Abstract
Objective: To establish a gas chromatography/mass spectrometry (GC/MS)-based metabolomics method to compare the metabolites in the follicular fluid (FF) from patients with in vitro fertilization (IVF) and repeated IVF failure (RIF). Methods: A prospective study was employed in Center for Reprodutive Medcine, Renji Hospital, Shanghai, China, between January and October 2010. FF samples were collected from 13 patients with RIF and 15 patients who achieved pregnancy after the first IVF cycle. Results: Partial least squares (PLS) discriminant analysis of the PCA data revealed that the samples were scattered into two different regions. FF from the two groups differed with respect to 20 metabolites. FF from RIF group showed elevated levels of several amino acids (valine, threonine, isoleucine, cysteine, serine, proline, alanine, phenylalanine, lysine, methionine and ornithine), and reduced levels of dicarboxylic acids, cholesterol and some organic acids. Conclusions: The studies corroborated successful determination of the levels of metabolite in the FF.
Keywords: In vitro fertilization (IVF), follicular fluid, repeated IVF failure (RIF), metabolomics
Introduction
Despite the advancements in infertility management that has been achieved using the in vitro fertilization (IVF) method, the success rate of IVF remains limited to 30-40% [1]. This may due to the report of repeated implnatation failuere (RIF) in many of the couples. RIF, in this regard, can be defined as the chances of failure to achieve a pregnancy following 2-6 IVF cycles post-inoculation with more than 10 high grade embryos [2]. In current clinical practice, there is a tendency to transfer only one or two embryos, and hence, this definition of RIF is no longer useful. For this reason, it has been suggested more recently that RIF may be considered as the failure of three consecutive cycles of IVF, in which reasonably good embryos were transferred [3].
Numerous factors tend to affect the etiology and success of RIF including reduced endometrial receptivity (due to uterine cavity abnormalities, immunological causes or thrombophilia), defective embryonic development (due to genetic abnormalities, zona pellucida hardening or suboptimal embryo culture conditions), endometriosis, hydrosalpinges or suboptimal ovarian stimulation [3,4]. Besides, oocyte quality has emerged as an important factor that affects the pregnancy outcomes [5]. Basically, FF forms the microenvironment of the developing oocyte, and has direct impact on the oocyte quality, sperm-oocyte interaction, sperm-mediated oocyte activation, implantation and early embryo development. Human FF is extremely useful for the assessment of the developmental competence of female gametes, as it is rich in low-molecular weight (LMW) metabolites that are responsible for the development and maturation of viable embryos [6], thus the outcome of pregnancy. Several studies have reported the correlation between components of FF and implantation rate to identify potential markers affecting the successful pregnancy. For instance, levels of metabolites (such as glucose, lactate, choline/phosphocholine and lipoproteins) in the FF have been reported to capable of distinguishing embryos that would develop into the early cleavage stage [7,8]. Furthermore, granulocyte colony-stimulating factor (G-CSF), vitamin D and hyaluronan levels in the FF have shown positive correlations with successful implantation, while anti-Mullerian hormone concentrations are found to be negatively correlated with reproductive outcome [9-12].
Metabolic profiling of FF could prove to be more useful for the assessment of oocyte quality than a targeted metabolic approach or the study of a selective class of substances. It has been observed that more than one metabolite is involved in determining the oocyte’s developmental competence and therefore a panel of biomarkers should be considered for clinical diagnostic purposes. Thus it is necessary to identify the reliable metabolic networks in FF using comprehensive and powerful technologies. This goal can be achieved employing the metabolomic platforms to identify and quantify a large array of metabolites simultaneously [13].
Gas chromatography/mass spectrometry (GC/MS)-based metabolomics are one of the most efficient and reliable platforms available, which can be used for separating and resolving the complex biological mixtures owing to their high efficiency, comprehensive database network and good reproducibility. As an integrated part of the system biology, metabolomics have numerous advantages over the traditional “omics” technologies, since metabolites are the end products of cellular biological processes and their levels ultimately reflect the integrated response of a biological system [13]. The low-molecular weight metabolites can reveal the response of the follicles to all the factors influencing its development. The assessment of metabolites, therefore, has been potentially more informative than the direct study of gene expression (genomics), mRNAs (transcriptomes) or proteins (proteomes) as the increasing gene activation with consequent mRNA and protein synthesis correspond to the altered cellular function, whereas metabolomes provide idea on the actual functional status of a biological system and its cells.
Metabolomics have been proven to be a consistent and informative technology for pattern recognition analysis of several biological systems, and presently being applied for studying the human embryos [6,14,15] and oocytes [16-18]. It helps in discriminating the metabolites secreted by the oocyte into the surrounding medium by investigating the FF, or alternatively, the compounds secreted by the oocyte into the culture medium in which it is suspended during in vitro culture after retrieval (“exometabolomics” or “secretomics”). To date, there are no literature reports available with the application of GC/MS analysis to FF, especially in the context of RIF.
