Abstract
Introduction: Cervical cancer is the second leading cause of cancer morbidity and mortality for women around the world. Long non-coding RNAs (lncRNAs) have been investigated as a new class of regulators of cellular processes, such as cell growth, apoptosis, and carcinogenesis. Although downregulation of lncRNA GAS5 in several cancers has been studied, its role in cervical cancer remains unknown. The aim of this study is to investigate the expression, clinical significance and biological role in cervical cancer. Methods: Expression of GAS5 was analyzed in cervical cancer tissues by quantitative Real-time PCR (qRT-PCR). And its association with overall survival of patients was analyzed by statistical analysis. Small interfering RNA (siRNA) was used to suppress GAS5 expression in cervical cancer cells. In vitro assays were performed to further explore the biological functions of GAS5 in cervical cancer. Results: We found that GAS5 expression was markedly downregulated in cervical cancer tissues than in corresponding adjacent normal tissues. Decreased GAS5 expression was significantly correlated with FIGO stage, vascular invasion and lymph node metastasis. Moreover, cervical cancer patients with GAS5 lower expression have shown significantly poorer overall survival than those with higher GAS5 expression. And GAS5 expression was an independent prognostic marker of overall survival in a multivariate analysis. In vitro assays our data indicated that knockdown of GAS5 promoted cell proliferation, migration, and invasion. Conclusions: Our study presents that lncRNA GAS5 is a novel molecule involved in cervical cancer progression, which provide a potential prognostic biomarker and therapeutic target.
Keywords: GAS5, cervical cancer, proliferation, migration, invasion
Introduction
Cervical cancer is the second most common female cancer and is still one of the main causes of cancer-related deaths worldwide [1]. In 2011, approximately 49,000 new cases of cervical cancer were diagnosed and 275,000 women were killed, which most occurred in developing countries [2]. Radiotherapy, chemotherapy and surgery have been recently used as standard treatment modalities for patients with cervical cancer, with consequent disease remission, clinical outcomes vary significantly between patients and can be difficult to predict [3,4]. Therefore, it is still urgently for us to find new and effective prognostic markers and therapeutic strategies to improve treatment of cervical cancer.
Recent genome sequencing studies have revealed that the human genome is comprised of less than 2% protein coding genes and more than 90% of the genome is transcribed as non-coding RNAs (ncRNA) [5]. NcRNAs are collectivey divided into three categories: housekeeping RNAs, small non-coding RNAs, and long non-coding RNAs [6]. Long non-coding RNAs (lncRNAs) is an RNA molecule that is longer than 200 nucleotides and is not translated into a protein [7]. Recent studies demonstrated that lncRNAs have an important role in numerous biological processes, including transcriptional regulation, cell growth and tumorigenesis [8,9]. In this regard, highlighting the potentially widespread functional roles of lncRNAs in human cancer is important. For example, Huang et al. showed lncRNA HOTAIR expression in cervical cancer tissues was significantly upregulated compared with the matched nontumorous tissues, and patients with HOTAIR higher expression have shown significantly poorer overall survival than those with lower HOTAIR expression [10]. Guo et al. demonstrated the biological significance of lncRNA MALAT1 in cervical cancer progression and provide novel evidence that MALAT1 may serve as a therapeutic target in the prevention of human cervical cancer [11]. Qin et al. showed that lncRNA MEG3 was high expression in non-neoplastic tissues than in cervical cancer tissues, and Ectopic expression of MEG3 inhibited the proliferation of human cervical carcinoma cells HeLa and C-33A in vitro [12]. Unfortunately, the emerging functional role of lncRNAs in cervical cancer remains largely unknown.
GAS5 (growth arrest-specific transcript 5) is originally isolated from NIH 3T3 cells using subtraction hybridization [13]. This gene is encoded at 1q25, a chromosomal locus which has been associated with lymphoma [14]. Recent studies indicated that GAS5 was a tumor-suppressor lncRNA. Maarabouni et al. found GAS5 transcript levels were significantly reduced in breast cancer samples relative to adjacent unaffected normal breast epithelial tissues. And had a critical role in mammalian apoptosis and cell population growth [15]. Qiao et al. found that a decrease in GAS5 expression is associated with renal cell carcinoma genesis and progression and overexpression of GAS5 can act as a tumor suppressor for renal cell carcinoma [16]. Tu et al. found that GAS5 was down-regulation in a majority of hepatocellular carcinoma patients. And GAS5 expression was an independent prognostic factor for patients with liver cancer [17]. However, the clinical and prognostic significance of lncRNA GAS5 expression in cervical cancer has not been reported yet.
