Abstract
Objective: The aim of the study is to assess the efficacy of the levonorgestrel-releasing intrauterine system (LNG-IUS) on the tamoxifen-induced endometrial lesions in breast cancer patients. Methods: PubMed and EMBASE databases were searched for eligible studies. Odds ratios were obtained to estimate the association between the LNG-IUS and tamoxifen-induced endometrial lesions. The fixed effects or random-effects model was used to combine data depending on heterogeneity. Results: With three eligible randomized clinical trials involving 359 patients, this analysis demonstrated tamoxifen-treated breast cancer patients using the LNG-IUS derived benefit from de novo polyps prevention (P < 0.0001, OR 0.18, 95% CI: 0.08-0.42). However, the LNG-IUS only showed a trend of maintaining endometrial proliferation or secretory status (P = 0.05, OR 0.36, 95% CI 0.13-1.02) and no statistical difference in atrophic or inactive changes (P = 0.13, OR 0.24, 95% CI 0.04-1.53) or endometrial hyperplasia without atypia (P = 0.08, OR 0.20, 95% CI 0.04-1.18). The LNG-IUS didn’t have an increased incidence in breast cancer recurrence (P = 0.28, OR 1.75, 95% CI: 0.64-4.80) and cancer-induced death (P = 0.71, OR 1.22, 95% CI: 0.42-3.52). Bleeding in the treatment group was statistically more frequent than that in the control group (OR 6.20, 95% CI: 2.99-12.85, P < 0.00001). Conclusions: This analysis verifies the efficacy of the LNG-IUS in preventing tamoxifen-induced polyps. The LNG-IUS didn’t have an increased incidence in breast cancer recurrence and cancer-induced death. Long-term, large randomized studies of the LNG-IUS will be necessary to determine the benefit and risk in tamoxifen-treated breast cancer patients.
Keywords: Breast cancer, tamoxifen, LNG-IUS, endometrial lesion
Introduction
Breast cancer, which is usually hormone-dependent, is regarded as the most common malignant tumor in women. Tamoxifen, a selective estrogen receptor modulator (SERM), is the preferred hormonal treatment [1] for estrogen receptor-positive subtypes of breast cancer. It plays a crucial role in the prevention of recurrence and metastasis and it also is used as salvage therapy for metastatic breast cancer [2].
Besides the antiestrogenic effects on the breast, tamoxifen has estrogenic effects on the skeletal system [3], lipid metabolism [4] and gynecological organs such as the cervix and uterus [5]. Chronic tamoxifen treatment may play a role in the carcinogenicity of the endometrium and increase the morbidity of endometrial cancer [6].
Uterine curettage is carried out to exclude the possibility of cancer when the thickness of the endometrium is more than 8 mm, even 5 mm for postmenopausal patients. Repeated curettage damages the endometrium and increases patient anxiousness. Therefore, it is essential to find an effective way to avoid unnecessary repeated curettage.
To address this important issue, we undertook a systematic review of all the relevant randomized clinical trials and performed a quantitative meta-analysis to assess the efficacy of the levonorgestrel-releasing intrauterine system (LNG-IUS) on the tamoxifen-induced endometrial lesions in breast cancer patients.
Methods
Search strategy
Relevant trials from the last two decades were identified using a computerized search of PUBMED and EMBASE databases. The search was restricted to English language articles using the following algorithm: (breast cancer OR breast carcinoma OR breast neoplasm) AND (tamoxifen AND (levonorgestrel- releasing intrauterine system OR LNG-IUS)) AND (randomized controlled trial OR clinical trial). All data were from intent-to-treat analyses. All the randomized controlled trials (RCTs) should be approved by the ethics committee.
Data extraction and synthesis
The following information was extracted from each publication: year of publication, first author, follow-up period, and number of patients analyzed per arm, patient demographics, and duration of tamoxifen treatment and methods of intervention. Data on endometrial lesions from the final assessment, as defined in the individual trials, were also extracted. Five endometrial changes were listed in this analysis as follows: polyps, submucosal fibroids, atrophic or inactive hyperplasia, proliferation or secretory endometrium, endometrial thickness. We further assessed the changes according to the menstruation. In addition, we abstracted side effects of the LNG-IUS and the recurrence and mortality incidences in these breast cancer patients. Two authors (F.Y and Z.Z.G) independently extracted all data from each eligible publication. Discrepancies were resolved by consensus. The meta-analysis was carried out in two arms: the treatment group (LNG-IUS) and the control group (endometrial surveillance only).
