Abstract
Previous studies demonstrated that the acquired drug resistance of non-small cell lung cancer (NSCLC) was related to deregulation of miRNAs. However, the effects of miR-107 and the mechanism through which miR-107 affects the cisplatin chemoresistance in NSCLC have not been reported. TaqMan RT-PCR or Western blot assay was performed to detect the expression of mature miR-107 and cyclin dependent kinase 8 (CDK8) protein. The viabilities of treated cells were analyzed using MTT assay. We found that the expression level of miR-107 in A549 cells was significantly lower than that in normal human bronchial epithelial cells (0.45 ± 0.26 vs. 1.00 ± 0.29, P = 0.032). The MTT assay showed that the A549 cells transfected with miR-107 mimics were significantly more sensitive to the therapy of cisplatin than control cells. A549 cells transfected with miR-107 mimics showed a decreased CDK8 protein expression. Downregulation of CDK8 expression by siRNAs, A549 cells became more sensitive to the therapy of cisplatin. In addition, the enhanced growth-inhibitory effect by the miR-107 mimic transfection was enhanced after the addition of CDK8 siRNA. In conclusion, the present study provides the first evidence that miR-107 plays a key role in cisplatin resistance by targeting the CDK8 protein in NSCLC cell lines, suggesting that miR-107 can be used to predict a patient’s response to chemotherapy as well as serve as a novel potential maker for NSCLC therapy.
Keywords: Non small cell lung cancer, miR-107, CDK8, cisplatin, chemosensitivity
Introduction
Non-small cell lung cancer (NSCLC) accounts for about 80% of all lung cancer cases, and is the most common cause of cancer death [1]. Despite the considerable advances in medical and surgical treatment of NSCLC patients, the prognosis associated with this disease remains dismal. NSCLC is still an incurable disease, and one of the most important factors that affect survival rate is resistance to therapeutic drugs [2]. Cisplatin (diammine dichloroplatinum), which is a platinum-coordinated complex, is widely used as the first-line chemo-therapeutic agent for NSCLC treatment [3]. Unfortunately, the clinical affectivity of cisplatin is limited because some tumors show resistance or become resistant to it after repeated cycles of chemotherapy which ultimately leads to relapse and poor prognosis [4].
MicroRNAs (miRNAs) are a new class of small, non-coding RNAs that range in size from 19 to 25 nucleotides and play important roles in a variety of biologic processes [5]. miRNAs are predicted to regulate the expression of up to one-third of human protein-coding genes and are involved in pathogenesis, diagnosis, treatment and prognosis [6,7]. An increasing body of evidence demonstrates that miRNAs regulate drug sensitivity and/or resistance to chemotherapeutic agents [8]. miR-107, located on 10th chromosome, is differentially expressed in a number of metabolic pathways, including adipogenesis, hypoxia, cell cycle arrest, angiogenesis and neurodegenerative diseases. In the past few decades, it has been shown that miR-107 acts as a tumor suppressor gene in several tumor development process [9-12]. Previously, Takahashi et al found that the expression of miR-107 was reduced in NSCLC tissues, and it was able to induce cell cycle arrest in human NSCLC cell lines [13]. These results indicated that miR-107 might be linked to lung cancer chemotherapy resistance. However, the effects of miR-107 and the mechanism through which miR-107 affects the cisplatin chemoresistance in NSCLC have not been reported.
Cyclin dependent kinase 8 (CDK8) locating on chromosome 13q12 has five transcripts and only one transcript encodes protein product containing 464 amino acid residues (molecular weight 53.2 kD). CDK8 has important function on the regulation of gene transcription [14]. Recent studies suggest that CDK8 is important in the process of tumor development [15]. CDK8 plays a key role in the regulation of cell cycle and cell growth on post-transcriptional level, and promotes the development and progression of colorectal cancer [16]. We found that miR-107 may modulate CDK8 using online prediction software Target Scan. Therefore, we speculate that miR-107 may play an important role in NSCLC chemoresistance by targeting CDK8.
Materials and methods
Drugs and reagents
Cisplatin was purchased from QiLu Pharmaceutical (Jinan, China). miR-107 mimics, CDK8 siRNA, and their negative control oligonucleotides were synthesized by Integrated Biotech Solutions Co, Ltd (Shanghai, China). These were used to transfect A549 cells using LipofectamineTM 2000 (Invitrogen, Carlsbad, CA, USA) according to the instructions provided by the manufacturer. CDK8 monoclonal antibody was purchased from Cell Signaling Company. BCA protein concentration assay kit was purchased from Beyotime Biotechnology Research Institute.