The attempts were made, therefore, to compare metabolite profiles in FF samples between patients with RIF and a control group of participants employing GC/MS and chemical derivatization techniques coupled with principal components analysis (PCA) to identify the metabolic biomarkers in the FF to differentiate between the patients with RIF and the subjects with successful pregnancy after the first cycle.
Materials and methods
Chemicals and reagents
1,-2-chlorophenylalanine (used as an internal standard) was obtained from M/s Shanghai Intechem Tech. Co. Ltd., (Shanghai, China); methanol (pesticide residue grade), bis-(trimethylsilyl)-trifluoroacetamide (BSTFA) plus 1% trimethylchlorosilane (TMCS) and amino acid standard solution were purchased from M/s Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and reagents used during the experimental procedure were purchased from M/s Ampu Company (Shanghai, China). Triple distilled water produced from the Milli-Q water purification system (Millipore, Billerica, MA, USA).
Enrolment of study participants
A case-control study was selected and the entire study was carried out at the Renji Hospital, Shanghai Jiaotong University, Shanghai, China. The study was approved by the Institutional Ethics Committee of Renji Hospital and written informed consents were taken from each participant. Thirteen patients with RIF were recruited between January 2010 and October 2010. The inclusion criteria for patients with RIF selected were age < 38 years followed by at least three IVF embryo transfer failures and the transferal rate of at least 10 high-quality embryos in total. All the male partners had normal semen quality according to World Health Organization (WHO, 1999) criteria. In addition, 15 patients who became pregnant in the first IVF cycle were recruited (over the same time period) as a control group. All patients had a good hormonal reserve and a good response to hormonal stimulation (more than eight oocytes/oocyte retrieval). The patients with premature ovarian failure and endometriosis were excluded. All patients were confirmed to have a normal uterine cavity by hysteroscopy and a normal endometrial thickness evidenced by ultrasound.
IVF procedure
Ovarian stimulation and oocyte retrieval were performed in all patients using a routine protocol consisting of mid-luteal pituitary down-regulation by daily dosing with gonadotropin-releasing hormone agonist (GnRH-a), followed by controlled ovarian stimulation in an individually-adjusted step-up protocol using daily injection of urinary or recombinant gonadotrophins. Oocytes were retrieved after 36-40 h of administration of human chorionic gonadotrophin (HCG), given according to the presence of at least two leading follicles of 18-20 mm. Oocyte pick-up was performed by ultrasound-guided transvaginal follicular aspiration. All mature oocytes retrieved underwent a routine IVF/intra-cytoplasmic sperm injection (ICSI) procedure. The number of cells represented the cleavage rate, and a morphological score was attributed to each embryo prior to embryo transfer based on the degree of fragmentation, granularity and similarity in the size of blastomeres as per the criteria described by Veeck [19]. All embryos were then cultured for 72 h and subsequently transferred back to the uterus. Luteal support was given to all patients. Only clinical pregnancies that included sonographic demonstration of a gestational sac were counted.
Collection of follicular fluid samples
The FF from an individual, mature, 18-20 mm follicle was aspirated and processed immediately. To obtain the exact metabolomics profiling within a single follicle and to avoid contamination from blood, flush medium or mixed follicular fluid during oocyte retrieval, only the follicular fluid retrieved follicle from bilateral ovaries was collected. The presence or absence of blood contamination was graded by visual inspection, and samples which seem to be cloudy or blood stained were discarded. Meticulous care was taken to include only uncontaminated samples. The FF was centrifuged at 2000 rpm for 20 min at room temperature and the supernatant was collected and stored at -80°C until further analysis.
Specimen processing and GC/MS analysis
The FF samples were thawed at 37°C for 3 min and vortex mixed for 15 s. One mL of a monophasic mixture of chloroform-methanol-water (2:5:2, %v/v/v) and 20 μL of 100 μM ribitol (as an internal standard) were added to 50 μL of the sample. An aliquot of 210 μL of HPLC-grade methanol was added to the mixture. The solution was vortex mixed for 5 min and ultrasonicated at room temperature for 20 min. After careful adjustment of the pH between 9 and 10 using NaOH (500 mM), the solution was filtered through a 0.45-μm membrane. Following this, 100 μL of the resulting filtrate was transferred to a screw top vial (2 mL) with PTFE-lined screw cap (Pyrex, UK) and evaporated to dryness under a stream of nitrogen gas. Subsequently, 80 μL of BSTFA with 1% TMCS was added to each vial and the mixture was allowed to react for 1 h in a microwave oven (Haier Co. Qidao, Shandong, China) at a temperature of 100°C [20].
