was found.
Figure 1.
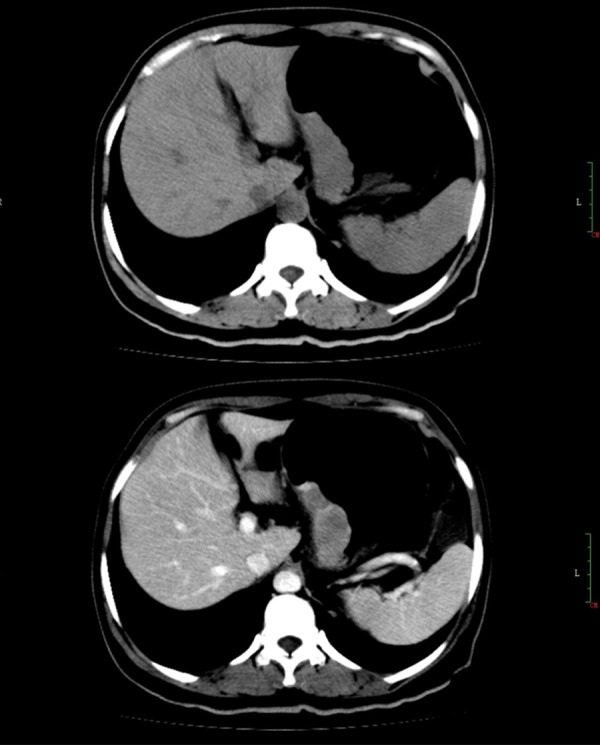
Abdominal CE-CT showing large mass involving the lesser curvature of stomach.
Sections of the biopsy specimens with hematoxylin-eosin stain from a local hospital were re-examined by pathologists in our hospital. Tumor cells were small in size, round in shape, with a little cytoplasm, round hyperchromatic nuclei and moderate mitotic figures. No pigmentation was found. The pathologists considered it as a poor differentiated gastric adenocarcinoma.
Repeat gastroscopy was performed. An easy bleeding mass, covered with blood scab, was found located at the lesser curvature of the stomach. 4 biopsy specimens were taken from the tumor. Histological features of the biopsy were similar with the first biopsy, and no pigmentation was found as well. Immunohistochemistry revealed SYN positive, CK5/6 negative, CK positive, CD20 negative, CD3 negative, CGA negative and Ki67 positive (40%). According to these findings, it was suggested as neuroendocrine carcinoma, G3.
Treatment and follow up
Since the diagnosis of malignancy was confirmed, radical surgery was therefore performed including total gastroectomy and D2 lymph node dissection. Surgery demonstrated a mass measuring 8 cm × 3.5 cm × 3 cm located at the lesser curvature of the stomach, protruding to the gastric lumen. Careful inspection of the dissected specimen found spotty and light pigmentation on the surface of the tumor. Enlarged lymph nodes were found in group 3 of paragastric lymph nodes.
Postoperative pathological inspection revealed a combined pattern of the tumor with epitheliod, spindled and small round cells. Abundant pigmentation was found in the cytoplasm of spindled cells, and less was found in the epitheliod cells. The area composed of small round cells had little pigmentation (Figure 2).
Figure 2.
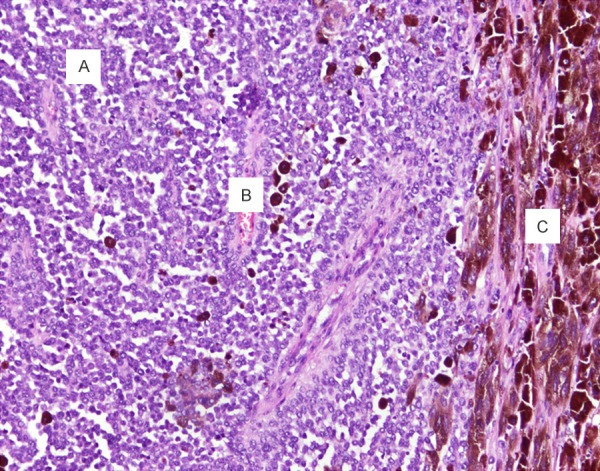
Photomicrograph of postoperative specimen showing combined pattern of the tumor composing of spindled (A), epitheliod (B), and small round cells (C). Melanin pigment distributes unevenly with the tumor.