NSCLC cancer cell line and cell culture
The human lung adenocarcinoma cell line A549 was purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). A549 cell line was cultured in DMEM containing 10% fetal bovine serum (Gibco®, Invitrogen, Carlsbad, CA, USA), 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in a 5% CO2 humidified incubator to the log phase of proliferation before harvesting the cells. Normal human bronchial epithelial cells (NHBE) (Clonetics™) were maintained in a culture medium according to the protocol provided by Clonetics™.
MTT assay
Cells transfected with miR-107 mimics or CDK8 siRNA, were seeded into 96-well plates at 6 × 103 cells/well and allowed to grow overnight, and then were treated with different concentrations of cisplatin. After 24 h of treatment, 20 μl of 5 mg/ml MTT reagent (Sigma-Aldrich, St.Louis, MO, USA) was added and incubated in the dark for 4 h. The absorbance of the plate was measured in a microplate reader at a wavelength of a 570-nm reference (BMG Lab Technologies, Germany), and the results were expressed as the percentage of absorbance relative to untreated controls. Each treatment was carried out in triplicate.
Real-time quantitative reverse transcription-PCR for miRNA expression and mRNA expression
For miRNA expression detection, reverse transcription (RT) reaction was performed with PrimeScript® RT reagent Kit (TAKARA Biotechnology CO., LTD., Dalin, China) and real-time quantitative RT-PCR (qRT-PCR) was performed using SYBR® Premix Ex TaqTM II (TAKARA Biotechnology CO., LTD., Dalin, China) on the basis of the protocol provided by the manufacturer. For mRNA expression detection, reverse transcription reaction was performed with PrimeScript® RT reagent kit (TAKARA Biotechnology CO., LTD., Dalin, China) and RT-PCR (qRT-PCR) was performed using SYBR® Premix Ex TaqTM II (TAKARA Biotechnology CO., LTD., Dalin, China). The primer sequences were as follows: 5’-G-GGATCTCTATGTCGGCATGTAG-3’ (forward) and 5’-AAATGACGTTTGGATGCTTAAGC-3’ (reverse) for CDK8; 5’-CATGAGAAGTATGACAACAGCCT-3’ (forward) and 5’-AGTCCTTCCACGATACCAAAGT-3’ (reverse) for GAPDH. The expression of the target miRNA was normalized relative to that of the internal control, U6 and the expression of the target gene was normalized relative to the expression of glyceraldehyde- 3-phosphate dehydrogenase (GAPDH), which was used as an internal control. Data were analyzed according to the comparative Ct method also referred to as the 2−ΔΔCT method.
Western blot assay
The proteins were resolved on an SDS denaturing polyacrylamide gel and then transferred onto a nitrocellulose membrane. Antibody to CDK8 or GAPDH was incubated with the membranes overnight at 4°C. The membranes were washed and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. Protein expression was assessed by enhanced chemiluminescence and exposure to chemiluminescent film. LabWorks™ Image Acquisition and Analysis Software (UVP, Upland, CA) were used to quantify the band intensities. All the antibodies were purchased from Abcam (Cambridge, MA).
Statistical analysis
All the data were shown as mean ± standard deviation (SD) and the experiments were repeated three times. The difference was determined by two-tailed students’ t-test and P < 0.05 was considered statistically significant. The data were assessed using the GraphPad Prism software 5.0 (GraphPad, USA) and SPSS version 18.0 (SPSS, Chicago, IL, USA).
Results
Expression level of miR-107 in A549 cell line
To define the role of miR-107 in human lung cancer tumorigenesis, we compared the expression levels of miR-107 in human lung cancer cell line A549 and NHBE cell line (normal human bronchial epithelial cells) by qRT-PCR. The expression level of miR-107 in A549 was significantly lower than that in NHBE cell line (0.45 ± 0.26 vs. 1.00 ± 0.29, P = 0.032, shown in Figure 1).
Figure 1.