An aliquot (1 μL) of the derivatized sample was injected into an Agilent 7890A GC system equipped with a 30.0 m × 0.25 mm internal diameter capillary column containing fused-silica as stationary phase with a thickness of 0.25 μm (M/s Agilent Technologies, Shanghai, China). The injector temperature was set at 270°C. Helium was used as the carrier gas. The column temperature was initially maintained at 80°C for 2 min and then increased upto 300°C @ 10°C per min, where it was held for 6 min. The column effluent was introduced into the ion source of an Agilent 5975C mass selective detector. The MS quadrupole temperature and ion source temperature was set at 150°C and 230°C , and the masses were acquired between m/z 50 to 600.
Data processing and pattern recognition
After GC/MS analysis, each sample was represented by a GC/MS total ion chromatogram (TIC), and the peak areas for each of the compounds were determined. The ratio of the peak area for each compound to that of the corresponding internal standard was calculated as the response. Two-sample t-test was used for comparison of metabolite levels to determine any differences between the RIF and control groups. The differences among the groups with P-value ≤ 0.05 were considered statistically significant. Multivariate statistical tools like PCA were used to differentiate the samples and were performed using MATLAB 7.2 software (M/s Math Works, Natick, MA USA). Further, the data obtained from the differentially expressed compounds were fitted in the PCA model. The score plots of the first three principal components were allowed for visualization of the data and statistical comparison of the samples between the study and control groups.
Results
Participant characteristics
The characteristics of the 13 patients with RIF and the 15 control participants in the control group selected in the present study are enlisted in Table 1. Significant differences between the two groups were found for the duration of the infertility and the number of previous cycles received. However, no significant differences were evident between the groups for the other clinical parameters, including age, endometrial thickness on the day of HCG administration and various parameters reflecting the outcome of the treatment cycle (fertilization rate, cleavage rate and good quality embryo rate). This can be better explained by categorizing the embryo type as Grade I and Grade II embryos as per the procedure described Veeck [19].
Table 1.
Clinical characteristics of the participants included in the study
| Parameter | RIF group (n = 13) | Control group (n = 15) |
|---|---|---|
| Age (years) | 34.0 ± 3.9 | 33.3 ± 3.2 |
| Duration of infertility (years) | 7.9 ± 4.3* | 3.5 ± 1.5 |
| Number of previous cycles received | 3.7 ± 1.1* | 1.0 ± 0.0 |
| Fertilization rate (%) | 57.6 | 64.3 |
| Cleavage rate (%) | 98.1 | 100 |
| Good quality embryo rate (%) | 63.5 | 69.7 |
| Number of embryos transferred | 3.1 ± 0.7 | 2.5 ± 0.6 |
| HCG-day endometrial thickness (mm) | 8.0 ± 0.8 | 8.9 ± 0.9 |
Data are presented as the mean ± standard deviation or as a percentage.
P < 0.05.
RIF, repeated in vitro fertilization failure; HCG, human chorionic gonadotropin.
Metabolomic profiling of FF samples
GC/MS TIC chromatograms of the FF samples from the control group and study group are portrayed in Figure 1 and representative TICs are shown in Figure 2. The majority of the peaks in the chromatograms were identified (using the NIST mass spectral library) as endogenous metabolites including amino acids, organic acids, carbohydrates and fatty acids. These metabolites are known to be involved in multiple biochemical processes, especially in energy and lipid metabolism.
Figure 1.
GC/MS TICs of the FF samples from the RIF (n = 13) and control (n = 15) groups. The upper panel (Group C) shows the TICs of the 15 FF samples of the control group, while the lower panel (Group R) illustrates the TICs of the 13 samples of RIF group. The ordinate shows the relative mass abundance and the abscissa shows the retention time.
Figure 2.
Representative GC/MS TICs of FF samples from the RIF and control groups. The upper panel (C1162) shows the TIC of a FF sample from the control group, while the lower panel (R1118) illustrates the TIC of a sample from the RIF group. The ordinate shows the relative mass abundance and the abscissa shows the retention time.
Pattern recognition and functional analysis
PCA, the most commonly used multivariate statistical algorithms in metabolomics studies was employed to process the GC/MS data. PCA is an unsupervised projection method used to visualize the dataset and to depict the similarities and differences among the study groups. The simultaneous comparison of a large number of complex objects was facilitated by reducing the dimensionality of the dataset via three dimensional mapping procedures. The resulting data were displayed as “score plots”, which represented the distribution of the samples in multivariate space. After standardization of the data, marker metabolites that were responsible for differentiation of the RIF group from the control group were determined, which are summarized in Figures 3, 4 and 5.
Figure 3.