In this study, we aimed to investigate the expression of lncRNA GAS5 in cervical cancer and further explore the clinical significance and biological functions of GAS5 in cervical cancer.
Materials and methods
Patients and specimens
A total of 102 cervical cancer tissues and matched adjacent normal tissues were obtained from Zhengzhou People’s Hospital (Zhengzhou, China) between January 2005 and October 2009. All patients recruited in this study were not subjected to preoperative radiotherapy and/or chemotherapy and were diagnosed as infiltrating carcinoma by pathology. The tumor stage was classified by two experienced gynecological oncologists according to the International Federation of Gynecology and Obstetrics (FIGO) staging system for cervical cancer. Clinical and pathological variables analyzed are shown in Table 1. All patients were regularly followed up, with a mean observation period of 44 months. Tumor specimens and corresponding adjacent normal tissues were collected and stored in liquid nitrogen until use. The study was approved by the Medical ethics committee of Zhengzhou People’s Hospital.
Table 1.
Association of GAS5 expression with clinicopathological features in cervical cancer
| Parameters | Group | Total | GAS5 expression | P value | |
|---|---|---|---|---|---|
|
| |||||
| High | Low | ||||
| Age (years) | < 50 | 48 | 24 | 24 | 0.187 |
| ≥ 50 | 54 | 34 | 20 | ||
| Tumor size (cm) | < 4 | 46 | 24 | 22 | 0.386 |
| ≥ 4 | 56 | 34 | 22 | ||
| Histology | Squamous | 82 | 47 | 35 | 0.851 |
| Adenocarcinoma | 20 | 11 | 9 | ||
| FIGO stage | Ib-IIa | 50 | 44 | 6 | 0.000 |
| IIb~IIIa | 52 | 14 | 38 | ||
| Differentiation | Well + moderate | 72 | 34 | 28 | 0.462 |
| Poor | 30 | 14 | 16 | ||
| Lymph nodes metastasis | No | 58 | 46 | 12 | 0.000 |
| Yes | 44 | 12 | 32 | ||
| Vascular invasion | No | 60 | 52 | 8 | 0.000 |
| Yes | 42 | 6 | 36 | ||
Cell culture
The human cervical cancer cell lines Caski were purchased from the American Type Culture Collection (ATCC, USA). Cells were cultured in RPMI-1640 medium (Gibco) with 10% fetal bovine serum (Gibco), 50 U/ml of penicillin and 50 μg/ml of streptomycin. All cells were cultured in a sterile incubator maintained at 37°C with 5% CO2.
siRNA transfection
Small interfering RNA that targeted GAS5 RNA (si-GAS5) and a scrambled negative control (si-NC) were generously provided by Life Technologies. The sequences of si-GAS5 were CUUGCCUGGACCAGCUUAAUU, Human cervical cancer cells were transfected with either 50 nmol si-GAS5 or si-NC using Lipofectamine 2000 transfection reagent according to the manufacturer’s instruction (Life Technologies).
Cell proliferation assays
Cells (2000 cells/well) were seeded in 96-well plates and stained at specified time points with 100 ml sterile MTT dye (0.5 mg/ml, Sigma) for 4 hours at 37°C. The culture medium was removed and 150 ml of dimethyl sulfoxide (DMSO, Sigma) was added. The absorbance was measured at 570 nm. All experiments were performed in triplicate.
Migration assay
To determine cell migration, cervical cancer cells transfected with siRNA were seeded into 12-well plates and allowed to grow to 90-95% confluence. Before scratching, cells were starved for 24 hours in the medium with 1% FBS. Similar sized wounds were introduced to monolayer cells using a sterile white pipette tip. Wounded monolayer cells were washed three times by PBS to remove cell debris and then cultured. The speed of wound closure was monitored and photographed at 48 hours. All experiments were performed in triplicate.
Invasion assay
The invasion assay was performed using a Biocoat Matrigel Invasion Chamber from Becton Dickson (8 µm pore size). The cells (4×104) were plated in the upper chamber with 200 μl serum-free medium. The bottom chamber contained 400 μl RPMI-1640 with 10% FBS. After 48 hours, the chambers were fixed using cold methanol and stained with crystal violet. The cells that migrated or invaded to the lower surface were counted in every five high power fields (100×) under a microscope. All experiments were performed in triplicate.