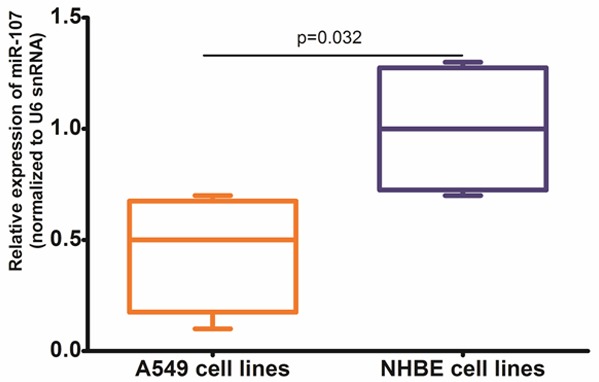
The expression level of miR-107 in A549 cells line was significantly lower than that in NHBE cell line (0.45 ± 0.26 vs. 1.00 ± 0.29, P = 0.032).
Transfection of miR-107 mimics induced sensitivity of A549 cells to cisplatin
To further assess the effect of miR-107, we transfected miR-107 mimics and its negative control oligonucleotides into A549 cells. Transfection of cells with miR-107 mimics increased miR-107 level compared with the control cells (P = 0.016, shown in Figure 2). The MTT assay showed that the A549 cells transfected with miR-107 mimics were significantly more sensitive to the therapy of cisplatin than control cells (shown in Figure 3).
Figure 2.
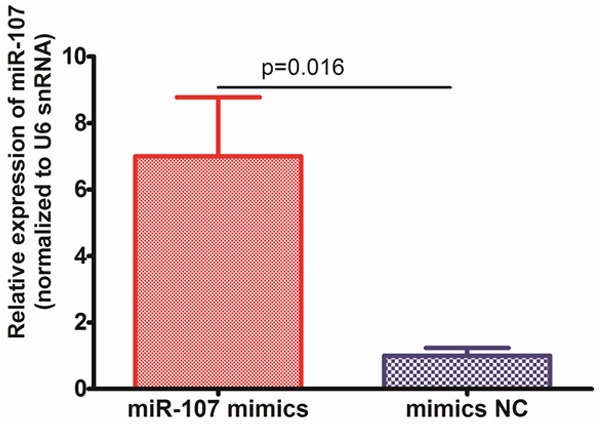
A549 cells were transfected with miR-107 mimics or mimics NC, and the levels of miR-107 were confirmed by qRT-PCR.
Figure 3.
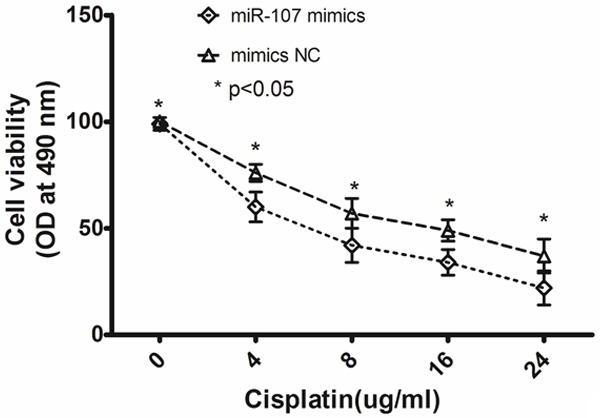
The MTT assay showed that the A549 cells transfected with miR-107 mimics were significantly more sensitive to the therapy of cisplatin than control cells (*P < 0.05). Data are mean ± SD of three experiments.
CDK8 was a target of miR-107 and responsible for the miR-107-induced resistance to cisplatin in A549 cells
We transfected A549 cells with miR-107 mimics or mimics NC. The CDK8 mRNA level was significantly decreased in A549 cells transfected with miR-107 mimics compared with controls (shown in Figure 4A). We then examined the protein levels of CDK8 following the transfection of miR-107 mimics in A549 cells by Western blot analysis and found that cells transfected with miR-107 mimics showed a decreased CDK8 protein expression (shown in Figure 4B). Downregulation of CDK8 expression by siRNAs, A549 cells became more sensitive to the therapy of cisplatin (shown in Figure 5). In addition, the enhanced growth-inhibitory effect by the miR-107 mimic transfection was enhanced after the addition of CDK8 siRNA (shown in Figure 5).
Figure 4.