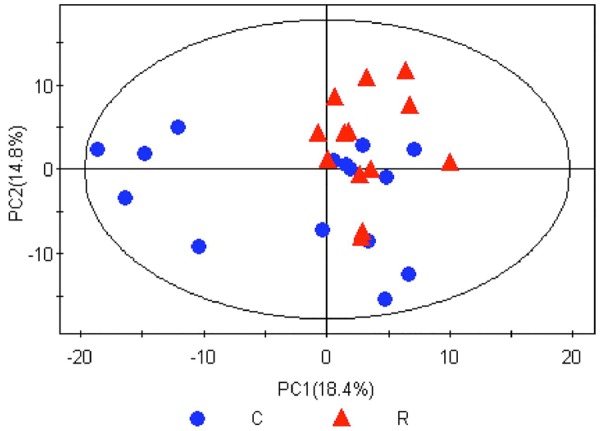
PCA score plot in the RIF group and control group sample. C, Control group; R, RIF group. After PCA analysis, 5 principal components were obtained underlying the variance of the dataset; the contributions of the top two principal components to the variance were 18.4% (PC1) and 14.8% (PC2). The score plot illustrates wide metabolic variation within the control samples, but less variation (i.e., good relatively good uniformity) in the RIF group.
Figure 4.
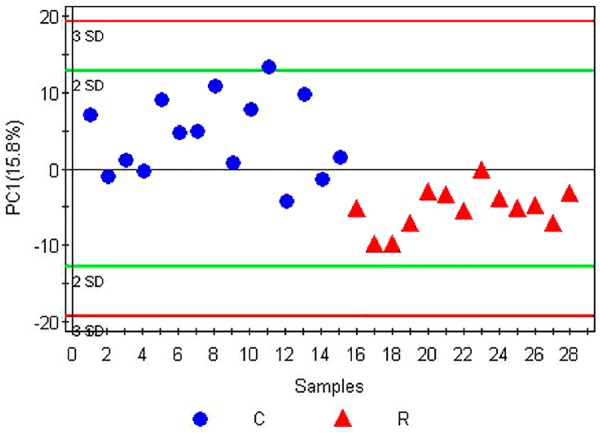
Partial least squares discriminant analysis (PLS-DA) score plot of the RIF group and the control group. C, Control group; R, RIF group. To test the difference between the experimental group and the control group, partial least squares discriminant analysis (PLS-DA) was employed for modeling of the samples. A principal component, PC1, was estimated, with parameters of R 2 Y = 0.581 and Q 2 = 0.413. Generally, a model with an R 2 Y value > 0.4 is considered to be reliable. Therefore, this PLS-DA model is suitable for interpretation of the differences in FF metabolite between the RIF group and the control group. Q 2 indicates the prediction rate of the currently established model; since Q 2 was > 0.4, this PLS-DA model could be used to predict the unknown samples, with a contribution rate of 15.8% to the differences between the RIF group and the control group. The score plots of the samples from the RIF group and the control group were distributed predominantly on the upper and lower sides of PC1, respectively, indicating different clustering between the two groups. Hence, PC1 could be reliably used to differentiate follicular fluid metabolite differences between the RIF group and the control group.
Figure 5.
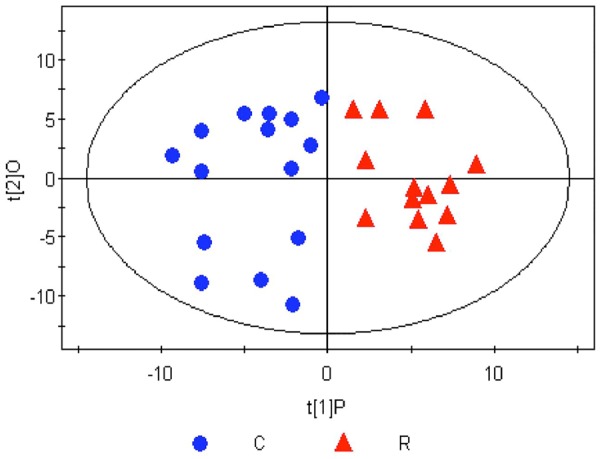
Orthogonal partial least squares discriminant analysis (OPLS-DA) score plot of the RIF group and the control group after successful modeling. C, Control group; R, RIF group. To identify the metabolites causing the significant differences, orthogonal partial least squares discriminant analysis (OPLS-DA) was employed to screen the signal unrelated to the model category, namely the orthogonal signal, so as to obtain the OPLS-DA model. The major parameters to assess the quality of the model involve a principal component and an orthogonal component. This OPLS-DA model is suitable for the interpretation of the difference in the metabolism of the samples between the RIF group and the control group. After screening out the noisy signal unrelated to the model category, metabolic profiling on PC1 yielded good separation between the two groups, with the score plots of the samples from the two groups distributed on the left (negative) and right sides of PC1 (t[1]P).