Quantitative real-time PCR assay
Total RNA was isolated tissue using TRIZOL reagent according to the manufacturer’s protocol (Invitrogen). RNA was reverse transcribed using SuperScript First Strand cDNA System (Invitrogen) according to the manufacturer’s instructions. The PCR amplification were performed for 40 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s, on a Applied Biosystems 7900HT (Applied Biosystems) with 1.0 μl of cDNA and SYBR Green Real-time PCR Master Mix (Takara). Data was collected and analyzed by SDS2.3 Software (Applied Biosystems). The expression level of each candidate gene was internally normalized against that of the RNU6B. The relative quantitative value was expressed by the 2-ΔΔCt method. Each experiment was performed in triplicates and repeated three times.
Statistical analysis
All computations were carried out using the software of SPSS version 18.0 for Windows. Data were expressed as mean ± SD. The data were analyzed using independent two-tailed t test. Categorical data were analyzed using the two-side chi-square test. Overall survival was estimated by using Kaplan-Meier method, and univariate analysis was conducted by log-rank test. The Cox proportional hazards model was used in the multivariate analysis. Values of P < 0.05 were considered statistically significant.
Results
Expression of GAS5 is downregulated in human cervical cancer tissues
We firstly examined lncRNA GAS5 expression level in 102 paired human cervical cancer and adjacent normal tissues by qRT-PCR. As shown in Figure 1, after normalization to RNU6B expression levels, the expression level of GAS5 in cervical cancer tissues was significantly lower than that in adjacent normal tissues (P < 0.05). The data indicated that abnormal GAS5 expression may be related to cervical cancer pathogenesis.
Figure 1.

GAS5 expression in 102 pairs of cervical cancer and adjacent normal tissues were respectively detected by qRT-PCR. After normalization to RNU6B, the expression level of GAS5 in cervical cancer tissues was significantly lower than that in adjacent normal tissues (*P < 0.05). Results are expressed as mean ± SD for three replicate determination. All data analyzed using Student’s t test.
Relationship between GAS5 expression and clinic pathological factors in patients with cervical cancer
The median expression level of GAS5 (0.29) was used as a cutoff point to divide all 102 patients into two groups: cervical cancer patients who express GAS5 at levels above than the cutoff value were assigned to the high expression group (n = 58, GAS5 expression level ≥ cutoff point), and those with expression less than the cutoff value were assigned to the low expression group (n = 44, GAS5 expression level < cutoff point). Clinic pathological factors were compared between the two groups (Table 1). The low GAS5 group was correlated with FIGO stage, vascular invasion and lymph node metastasis than the high GAS5 group (P < 0.05). However, GAS5 expression level was not associated with other parameters such as age, tumor size, histology and differentiation (P > 0.05).
GAS5 downregulation associates with poor prognosis in patients with cervical cancer
The association between GAS5 expression and survival of cervical cancer patients was investigated by Kaplan-Meier analysis and log-rank test. As shown in Figure 2, cervical cancer patients with low GAS5 expression tend to have shorter overall survival than those with high GAS5 expression (log-rank test, P < 0.05). Univariate analysis demonstrated that FIGO stage, vascular invasion, lymph node metastasis and GAS5 expression were significantly associated with overall survival of cervical cancer patients (Table 2). Multivariate analysis using the Cox proportional hazards model for all variables that were significant in the univariate analysis showed that FIGO stage, vascular invasion, lymph node metastasis and GAS5 expression were independent prognostic factors for patients with cervical cancer (Table 2).
Figure 2.