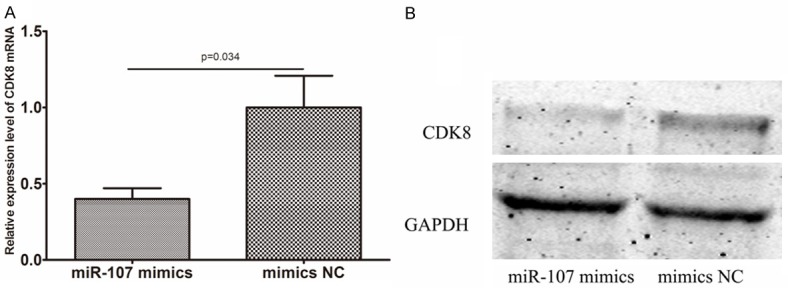
Evaluation of CDK8 in A549 cells transfected with miR-107 mimics and its negative control oligonucleotides (NC). A. qRT-PCR showed significant downregulation of CDK8 mRNAs in the transfected cells. B. Western blot analysis demonstrated significantly lower expression of CDK8 proteins in the transfected cells (*P < 0.05). Data are mean ± SD of three experiments.
Figure 5.
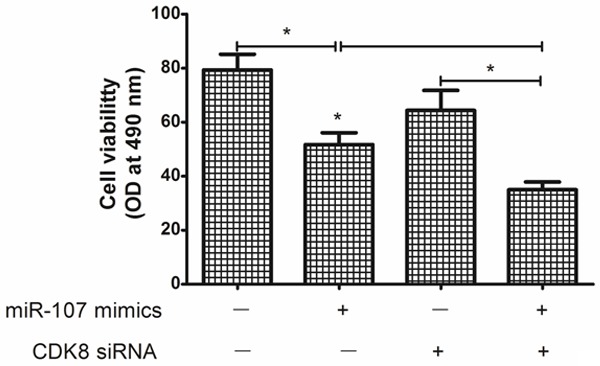
Changes in anti-tumor effects of the cisplatin after transfection of miR-107 mimics and/or siRNA against CDK8 in A549 cells. Downregulation of CDK8 expression by siRNAs, A549 cells became more sensitive to the therapy of cisplatin. In addition, the enhanced growth-inhibitory effect by the miR-107 mimics transfection was enhanced after the addition of CDK8 siRNA (*P < 0.05).
Discussion
Management of patients with lung cancer continues to pose a considerable challenge to today’s oncologist. While treatment may be curative in the early stages of the disease, the majority of patients are not diagnosed until the tumor has progressed beyond the primary site. Most patients face an intensive and invasive treatment regimen comprising surgery, radiotherapy, or chemotherapy, or combinations thereof depending on disease stage/performance status. Most will require chemotherapy even if their initial surgery is potentially curative; for those with advanced disease, chemotherapy may be their only treatment option. Cisplatin is still one of the most useful management therapies for NSCLC patients, although resistance to cisplatin represents a significant barrier to the improvement of the long-term overcome of patients with NSCLC [4]. Factors that enhance the sensitivity of NSCLC cells to cisplatin may highlight predictive biomarkers or targets for therapy; however, the molecular mechanisms leading to cisplatin chemoresistance are poorly understood.
The dysregulation of miRNAs is common in various carcinomas and plays an important role in cancer progression by altering normal gene expression. miR-107, located on 10th chromosome, is differentially expressed in a number of metabolic pathways, including adipogenesis, hypoxia, cell cycle arrest, angiogenesis and neurodegenerative diseases. miR-103/miR-107 are highly conserved miRNAs that map to intron 5 of pantothenate kinase (Pank) genes. Pank2 and Pank3 host the pre-miRNA sequences of miR-103, whereas Pank1 encodes miR-107, which differs from miR-103 by a single nucleotide [17,18]. Pantothenate kinase is the rate limiting enzyme in the biosynthesis of coenzyme A, a cofactor that is involved in over 100 metabolic reactions. p53 can induce expression of Pank-1 and miR-107, presumably, through a p53 element located~1 kb upstream of the Pank-1 transcriptional start site [19]. Yamakuchi et al demonstrated that expression of miR-107 is inversely associated with expression of hypoxia inducible factor-1β (HIF-1β) and can mediate p53 regulation of hypoxic signaling and tumor angiogenesis in colon cancer. Members of the miR-107 gene group have also been shown to repress granulin (GRN) protein, a potent mitogen and growth factor, in prostate cancer cells [20]. miR-107 induces cell cycle G1 arrest and inhibits invasion by targeting cyclin dependent kinase 6 (CDK6), thereby inhibiting tumor progression in gastric cancer, pancreatic cancer, and non-small cell lung cancer cell lines [21]. Another study revealed that Toll-like Receptor-4 down-regulated miR-107, increasing macrophage adhesion via CDK6 [22]. On the contrary, miR-107 has also been implicated in tumor progression. miR-107 showed over-expression in pancreatic cancer and breast cancer suggesting some positive role in carcinogenesis and its expression was inversely correlated with let-7 expression in breast tumors and cancer cell lines [23].