Differences in metabolites between the RIF and control groups
The study demonstrated the variable importance in the projection (VIP) statistic of the first principal component of the orthogonal partial least squares discriminant analysis (OPLS-DA) model (threshold > 1), together with the p-value of the Student’s t-test (threshold 0.05), to identify differences in the expressions of the metabolites. The differences in metabolites among the groups were qualitatively demarcated using self-built standard material database and NIST commercial databases. As detailed in Table 2, significant augmentation in the levels of metabolites were observed in the RIF group compared with the control group (i.e., with respect to the levels of valine, threonine, isoleucine, cysteine, serine, proline, alanine, phenylalanine, lysine, methionine and ornithine), while levels of other components like dicarboxylic acids, cholesterol, and some organic acids were significantly reduced.
Table 2.
Differences in metabolite levels in the RIF group, compared with the control group
| No. | Compound | VIP-value (OPLS) | P-value (t-test) | Fold change* [Log2(R/C)] |
|---|---|---|---|---|
| 1 | α-Aminoadipic acid | 1.51 | 2.51E-02 | -2.74 ↓ |
| 2 | 6-Hydroxy-2-aminohexanoic acid | 1.36 | 4.54E-02 | -2.44 ↓ |
| 3 | Aminomalonic acid | 1.59 | 1.73E-02 | -1.23 ↓ |
| 4 | L-Aspartic acid | 1.43 | 2.76E-02 | -1.20 ↓ |
| 5 | 3-Hydroxybutyric acid | 1.34 | 4.94E-02 | -0.91 ↓ |
| 6 | Cholesterol | 1.36 | 4.53E-02 | -0.45 ↓ |
| 7 | Glutaric acid | 1.34 | 4.84E-02 | -0.45 ↓ |
| 8 | Succinic acid | 1.49 | 3.25E-02 | -0.43 ↓ |
| 9 | 3-Methyl-2-ketobutyric acid | 1.49 | 2.73E-02 | -0.20 ↓ |
| 10 | L-Valine | 1.69 | 1.07E-02 | 0.28 ↑ |
| 11 | L-Threonine | 1.68 | 1.17E-02 | 0.34 ↑ |
| 12 | L-Isoleucine | 1.72 | 9.50E-03 | 0.38 ↑ |
| 13 | L-Cysteine | 1.34 | 4.94E-02 | 0.46 ↑ |
| 14 | L-Serine | 1.77 | 7.38E-03 | 0.55 ↑ |
| 15 | L-Proline | 1.73 | 9.10E-03 | 0.59 ↑ |
| 16 | L-Alanine | 2.47 | 4.56E-05 | 0.63 ↑ |
| 17 | L-Phenylalanine | 1.68 | 1.16E-02 | 0.66 ↑ |
| 18 | L-Lysine | 1.45 | 3.20E-02 | 0.83 ↑ |
| 19 | L-Methionine | 1.99 | 2.03E-03 | 0.87 ↑ |
| 20 | L-Ornithine | 1.38 | 4.21E-02 | 1.00 ↑ |
The data represent metabolite levels in the RIF group, relative to those in the control group. VIP, variable importance in projection statistic; OPLS, orthogonal partial least squares.
Discussion
The rationale of present study was for the application of GC/MS and metabolomics techniques to identify the metabolites in FF and the difference in their levels between the patients with RIF and individuals who have not suffered from RIF. The major findings of the study include elevated levels of the key amino acids like valine, threonine, isoleucine, cysteine, serine, proline, alanine, phenylalanine, lysine, methionine, and ornithine) and reduced levels of dicarboxylic acids, cholesterol, and some organic acids among the patients in the RIF group vis-à-vis the participants in the control group. To the best of our knowledge, this is the first study which demonstrates the difference in the composition of FF metabolite in patients with RIF compared to that of the individuals without RIF. Since all the embryos transferred were considered to be of good quality from a morphological standpoint. The variation in the metabolites between the RIF group and control group may correlate with the developmental competence of the human oocyte. Hence, we suggest that determination of the levels of certain metabolites may be useful for early assessment of the developmental potential of oocytes and subsequent metabolomic profiling of FF may prove to be a valuable technique for the selection of competent oocytes and viable embryos in assisted reproductive techniques (ART).
Despite the immense applicability of this technique, there are few shortcomings which are as follows. Follicular fluid (FF) is superfluous, abundant and easily available during oocyte pick-up, and theoretically represents an optimal source of noninvasive biochemical predictors of oocyte quality [17]. However the procedure of single follicle aspiration is uncomfortable both for the patient and for the physician. Moreover, it is not completely clear if the FF concentration of a given substance is a variable related to the quality of the follicle and of the oocyte or to the clinical characteristics of the patient, such as age or type of ovarian stimulation.
The mechanisms by which amino acids promote human pre-implantation embryo development are largely unknown. In early embryos of other species, notably the mouse, amino acids have been shown to serve a variety of functions in the synthesis of proteins and nucleotides [21], acts as a source of energy [22], osmolytes [23], antioxidants [24], pH regulators [25], chelators [26] and precursor for synthesis of signaling molecule, nitric oxide [27].