Assessment of risk of bias
Risk of bias was assessed using the Cochrane risk of bias tool. Two reviewers independently assessed methodological quality and resolved disagreements through discussion. The evaluation focused on randomization, blinding, percentage of lost to follow-up, intention-to-treat (ITT) principle, incomplete outcome data and selective reporting. We planned to assess publication bias using funnel plots and visual inspection for asymmetry [7]. However, it was not appropriate to construct funnel plots to detect publication bias because of the small number of included studies. We considered the research strategies used to be rigorous and therefore capable of locating all relevant available RCTs.
Statistical analysis
All data were analyzed using RevMan 5 (The Cochrane Collaboration, Copenhagen, Denmark) and Stata 12.0 analysis software (Stata Corporation, College Station, TX, USA). The relative frequency of an outcome event per arm was expressed as an odds ratio (OR) and a 95% confidence interval (CI). The heterogeneity of the study results was assessed using Conchran chi-squared statistics and an I-squared test, which determined the use of either a fixed-effects or random-effects (Mantel-Haenszel method or inverse variance methods) model. Heterogeneity was considered when either the P-value < 0.05 or the I-square > 50% [8]. All statistical tests were two-sided, and statistical significance was defined as a p-value less than 0.05.
Results
Description of eligible studies
There were three eligible randomized clinical trials [9-11] enrolling 359 patients that met the inclusion criteria, two of which had long-term follow-up and updated the prior results [12,13]. Patient characteristics are listed in Table 1. Among the three trials, 118 patients did not take any tamoxifen or any other hormonal therapy before the start of the study, while the other 241 patients were exposed to tamoxifen treatment for at least one year.
Table 1.
Characteristics of eligible studies included in the meta-analysis
| Author (year) | Median follow up | Samples | Duration of tamoxifen treatment (weeks) | Intervention |
|---|---|---|---|---|
| Gardner 2009 [9] | 4.5 years | Treatment group (surveillance + LNG-IUS): 47 | Treatment group: 148.8±67.8 | 1. Mammography: annually |
| Control group (surveillance): 52 | The control group: 152.6±72.0 | 2. Transvaginal ultrasonography | ||
| 3. Hysteroscopy and endometrial biopsy | ||||
| Kesim 2008 [11] | 3 years | Treatment group (surveillance + LNG-IUS): 70 | Treatment group: 100.0±53.5 | 1. Transvaginal ultrasound |
| Control group (surveillance): 72 | The control group: 114.4±63.9 | 2. Hysteroscopy and endometrial biopsy | ||
| Wong 2013 [10] | 5 years | Treatment group (surveillance + LNG-IUS): 58 | All patients didn’t take tamoxifen before trial | 1. Transvaginal ultrasonography |
| Control group (surveillance): 60 | 2. Hysteroscopy and endometrial biopsy |
Exclusion criteria included pelvic inflammatory disease [9,10], a history of malignant disease other than breast cancer [9,11], active liver disease [9], congenital uterine anomaly [10], the presence of grade III submucosal fibroids or endometrial polyps [9] and a uterine cavity length > 10 cm [10].
Ultrasonography, hysteroscopy and endometrial biopsy were carried out in all the three trials (Table 1). Besides, Gardner et al. [9] also used mammography annually to surveillance the candidates.
Risk of bias in included studies
Overall results of risk of bias assessment appear in Figure 1. In the three RCTs, randomization was computer-generated in two of them and numbered and sealed envelope-generated in the third (Gardner’s) trial. The method of allocation concealment was assessed as adequate in all three trials. Participating women were not blind to treatment assignment because there was no use of placebo. Moreover, as the providers of each trial performed the insertion of the LNG-IUS in the participants, it was not possible to be blinded. However, outcome assessors for the trials were blinded.
Figure 1.
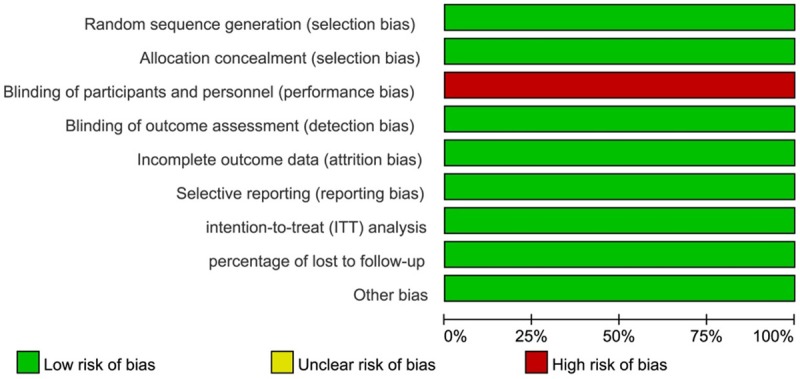
Risk of bias graph: reviewers’ judgments about each methodological quality item are presented as percentages across all included studies.