Immunohistochemistry staining found the tumor cells were positive for HMB45- (Figure 3A), Melan-A (Figure 3B), and S-100 protein (Figure 3C).
Figure 3.
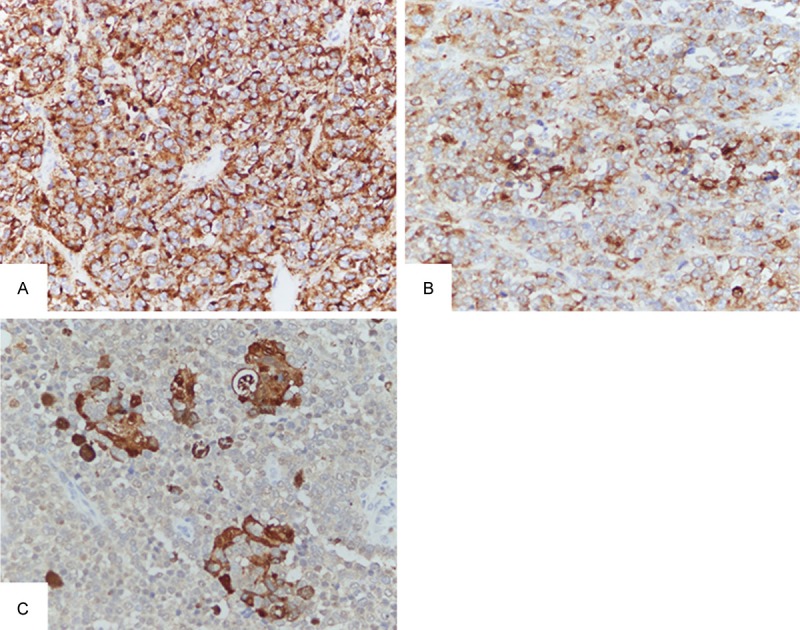
Photomicrograph of postoperative specimen showing strong positive for HMB-45, scattered positive for Melan-A and nests of staining of S-100.
Lymph nodes were carefully isolated from the specimen and were examined by the pathologist. Metastatic tumor was found in 4 out of 42 lymph nodes harvested.
While the diagnosis of melanoma was confirmed, further inspections were performed to assess whether the tumor was metastatic or primary. A careful medical history was retaken and no history of melanoma or regression of cutaneous pigmentation was found. Whole body skin and mucus, including the genital tract, were carefully examined by a dermatologist, but no significant pigmentation of the skin or mucous was found. Retinas were examined by an ophthalmologist, but no lesion of pigmentation was found either. Image of CE-CT was reviewed by an experienced radiologist, and no signs of tumor were found at other sites of the digestive tract. PET-CT was suggested, but the patient declined due to financial consideration. These findings suggested that it was a primary melanoma of the stomach.
Systemic adjuvant chemotherapy was offered, but the patient declined. Follow up is undergoing, and no signs of recurrence were found in the first 4 months.
Discussion
Malignant melanoma is a highly aggressive disease that can arise from skin, retina and mucus. It was reported that melanoma can arise from all sites of the GI mucus. Melanocytes, the histological origin of melanoma, have been demonstrated in the oral, esophageal and anorectal mucus [2]. However, it is still controversial whether malignant melanoma can arise from stomach, small intestine and colon, since there are no melanocytes in these regions. A hypothesis was proposed to explain the potential mechanism. One theory suggested that mutipotential neural crest cells might migrate to these regions and develop into melanoma after differentiation, while another proposed that amine precursor uptake and decarboxylation (APUD) cells in the gastrointestinal tract might develop to melanoma [3]. Melanosis of the stomach has been demonstrated in anal and esophageal melanoma [4], and this provides strong evidence supporting the existence of melanocytes in stomach under specific conditions.
Melanoma is the most common tumor that develops gastrointestinal metastasis. Most melanomas found in the gastrointestinal tract are metastatic. Small intestine, colon, and stomach are the most common sites of metastasis [2]. Up to 4% of patients with cutaneous melanoma had metastasis to the gastrointestinal tract ante mortem and up to 60% at autopsy [5]. As a result, metastasis from other sites must be ruled out before making a diagnosis of primary gastric melanoma. The criteria for diagnosing primary gastric melanoma includes [6,7]: 1) single lesion of melanoma in the stomach proven by pathology; 2) no concurrent lesions in other site of the body; 3) no history of melanoma; 4) disease-free survival of at least 12 months after curative surgery. In this case, a single lesion was found in the stomach and thorough post-operative inspection failed to find any potential lesion on the skin, mucus or other organs, suggesting the diagnosis of primary gastric melanoma. However, follow up is still required to confirm the diagnosis according to these criteria.