Several studies have investigated the effects of individual or small numbers of amino acids on embryo development [28-30]. Hence, in the female tract, oocytes and embryos were exposed to a physiological mixture of amino acids. To circumvent the limitations of the previous studies, which have focused only on a small number of amino acids, we applied a GC/MS-based technique to measure the metabolites in the FF samples. Our study identified marked differences in the amino acid composition of the FF between the RIF group and the control group, which tend to achieve pregnancy successfully in the first IVF cycle. It is likely that the oocyte quality plays a major role in determining embryo viability and our results showed potential implications in the selection of human embryos for transfer to attain successful IVF. Besides, our study ratified that the elevated levels of 12 amino acids may be strongly responsible for plausible failure of pregnancy during IVF. The findings revealed that the elevated levels of valine, threonine, isoleucine, cysteine, serine, proline, alanine, phenylalanine, lysine, methionine, and ornithine in RIF. Although the underlying mechanisms responsible for such biochemical changes are currently unknown, yet this may be due to the number of reasons like such as oocytes of the RIF group may show a weakened ability to complete egg maturation and carry out protein synthesis, and have lower energy consumption compared with the control group. This may result in an increase in the levels of essential amino acids in the FF of the RIF group. Secondly, in the bovine embryo, alanine is produced from the transamination of pyruvate [31], and this is associated with sequestration of the potentially toxic effects of ammonia. In general, oocyte quality is affected by the environment in which it grows and within this environment amino acid metabolism can protect embryos from oxidative damage [24] and regulate intracellular osmotic pressure to maintain a normal cell volume, thereby ensuring normal development [23]. Apart from these, other plausible mechanisms include increase in the osmotic pressure external to the egg may be imbalanced leading to the increased in the amino acid load in the RIF group. Also generation of a substantial quantity of ammonium during oxidative metabolism causes loss of growth of oocytes in the RIF group. The build-up of ammonium in the FF can lead to metabolic perturbations, alterations in gene expression patterns and a reduction of embryo viability [32]. In normal metabolism, the ammonium produced by cells is removed by the synthesis of alanine, glutamic amide and the urea cycle. A likely role for the increased alanine and ornithine in the FF, observed in this study, is in the removal of excess ammonium [33]. Consistent with this idea, Humpherson and colleagues [34] found that the pig embryo culture releases large amounts of alanine. Many a time the amino acid transport system may likely be dysfunctional in the RIF group due to which the egg losses selectivity for transport of amino acids, which are considered to be essential for oocyte development.
Another important finding of the present study was reduction in the level of FF cholesterol in the RIF group compared to the control. Estrogen is synthesized from testosterone and androstenedione, which in turn are synthesized from cholesterol. The reduced cholesterol level in the RIF group may ascribed to the consequence of abnormalities in lipid metabolism and cholesterol utilization, thereby leading to the alteration in the levels of estrogen, which is crucial for growth and development of follicles.
The decreased level of cholesterol in the FF of the RIF group may be due to the excessive cholesterol consumption during the synthesis of estrogen, thereby resulting in a higher estrogen level in the RIF group than the normal group. It has been reported that in a successful IVF-embryo transfer cycle, the area of the estradiol plasma concentration-time curve is significantly lower than that of an unsuccessful cycle [35]. Estrogen can promote follicular maturation, but if levels are too high, it can damage the uterine and ovarian blood flow. This can result in follicular hypoxia, with adverse effects on the egg spindle that impair the developmental potential of embryos. In addition, the elevated estrogen levels in the ovary, pituitary, and hypothalamus may shorten the luteal phase, lower the mid-luteal progesterone concentration and change the endometrial tissue. Hence, even if a good quality embryo is transferred, this may not improve the pregnancy outcome. However, the precise role of estrogen in follicular development and in the oocyte maturation process remains unclear. Further research in this domain is required to determine the mechanism underlying the low level of cholesterol in the FF of the RIF group.
In conclusion, metabolomic profiling of human FF from IVF patients containing several metabolites were identified by GC/MS, which furnished idea on the total increase or decrease in their levels in the FF of patients with RIF compared with those achieving successful implantation. This work raises the exciting possibility that metabolomic profiling may be a realistic approach to optimize the selection of embryos to achieve a successful pregnancy with potential advantages over traditional morphology-based selection techniques. This method is non-invasive, rapid, sensitive, and reliable, only requires 1 mL of FF for each test procedure, thus make it highly suitable for clinical setting. Large-scale, prospective studies are required to relate the metabolic profiling of the FF to evaluate the developmental potential of human oocytes and applying this non-invasive technique in routine clinical practice.
Acknowledgements
This work was supported by grants from National Natural Science Foundation China (81370687, 81200468), Shanghai Nature Science Foundation China (13ZR1424600), the Shanghai Key Laboratory for Assisted Reproduction and Reproductive Genetics (12DZ2260600).