The number of participants randomized to treatment assignment in Gardner’s and Wong’s trials did not exactly match with the number of participants followed up because of the exclusion of women after randomization. Reasons for exclusion were described in details. We could not identify further potential sources of bias for the included studies.
Baseline characteristics and endometrial pathology at recruitment
A total of 359 women recruited in three RCTs, 175 in the treatment group and 184 in the control group. Age, Menstruation, endometrial pathology at baseline assessment was similar (Table 2). As one of the three RCTs [11] didn’t list the exact number of each breast cancer staging, we couldn’t analyze the overall comparison. But Kesim et al [11] mentioned the breast cancer staging of two arms in their trial was similar. Besides, we analyzed the other two trials at the aspect of breast cancer staging at recruitment and there was no significant difference found in the two arms (P = 0.143).
Table 2.
Baseline characteristics and endometrial pathology at recruitment
| Treatment group | Control group | P value | |
|---|---|---|---|
| N | 175 | 184 | |
| Age | 51.7 ± 8.3 | 53.4 ± 8.8 | 0.06 |
| Menstruation | 0.51 | ||
| Pre-menopause | 37 (21.1%) | 33 (17.9%) | |
| Post-menopause | 138 (78.9%) | 151 (82.0%) | |
| Endometrial pathology | |||
| Polyps | 9 (5.1%) | 12 (6.5%) | 0.59 |
| Submucosal fibroids | 8 (4.6%) | 13 (7.0%) | 0.35 |
| Atrophic or inactive | 101 (57.7%) | 117 (63.6%) | 0.26 |
| Proliferative or secretory | 56 (32.0%) | 49 (26.6%) | 0.27 |
| Insuffient for diagnosis | 23 (13.1%) | 27 (14.7%) | 0.67 |
Effects of the LNG-IUS on endometrial polyps
All patients who had endometrial polyps at entry into the three RCTs underwent a hysteroscopic polypectomy. There was a significant difference among the three studies in the overall effect of the LNG-IUS on de novo endometrial polyps (P < 0.0001). The outcome indicated that there was a significant reduction in the number of endometrial polyps in the LNG-IUS treatment group (5.0%) compared with the surveillance group (20.7%): OR 0.21, 95% CI: 0.10-0.45; heterogeneity chi-squared = 1.71, I-squared = 0%, P = 0.42 (Figure 2). Among all the patients had the de novo polyps, only 6 patients had an abnormal vaginal bleeding, while the rest were asymptomatic. When data were analyzed based on the menstruation, menopausal status was not found to have a significant impact on the de novo polyps prevention due to the LNG-IUS intervention (OR: 1.93, 95% CI: 0.93-4.00, P = 0.08).
Figure 2.
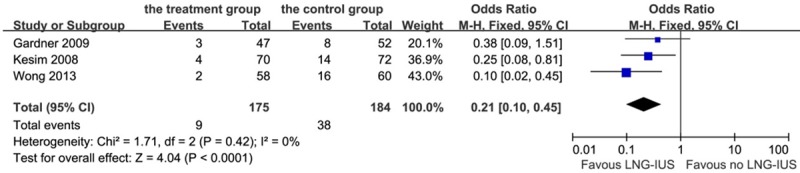
Forest plot of OR for the association between LNG-IUS intervention and endometrial polyps. The size of the square box is proportional to the weight that each study contributes in the meta-analysis. A diamond marks the overall estimate and confidence interval. Symbols on the right of the solid line indicate an OR > 1 and symbols on the left of the line indicate an OR < 1. Abbreviations: M-H = Mantel-Haenszel (fixed-effects model); 95% CI: 95% confidential interval.
Effects of the LNG-IUS on submucosal fibroids
Only patients in the Wong’s trial who had submucosal fibroids underwent a hysteroscopic resection at recruitment. Although the analysis showed a decreased effect of fibrosis prevention with 1.14% of patients in the treatment group and 2.7% in the control group, the result was of no statistical significance (P = 0.30): OR 0.42, 95% CI: 0.08-2.21; heterogeneity chi-squared = 0.04, I-squared = 0%, P = 0.83 (Figure 3).
Figure 3.
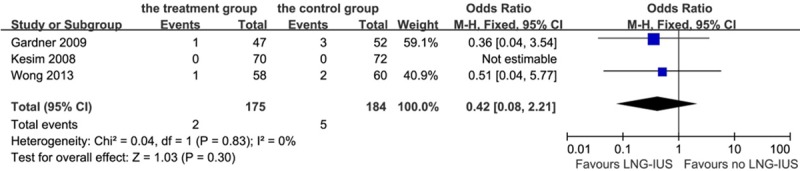
Forest plot of OR for the association between LNG-IUS intervention and submucosal fibroids. The size of the square box is proportional to the weight that each study contributes in the meta-analysis. A diamond marks the overall estimate and confidence interval. Symbols on the right of the solid line indicate an OR > 1 and symbols on the left of the line indicate an OR < 1. Abbreviations: M-H = Mantel-Haenszel (fixed-effects model); 95% CI: 95% confidential interval.