Pathologic diagnosis of melanoma is dependent on the identification of melanin in cytoplasm. Microscopically, most of the typical advanced melanomas are built up in a complex pattern, in which the tumor is composed of different cell types, such as spindled, plasmacytoid and epithelioid tumor cells. They are arranged in a sheet like, organoid/alveolar, neurotropic, or desmoplastic configuration. Immunohistochemistry is usually required to confirm the diagnosis since a portion of melanoma contains no melanin pigment. Although the pathologic diagnosis of primary gastric melanoma is similar with cutaneous melanoma, preoperative diagnosis of primary gastric melanoma is usually challenging [8-10]. Primary gastric melanoma with visible pigmentation is easy to diagnosed, but those with little or no melanin is usually misleading, since it may be highly similar with other tumors in gastrointestinal tract, such as lymphoma, poorly differentiated carcinoma, NETs and GISTs. Misdiagnosis is common in such conditions [11-13].
Carefully reviewing each steps of diagnosing this case, we summarize the reasons for the failure to identify melanoma preoperatively. Firstly, unawareness of both the physician who performed gastroscopy and the pathologist who made a diagnosis was a major cause. Although the spotty pigmentation of the tumor might be covered by the blood scab, it could still be recognized if the physician thought of this disease before taking biopsy. Biopsy around the pigmented region will certainly help the pathologist to make a correct diagnosis. Similarly, the unawareness of the pathologists made them neglect immunohistochemistry testing for melanoma, even thought they were aware of the differential diagnosis of lymphoma, carcinoma and NET for a tumor composed of small and round cells. Secondly, the composition of this tumor was complex and the distribution of pigment in this tumor was uneven. Postoperative pathological inspection demonstrated that the tumor was organized in a combined pattern with uneven pigment distribution. It was unlucky that both preoperative biopsies didn’t get tumor tissue with melanin pigment although the tumors contained a lot of melanin at other regions. Bleeding of the tumor might be another factor for the failure of biopsy. Physicians usually take biopsy at the edge of the tumor to avoid bleeding, especially when the tumor is already bleeding. However, the edge or surface of the tumor didn’t contain visible pigment in this case.
The diagnosis of melanoma with invisible melanin usually relays on immunohistochemical stains with some specific markers, such as S100, tyrosinase, Melan A and HMB-45. However, immunohistochemical stains may sometimes be misleading, since some uncommon melanoma may express markers for other tumor types. A special subgroup of melanoma with neuroendocrine differentiation can be positive for the neuroendocrine markers chromogranin, synaptophysin and neurofilament protein [12]. Similarly, uncommon variants of melanoma may express the maker for gastrointestinal stromal tumors (GISTs), including CD117 and CD34 [14]. In the case of this patient, NET was diagnosed in the second biopsy due to the similarity in morphology and some positive neural biomarkers. The misdiagnosis could have been avoided if melanoma was considered and IHC for melanoma was performed.
The prognosis of primary gastric melanoma is extremely poor, with a median survival time (MST) of 5 months [15]. The 5 year survival rate is only 3% [1]. Independent prognosis predictors include: advanced tumor stage, failure to undertake surgical resection, positive lymph node status, and age. No standard protocol for treatment has been established. For all the gastrointestinal malignant melanomas, improvement in MST was observed for those patients undergoing surgical resection [15], and curative surgery is the only approach to cure resectable disease. The role of adjuvant chemotherapy and radiotherapy has not been well elucidated on this disease, though most of the cases in literature suggested systemic chemotherapy following surgery [16-18]. Radiation was also reported to be beneficial in some cases [19].
In conclusion, primary gastric melanoma is extremely rare in clinical practice. Preoperative diagnosis is sometimes very difficult. The awareness of physician and pathologist on this disease, appropriate biopsy orientating at the pigmented regions, careful observation, multiple sectioning to search for melanin pigment and immunohistochemistry targeted on melanoma will improve the preoperative diagnosis of primary gastric melanoma, especially for those with little or no melanin.
Disclosure of conflict of interest
None.