Disclosure of conflict of interest
None.
References
- 1.Gerris J, De Neubourg D, Mangelschots K, Van Royen E, Van de Meerssche M, Valkenburg M. Prevention of twin pregnancy after in-vitro fertilization or intracytoplasmic sperm injection based on strict embryo criteria: a prospective randomized clinical trial. Hum Reprod. 1999;14:2581–2587. doi: 10.1093/humrep/14.10.2581. [DOI] [PubMed] [Google Scholar]
- 2.Tan BK, Vandekerckhove P, Kennedy R, Keay SD. Investigation and current management of recurrent IVF treatment failure in the UK. BJOG. 2005;112:773–780. doi: 10.1111/j.1471-0528.2005.00523.x. [DOI] [PubMed] [Google Scholar]
- 3.Margalioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod. 2006;21:3036–3043. doi: 10.1093/humrep/del305. [DOI] [PubMed] [Google Scholar]
- 4.Simon A, Laufer N. Repeated implantation failure: clinical approach. Fertil Steril. 2012;97:1039–1043. doi: 10.1016/j.fertnstert.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Das M, Holzer HE. Recurrent implantation failure: gamete and embryo factors. Fertil Steril. 2012;97:1021–1027. doi: 10.1016/j.fertnstert.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 6.Botros L, Sakkas D, Seli E. Metabolomics and its application for non-invasive embryo assessment in IVF. Mol Hum Reprod. 2008;14:679–690. doi: 10.1093/molehr/gan066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace M, Cottell E, Gibney MJ, McAuliffe FM, Wingfield M, Brennan L. An investigation into the relationship between the metabolic profile of follicular fluid, oocyte developmental potential, and implantation outcome. Fertil Steril. 2012;97:1078–1084. e1078. doi: 10.1016/j.fertnstert.2012.01.122. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi L, Gagliardi A, Campanella G, Landi C, Capaldo A, Carleo A, Armini A, De Leo V, Piomboni P, Focarelli R, Bini L. A methodological and functional proteomic approach of human follicular fluid en route for oocyte quality evaluation. J Proteomics. 2013;90:61–76. doi: 10.1016/j.jprot.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Lédée N, Gridelet V, Ravet S, Jouan C, Gaspard O, Wenders F, Thonon F, Hincourt N, Dubois M, Foidart JM. Impact of follicular G-CSF quantification on subsequent embryo transfer decisions: a proof of concept study. Hum Reprod. 2013;28:406–413. doi: 10.1093/humrep/des354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pabuccu R, Kaya C, Cağlar GS, Oztas E, Satiroglu H. Follicular-fluid anti-Mullerian hormone concentrations are predictive of assisted reproduction outcome in PCOS patients. Reprod Biomed Online. 2009;19:631–637. doi: 10.1016/j.rbmo.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, Pal L. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril. 2010;94:1314–1319. doi: 10.1016/j.fertnstert.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babayan A, Neuer A, Dieterle S, Bongiovanni AM, Witkin SS. Hyaluronan in follicular fluid and embryo implantation following in vitro fertilization and embryo transfer. J Assist Reprod Genet. 2008;25:473–476. doi: 10.1007/s10815-008-9268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang M, Joseph JW. Metabolomic analysis of pancreatic beta-cell insulin release in response to glucose. Islets. 2012;4:210–222. doi: 10.4161/isl.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vergouw CG, Botros LL, Roos P, Lens JW, Schats R, Hompes PG, Burns DH, Lambalk CB. Metabolomic profiling by near-infrared spectroscopy as a tool to assess embryo viability: a novel, non-invasive method for embryo selection. Hum Reprod. 2008;23:1499–1504. doi: 10.1093/humrep/den111. [DOI] [PubMed] [Google Scholar]
- 15.Pinero-Sagredo E, Nunes S, de Los Santos MJ, Celda B, Esteve V. NMR metabolic profile of human follicular fluid. NMR Biomed. 2010;23:485–495. doi: 10.1002/nbm.1488. [DOI] [PubMed] [Google Scholar]
- 16.Nagy ZP, Jones-Colon S, Roos P, Botros L, Greco E, Dasig J, Behr B. Metabolomic assessment of oocyte viability. Reprod Biomed Online. 2009;18:219–225. doi: 10.1016/s1472-6483(10)60259-3. [DOI] [PubMed] [Google Scholar]