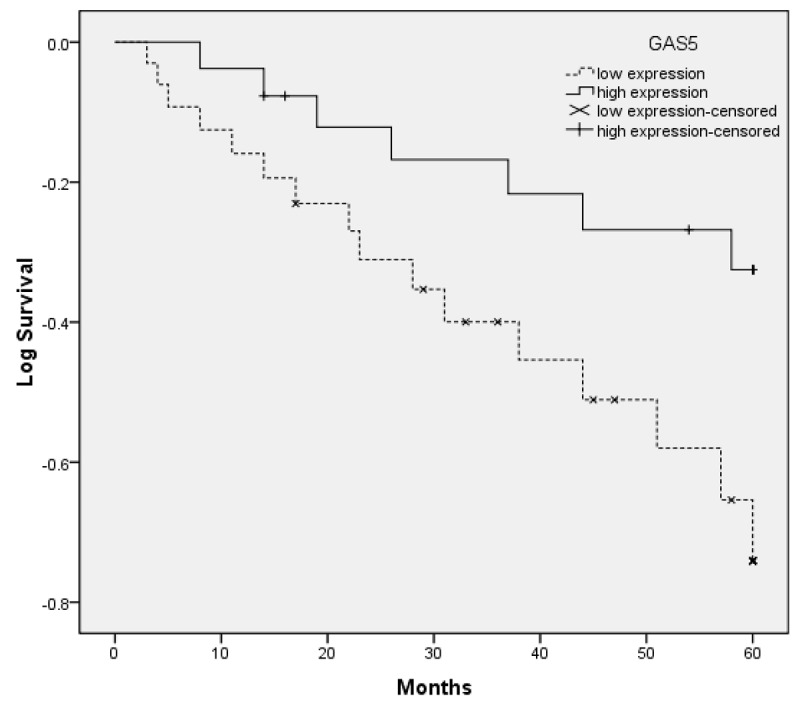
Kaplan-Meier curves for survival time in patients with cervical cancers divided according to GAS5 expression: significantly shorter survival times for patients with low GAS5 expression than for those with high GAS5 expression (*P < 0.05).
Table 2.
Prognostic factors in Cox proportional hazards model
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Risk ratio | 95% CI | P | Risk ratio | 95% CI | P | |
| Age (years) | 2.013 | 0.512-3.617 | 0.297 | |||
| ≥ 50 vs. < 50 | ||||||
| Tumor size | 2.642 | 0.373-4.117 | 0.218 | |||
| ≥ 4 vs. < 4 | ||||||
| Histology | 1.315 | 0.712-1.973 | 0.517 | |||
| Squamous vs. Adenocarcinoma | ||||||
| Differentiation | 2.614 | 0.431-4.714 | 0.089 | |||
| Poor vs. well + moderate | ||||||
| FIGO stage | 4.916 | 1.517-8.229 | 0.018 | 3.882 | 1.427-6.818 | 0.014 |
| IIb~IIIa vs. Ib-IIa | ||||||
| Lymph nodes metastasis | 5.272 | 2.371-9.768 | < 0.001 | 4.131 | 2.131-6.926 | 0.009 |
| Yes vs. No | ||||||
| Vascular invasion | 3.376 | 1.946-6.791 | 0.010 | 2.852 | 1.426-5.618 | 0.005 |
| Yes vs. No | ||||||
| GAS5 | 3.644 | 2.012-8.729 | 0.004 | 3.217 | 1.684-6.964 | < 0.001 |
| high vs. low | ||||||
si-GAS5 significantly downregulated the expression of GAS5 in cervical cancer cells
To further investigate the role of GAS5 in human cervical cancer, GAS5 specific siRNA (si-GAS5) was transfected into Caski cells, respectively. Nonspecific siRNA was used as a negative control (si-NC). As shown in Figure 3A, after transfection with si-GAS5 Caski cells showed a significant decreased mRNA expression of GAS5 compared to the si-NC group (P < 0.05). The result suggested that we successfully downregulated the GAS5 expression in human cervical cancer cells.
Figure 3.
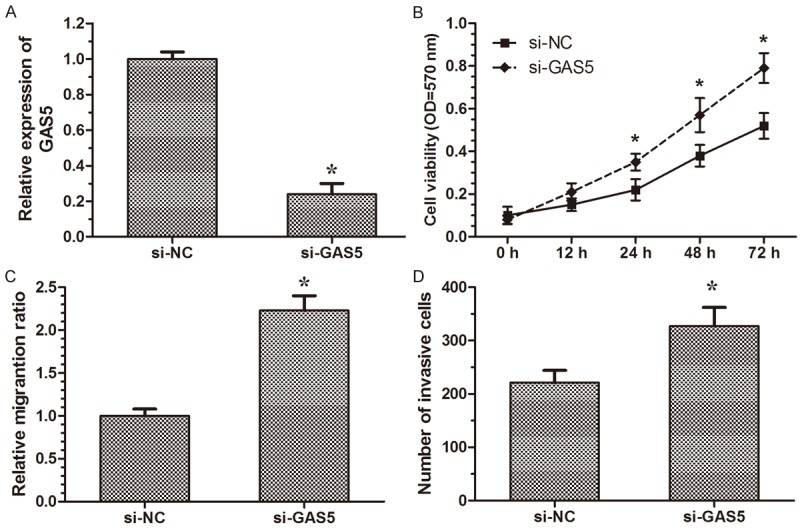
Knockdown of GAS5 promote cell proliferation, migration and invasion in cervical cancer cells. A. The relative expression level of GAS5 in Caski cells are significantly decreased by si-GAS5 compared with the si-NC. B. 48 hours after transfection, MTT assays are conducted to determine the proliferation of Caski cells. C. Cell scratch assay showed Caski cells transfected with si-GAS5 displayed significantly higher migration capacity compared with the si-NC group. D. Cell invasion assay showed Caski cells transfected with si-GAS5 displayed significantly higher invasion capacity compared with the si-NC group. Five areas were randomly selected in each chamber. The number of cells in these areas was counted, and results are expressed as means ± SD for three replicate determination. All data analyzed using Student’s t test *P < 0.05.