References
- 1.Bishop KD, Olszewski AJ. Epidemiology and survival outcomes of ocular and mucosal melanomas: a population-based analysis. Int J Cancer. 2013;134:2961–71. doi: 10.1002/ijc.28625. [DOI] [PubMed] [Google Scholar]
- 2.Mihajlovic M, Vlajkovic S, Jovanovic P, Stefanovic V. Primary mucosal melanomas: a comprehensive review. Int J Clin Exp Pathol. 2012;5:739–753. [PMC free article] [PubMed] [Google Scholar]
- 3.Li H, Fan Q, Wang Z, Xu H, Li X, Zhang W, Zhang Z. Primary malignant melanoma of the duodenum without visible melanin pigment: a mimicker of lymphoma or carcinoma. Diagn Pathol. 2012;7:74. doi: 10.1186/1746-1596-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravi A. Primary gastric melanoma: a rare cause of upper gastrointestinal bleeding. Gastroenterol Hepatol (N Y) 2008;4:795–797. [PMC free article] [PubMed] [Google Scholar]
- 5.Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–810. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- 6.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–1678. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 7.Elsayed AM, Albahra M, Nzeako UC, Sobin LH. Malignant melanomas in the small intestine: a study of 103 patients. Am J Gastroenterol. 1996;91:1001–1006. [PubMed] [Google Scholar]
- 8.Aggarwal R, Dhawan S, Chopra P. Primary Gastric Melanoma: a Diagnostic Challenge. J Gastrointest Cancer. 2013 doi: 10.1007/s12029-013-9530-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Noraidah M, Jasmi AY. Malignant melanoma of the gastrointestinal tract presenting as a bleeding gastric ulcer. Malays J Pathol. 2003;25:57–61. [PubMed] [Google Scholar]
- 10.Goldman SL, Pollak EW, Wolfman EF. Gastric ulcer. An unusual presentation of malignant melanoma. JAMA. 1977;237:52. doi: 10.1001/jama.237.1.52. [DOI] [PubMed] [Google Scholar]
- 11.Alghisi F, Crispino P, Cocco A, Richetta AG, Nardi F, Paoluzi P, Badiali D. Morphologically and immunohistochemically undifferentiated gastric neoplasia in a patient with multiple metastatic malignant melanomas: a case report. J Med Case Rep. 2008;2:134. doi: 10.1186/1752-1947-2-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402–409. doi: 10.1111/j.1365-2559.2005.02240.x. [DOI] [PubMed] [Google Scholar]
- 13.Dow N, Giblen G, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: differential diagnosis. Semin Diagn Pathol. 2006;23:111–119. doi: 10.1053/j.semdp.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Magro CM, Crowson AN, Mihm MC. Unusual variants of malignant melanoma. Mod Pathol. 2006;19(Suppl 2):S41–70. doi: 10.1038/modpathol.3800516. [DOI] [PubMed] [Google Scholar]
- 15.Cheung MC, Perez EA, Molina MA, Jin X, Gutierrez JC, Franceschi D, Livingstone AS, Koniaris LG. Defining the role of surgery for primary gastrointestinal tract melanoma. J Gastrointest Surg. 2008;12:731–738. doi: 10.1007/s11605-007-0417-3. [DOI] [PubMed] [Google Scholar]
- 16.Kim NR, Lee WK, Chung DH. Primary gastric melanoma with rhabdoid features: a case report. Korean J Pathol. 2013;47:606–609. doi: 10.4132/KoreanJPathol.2013.47.6.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alazmi WM, Nehme OS, Regalado JJ, Rogers AI. Primary gastric melanoma presenting as a nonhealing ulcer. Gastrointest Endosc. 2003;57:431–433. doi: 10.1067/mge.2003.120. [DOI] [PubMed] [Google Scholar]
- 18.Vettoretto N, De Cesare V, Cervi E, Villanacci V, Ruzzenenti N, Cervi G. [Gastric metastasis from melanoma. Report of 2 surgically treated cases] . Minerva Chir. 2000;55:787–791. [PubMed] [Google Scholar]
- 19.Slater JM, Ling TC, Slater JD, Yang GY. Palliative radiation therapy for primary gastric melanoma. J Gastrointest Oncol. 2014;5:E22–26. doi: 10.3978/j.issn.2078-6891.2013.057. [DOI] [PMC free article] [PubMed] [Google Scholar]