In the present study, to explore whether miR-107 was involved in the NSCLC cells resistant to cisplatin, we transfected miR-107 mimics and its negative control oligonucleotides into A549 cells. The MTT assay showed that the A549 cells transfected with miR-107 mimics were significantly more sensitive to the therapy of cisplatin than control cells, indicating that miR-107 may involve in chemoresistance of NSCLC cells to cisplatin. In NSCLC tissues, many onco-miRs/tumor suppressor-target or tumor suppressor-miRs/onco-target pathways have been demonstrated to participate in the tumorigenesis of lung cancer. However, miRNA/target network was so complex that more and more miRNA/target axis needs to be elucidated in lung cancer especially NSCLC. In the present study, we transfected A549 cells with miR-107 mimics and its negative control oligonucleotides. The expression of CDK8 mRNA and protein were decreased in cells transfected with miR-107 mimics compared with controls, indicating that miR-107 was a negative regulator of CDK8. Furthermore, we found that downregulation of CDK8 expression by siRNAs, A549 cells became more sensitive to the therapy of cisplatin. In addition, the enhanced growth-inhibitory effect by the miR-107 mimic transfection was enhanced after the addition of CDK8 siRNA. These findings suggested that CDK8 was responsible for the miR-107-induced resistance to cisplatin.
In conclusion, the present study provides the first evidence that miR-107 plays a key role in cisplatin resistance by targeting the CDK8 protein in NSCLC cell lines, suggesting that miR-107 can be used to predict a patient’s response to chemotherapy as well as serve as a novel potential maker for NSCLC therapy.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Rolfo C, Fanale D, Hong DS, Tsimberidou AM, Piha-Paul SA, Pauwels P, Van Meerbeeck JP, Caruso S, Bazan V, Cicero G, Russo A, Giovannetti E. Impact of MicroRNAs in Resistance to Chemotherapy and Novel Targeted Agents in Non-Small Cell Lung Cancer. Curr Pharm Biotechnol. 2014;15:475–85. doi: 10.2174/1389201015666140519123219. [DOI] [PubMed] [Google Scholar]
- 3.Eaton KD, Martins RG. Maintenance chemotherapy in non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8:815–821. doi: 10.6004/jnccn.2010.0058. [DOI] [PubMed] [Google Scholar]
- 4.Chang A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer. 2011;71:3–10. doi: 10.1016/j.lungcan.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Boeri M, Pastorino U, Sozzi G. Role of microRNAs in lung cancer: microRNA signatures in cancer prognosis. Cancer J. 2012;18:268–274. doi: 10.1097/PPO.0b013e318258b743. [DOI] [PubMed] [Google Scholar]
- 7.Melo SA, Kalluri R. Molecular pathways: microRNAs as cancer therapeutics. Clin Cancer Res. 2012;18:4234–4239. doi: 10.1158/1078-0432.CCR-11-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blower PE, Chung JH, Verducci JS, Lin S, Park JK, Dai Z, Liu CG, Schmittgen TD, Reinhold WC, Croce CM, Weinstein JN, Sadee W. MicroRNAs modulate the chemosensitivity of tumor cells. Mol Cancer Ther. 2008;7:1–9. doi: 10.1158/1535-7163.MCT-07-0573. [DOI] [PubMed] [Google Scholar]
- 9.Li F, Liu B, Gao Y, Liu Y, Xu Y, Tong W, Zhang A. Upregulation of microRNA-107 induces proliferation in human gastric cancer cells by targeting the transcription factor FOXO1. FEBS Lett. 2014;588:538–544. doi: 10.1016/j.febslet.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Sharma P, Saraya A, Gupta P, Sharma R. Decreased levels of circulating and tissue miR-107 in human esophageal cancer. Biomarkers. 2013;18:322–330. doi: 10.3109/1354750X.2013.781677. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Chen XR, Zhang R, Li P, Liu Y, Yan K, Jiang XD. MicroRNA-107 inhibits glioma cell migration and invasion by modulating Notch2 expression. J Neurooncol. 2013;112:59–66. doi: 10.1007/s11060-012-1037-7. [DOI] [PubMed] [Google Scholar]