Effect of GAS5 on cervical cancer cell proliferation in vitro
To assess the biological role of GAS5 in cervical cancer, we investigated the effect of GAS5 on cell proliferation. MTT assay revealed that cell growth was significantly increased in si-GAS5 transfected cells compared with the si-NC group (Figure 3B, P < 0.05). The result indicated that downregulation of GAS5 increase cervical cancer cell proliferation.
Effect of GAS5 on cervical cancer cell migration and invasion
We then performed cell wound healing assay and transwell invasion assays to investigate the role of GAS5 in the regulation of cell migration and invasion in human cervical cancer cells. Wound healing assays showed that the migratory rate of cervical cancer cells transfected with si-GAS5 was significantly upregulated compared with si-NC group (Figure 3C, P < 0.05). Transwell invasion assays showed that the invasion of cervical cancer cells transfected with si-GAS5 was notably upregulated compared with si-NC group (Figure 3D, P < 0.05). These data indicated that downregulation of GAS5 may promote the migration and invasion of cervical caner cells.
Discussion
Cervical cancer remains to be one of the leading causes of death, so finding new molecular targets for its diagnosis, prognosis and treatment has the potential to improve the clinical strategies and outcomes of this disease. More and more studies indicated that the molecular mechanisms of carcinogenesis are not only relevant to protein coding genes but also to non-coding regulatory RNAs [18]. Some lncRNAs have been identified to play a pivotal role in the happening of cancers. Recent studies showed that numerous lncRNAs were deregulated in various solid tumors and several lncRNAs can regulate cancer metastasis which indicating that the abnormal expression of lncRNAs adds the chances to tumorigenesis and cancer development [9]. However, at present only a few lncRNAs have been functionally studied in detail and many important questions remain to be addressed [19]. In the present study, our attention focused on the lncRNA GAS5.
In our study, we investigated the clinical significance of GAS5 in cervical cancer patients for the first time. By using qRT-PCR, our data showed that GAS5 expression was significantly lower in cervical cancer tissues compared with that in adjacent normal tissues. In addition, we also proved that the relative expression level of GAS5 was associated with FIGO stage, vascular invasion, and lymph node metastasis. However, LncRNA GAS5 expression was not associated with patient’s age, tumor size, histology and differentiation. More importantly, we proved that GAS5 expression was significantly associated with overall survival and could be an independent prognostic factor in patients with cervical cancer. These data indicated that GAS5 expression was an independent prognostic factor for patients with cervical cancer, and play an important role in tumorigenesis, and progression of cervical cancer.
Liu et al. found that the GAS5 expression was commonly downregulated in bladder cancer cell lines and human specimens, gain-of-function and loss-of-function studies showed that GAS5 inhibits bladder cancer cell proliferation, at least in part, by regulating CDK6 expression [20]. Sun et al. showed GAS5 was significantly downregulated in gastric cancer tissues, ectopic expression of GAS5 was demonstrated to decrease gastric cancer cell proliferation and induce apoptosis in vitro and in vivo [21]. From our clinical pathological data, we found that low GAS5 expression was closely associated with FIGO stage, vascular invasion, and lymph node metastasis, thus we supposed GAS5 may also regulate the growth and metastasis of cervical cancer cells. So, it is necessary to identify the biological function of GAS5 in cervical cancer cells.