- 17.Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7:40. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh R, Sinclair KD. Metabolomics: approaches to assessing oocyte and embryo quality. Theriogenology. 2007;68(Suppl 1):S56–62. doi: 10.1016/j.theriogenology.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Veeck LL. An Atlas of Human Gametes and Conceptuses: An Illustrated Reference for Assisted Reproductive Technology. Boca Raton, Florida: CRC Press; 1999. [Google Scholar]
- 20.Wu H, Xue R, Dong L, Liu T, Deng C, Zeng H, Shen X. Metabolomic profiling of human urine in hepatocellular carcinoma patients using gas chromatography/mass spectrometry. Anal Chim Acta. 2009;648:98–104. doi: 10.1016/j.aca.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki C, Yoshioka K, Sakatani M, Takahashi M. Glutamine and hypotaurine improves intracellular oxidative status and in vitro development of porcine preimplantation embryos. Zygote. 2007;15:317–324. doi: 10.1017/S0967199407004273. [DOI] [PubMed] [Google Scholar]
- 22.Gottlob RO, DeRouchey JM, Tokach MD, Goodband RD, Dritz SS, Nelssen JL, Hastad CW, Knabe DA. Amino acid and energy digestibility of protein sources for growing pigs. J Anim Sci. 2006;84:1396–1402. doi: 10.2527/2006.8461396x. [DOI] [PubMed] [Google Scholar]
- 23.Lane M, Gardner DK. Mitochondrial malate-aspartate shuttle regulates mouse embryo nutrient consumption. J Biol Chem. 2005;280:18361–18367. doi: 10.1074/jbc.M500174200. [DOI] [PubMed] [Google Scholar]
- 24.Baltz JM, Tartia AP. Cell volume regulation in oocytes and early embryos: connecting physiology to successful culture media. Hum Reprod Update. 2010;16:166–176. doi: 10.1093/humupd/dmp045. [DOI] [PubMed] [Google Scholar]
- 25.Edwards LJ, Williams DA, Gardner DK. Intracellular pH of the mouse preimplantation embryo: amino acids act as buffers of intracellular pH. Hum Reprod. 1998;13:3441–3448. doi: 10.1093/humrep/13.12.3441. [DOI] [PubMed] [Google Scholar]
- 26.Van Winkle LJ, Haghighat N, Campione AL. Glycine protects preimplantation mouse conceptuses from a detrimental effect on development of the inorganic ions in oviductal fluid. J Exp Zool. 1990;253:215–219. doi: 10.1002/jez.1402530211. [DOI] [PubMed] [Google Scholar]
- 27.Brosnan J, Rooyackers O. The importance of amino acids as independent metabolites, signalling molecules and as building blocks for protein. Curr Opin Clin Nutr Metab Care. 2012;15:47–48. doi: 10.1097/MCO.0b013e32834de431. [DOI] [PubMed] [Google Scholar]
- 28.Scott R, Seli E, Miller K, Sakkas D, Scott K, Burns DH. Noninvasive metabolomic profiling of human embryo culture media using Raman spectroscopy predicts embryonic reproductive potential: a prospective blinded pilot study. Fertil Steril. 2008;90:77–83. doi: 10.1016/j.fertnstert.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 29.Seli E, Vergouw CG, Morita H, Botros L, Roos P, Lambalk CB, Yamashita N, Kato O, Sakkas D. Noninvasive metabolomic profiling as an adjunct to morphology for noninvasive embryo assessment in women undergoing single embryo transfer. Fertil Steril. 2010;94:535–542. doi: 10.1016/j.fertnstert.2009.03.078. [DOI] [PubMed] [Google Scholar]
- 30.Wale PL, Gardner DK. Oxygen regulates amino acid turnover and carbohydrate uptake during the preimplantation period of mouse embryo development. Biol Reprod. 2012;87:24, 1–8. doi: 10.1095/biolreprod.112.100552. [DOI] [PubMed] [Google Scholar]
- 31.Tan LL, Musa A, Lee YH. Determination of ammonium ion using a reagentless amperometric biosensor based on immobilized alanine dehydrogenase. Sensors (Basel) 2011;11:9344–9360. doi: 10.3390/s111009344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lane M, Gardner DK. Ammonium induces aberrant blastocyst differentiation, metabolism, pH regulation, gene expression and subsequently alters fetal development in the mouse. Biol Reprod. 2003;69:1109–1117. doi: 10.1095/biolreprod.103.018093. [DOI] [PubMed] [Google Scholar]
- 33.Orsi NM, Leese HJ. Ammonium exposure and pyruvate affect the amino acid metabolism of bovine blastocysts in vitro. Reproduction. 2004;127:131–140. doi: 10.1530/rep.1.00031. [DOI] [PubMed] [Google Scholar]
- 34.Humpherson PG, Leese HJ, Sturmey RG. Amino acid metabolism of the porcine blastocyst. Theriogenology. 2005;64:1852–1866. doi: 10.1016/j.theriogenology.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 35.Holzer H, Casper R, Tulandi T. A new era in ovulation induction. Fertil Steril. 2006;85:277–284. doi: 10.1016/j.fertnstert.2005.05.078. [DOI] [PubMed] [Google Scholar]