- 12.Chen HY, Lin YM, Chung HC, Lang YD, Lin CJ, Huang J, Wang WC, Lin FM, Chen Z, Huang HD, Shyy JY, Liang JT, Chen RH. miR-103/107 promote metastasis of colorectal cancer by targeting the metastasis suppressors DAPK and KLF4. Cancer Res. 2012;72:3631–3641. doi: 10.1158/0008-5472.CAN-12-0667. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi Y, Forrest AR, Maeno E, Hashimoto T, Daub CO, Yasuda J. MiR-107 and MiR-185 can induce cell cycle arrest in human non small cell lung cancer cell lines. PLoS One. 2009;4:e6677. doi: 10.1371/journal.pone.0006677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adler AS, McCleland ML, Truong T, Lau S, Modrusan Z, Soukup TM, Roose-Girma M, Blackwood EM, Firestein R. CDK8 maintains tumor dedifferentiation and embryonic stem cell pluripotency. Cancer Res. 2012;72:2129–2139. doi: 10.1158/0008-5472.CAN-11-3886. [DOI] [PubMed] [Google Scholar]
- 15.Morris EJ, Ji JY, Yang F, Di Stefano L, Herr A, Moon NS, Kwon EJ, Haigis KM, Naar AM, Dyson NJ. E2F1 represses beta-catenin transcription and is antagonized by both pRB and CDK8. Nature. 2008;455:552–556. doi: 10.1038/nature07310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Firestein R, Bass AJ, Kim SY, Dunn IF, Silver SJ, Guney I, Freed E, Ligon AH, Vena N, Ogino S, Chheda MG, Tamayo P, Finn S, Shrestha Y, Boehm JS, Jain S, Bojarski E, Mermel C, Barretina J, Chan JA, Baselga J, Tabernero J, Root DE, Fuchs CS, Loda M, Shivdasani RA, Meyerson M, Hahn WC. CDK8 is a colorectal cancer oncogene that regulates beta-catenin activity. Nature. 2008;455:547–551. doi: 10.1038/nature07179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polster BJ, Westaway SK, Nguyen TM, Yoon MY, Hayflick SJ. Discordant expression of miR-103/7 and pantothenate kinase host genes in mouse. Mol Genet Metab. 2010;101:292–295. doi: 10.1016/j.ymgme.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang WX, Rajeev BW, Stromberg AJ, Ren N, Tang G, Huang Q, Rigoutsos I, Nelson PT. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci. 2008;28:1213–1223. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamakuchi M, Lotterman CD, Bao C, Hruban RH, Karim B, Mendell JT, Huso D, Lowenstein CJ. P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc Natl Acad Sci U S A. 2010;107:6334–6339. doi: 10.1073/pnas.0911082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang WX, Wilfred BR, Madathil SK, Tang G, Hu Y, Dimayuga J, Stromberg AJ, Huang Q, Saatman KE, Nelson PT. miR-107 regulates granulin/progranulin with implications for traumatic brain injury and neurodegenerative disease. Am J Pathol. 2010;177:334–345. doi: 10.2353/ajpath.2010.091202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng L, Xie Y, Zhang H, Wu Y. miR-107 targets cyclin-dependent kinase 6 expression, induces cell cycle G1 arrest and inhibits invasion in gastric cancer cells. Med Oncol. 2012;29:856–863. doi: 10.1007/s12032-011-9823-1. [DOI] [PubMed] [Google Scholar]
- 22.Hennessy EJ, Sheedy FJ, Santamaria D, Barbacid M, O’Neill LA. Toll-like receptor-4 (TLR4) down-regulates microRNA-107, increasing macrophage adhesion via cyclin-dependent kinase 6. J Biol Chem. 2011;286:25531–25539. doi: 10.1074/jbc.M111.256206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li XY, Luo QF, Wei CK, Li DF, Li J, Fang L. MiRNA-107 inhibits proliferation and migration by targeting CDK8 in breast cancer. Int J Clin Exp Med. 2014;7:32–40. [PMC free article] [PubMed] [Google Scholar]