To further understand the mechanism of GAS5 in cervical cancer process, in vitro experiments were conducted. siRNA-mediated knockdown of GAS5 significantly increased proliferation, migration and invasion capability of cervical cancer cells compared with control group, suggested that GAS5 can affect the tumorigenesis, and progression of cervical cancer. Our findings indicated that lncRNA GAS5 may function as a tumor suppressor and its deficiency or decreased expression could contribute to cervical cancer development.
In summary, we demonstrate for the first time that lncRNA GAS5 was downregulated in cervical cancer tissues and significantly associated with advanced tumor progression. Furthermore, GAS5 was identified as an independent marker for predicting the clinical outcome of cervical cancer patients. The downregulation of GAS5 plays key role in cervical cancer progression. These results suggest that GAS5 is a promising biomarker and a therapeutic target for cervical cancer in future.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103:368–383. doi: 10.1093/jnci/djq562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waggoner SE. Cervical cancer. Lancet. 2003;361:2217–2225. doi: 10.1016/S0140-6736(03)13778-6. [DOI] [PubMed] [Google Scholar]
- 4.Biewenga P, van der Velden J, Mol BW, Stalpers LJ, Schilthuis MS, van der Steeg JW, Burger MP, Buist MR. Prognostic model for survival in patients with early stage cervical cancer. Cancer. 2011;117:768–776. doi: 10.1002/cncr.25658. [DOI] [PubMed] [Google Scholar]
- 5.Lander ES. Initial impact of the sequencing of the human genome. Nature. 2011;470:187–197. doi: 10.1038/nature09792. [DOI] [PubMed] [Google Scholar]
- 6.Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15 Spec No 1:R17–29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 7.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L, Liao LM, Liu AW, Wu JB, Cheng XL, Lin JX, Zheng M. Overexpression of long noncoding RNA HOTAIR predicts a poor prognosis in patients with cervical cancer. Arch Gynecol Obstet. 2014;290:717–23. doi: 10.1007/s00404-014-3236-2. [DOI] [PubMed] [Google Scholar]
- 11.Guo F, Li Y, Liu Y, Wang J, Li G. Inhibition of metastasis-associated lung adenocarcinoma transcript 1 in CaSki human cervical cancer cells suppresses cell proliferation and invasion. Acta Biochim Biophys Sin (Shanghai) 2010;42:224–229. doi: 10.1093/abbs/gmq008. [DOI] [PubMed] [Google Scholar]
- 12.Qin R, Chen Z, Ding Y, Hao J, Hu J, Guo F. Long non-coding RNA MEG3 inhibits the proliferation of cervical carcinoma cells through the induction of cell cycle arrest and apoptosis. Neoplasma. 2012;60:486–492. doi: 10.4149/neo_2013_063. [DOI] [PubMed] [Google Scholar]
- 13.Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y, Takahashi N, Kakegawa E, Yoshida K, Ito Y, Kayano H, Niitsu N, Jinnai I, Bessho M. The GAS5 (growth arrest-specific transcript 5) gene fuses to BCL6 as a result of t (1; 3) (q25; q27) in a patient with B-cell lymphoma. Cancer Genet Cytogenet. 2008;182:144–149. doi: 10.1016/j.cancergencyto.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Mourtada-Maarabouni M, Pickard M, Hedge V, Farzaneh F, Williams G. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2008;28:195–208. doi: 10.1038/onc.2008.373. [DOI] [PubMed] [Google Scholar]
- 16.Qin R, Chen Z, Ding Y, Hao J, Hu J, Guo F. Long non-coding RNA MEG3 inhibits the proliferation of cervical carcinoma cells through the induction of cell cycle arrest and apoptosis. Neoplasma. 2012;60:486–492. doi: 10.4149/neo_2013_063. [DOI] [PubMed] [Google Scholar]
- 17.Tu ZQ, Li RJ, Mei JZ, Li XH. Down-regulation of long non-coding RNA GAS5 is associated with the prognosis of hepatocellular carcinoma. Int J Clin Exp Pathol. 2014;7:4303–4309. [PMC free article] [PubMed] [Google Scholar]
- 18.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 19.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Wang W, Jiang J, Bao E, Xu D, Zeng Y, Tao L, Qiu J. Downregulation of GAS5 promotes bladder cancer cell proliferation, partly by regulating CDK6. PLoS One. 2013;8:e73991. doi: 10.1371/journal.pone.0073991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun M, Jin FY, Xia R, Kong R, Li JH, Xu TP, Liu YW, Zhang EB, Liu XH, De W. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer. 2014;14:319. doi: 10.1186/1471-2407-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]