Effects of the LNG-IUS on endometrial proliferation or secretory changes
Four cases of proliferation or secretory hyperplasia were noted in the treatment groups (2.3%), while 13 cases occurred in the control group (7.1%). The LNG-IUS showed a beneficial trend in maintaining endometrial proliferation or secretory status (P = 0.05, OR 0.36, 95% CI 0.13-1.02; heterogeneity chi-squared = 3.59, I-squared = 44%, P = 0.17, Figure 4). When we analyzed the patients into subgroups according to the menstruation, LNG-IUS didn’t do a significant favor on the postmenopausal patients (OR: 1.46, 95% CI: 0.48-4.41, P = 0.05).
Figure 4.
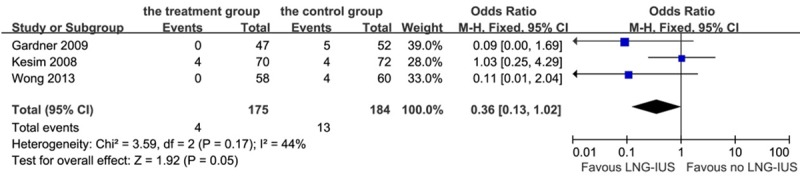
Forest plot of OR for the association between LNG-IUS intervention and endometrial proliferation or secretory changes. The size of the square box is proportional to the weight that each study contributes in the meta-analysis. A diamond marks the overall estimate and confidence interval. Symbols on the right of the solid line indicate an OR > 1 and symbols on the left of the line indicate an OR < 1. Abbreviations: M-H = Mantel-Haenszel (fixed-effects model); 95% CI: 95% confidential interval.
Effects of the LNG-IUS on endometrial atrophic or inactive changes
From Figure 5A, it is apparent that 42.9% of patients in the treatment group had atrophic or inactive hyperplasia according to pathological reports of the endometrium, while 69.6% of patients in the control group had the same diagnosis. The overall effect was highly significant between the two groups (P < 0.01). However, there was significant heterogeneity among the three studies (Chi-squared = 25.75, I-squared = 92%, P < 0.01). We performed influence analysis on this condition to explore the reason for the heterogeneity, as shown in Appendix 1. It showed that the study of Gardner et al. [9] and Wong et al. [10] substantially influenced the pooled OR. Whichever study we omitted would result in a high degree of heterogeneity. Therefore, the random-effects model was used to analyze the data and demonstrated that the LNG-IUS did not show a result of endometrial atrophy or inactiveness (P = 0.13, OR 0.24, 95% CI 0.04-1.53, Figure 5B). But when we analyzed these patients according to the menstruation, we found a significant difference that LNG-IUS resulted in endometrial atrophy or inactiveness more frequently in the postmenopausal patients than the premenopausal patients (OR: 1.88, 95% CI: 1.11-3.20, P = 0.02).
Figure 5.
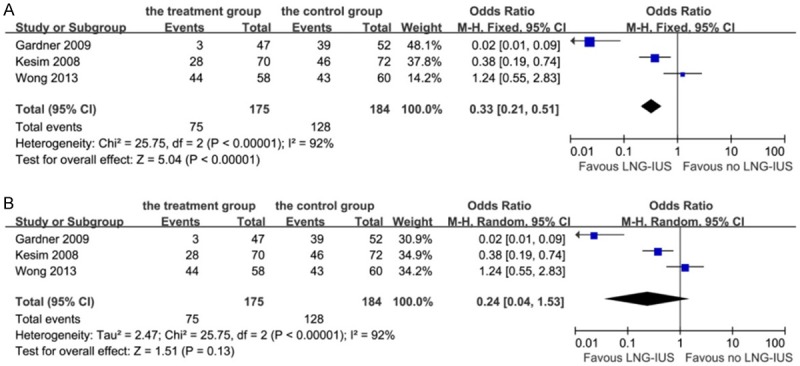
Forest plot of OR for the association between LNG-IUS intervention and endometrial atrophic or inactive changes. A: Fixed-effects model. B: Random-effects model. The size of the square box is proportional to the weight that each study contributes in the meta-analysis. A diamond marks the overall estimate and confidence interval. Symbols on the right of the solid line indicate an OR > 1 and symbols on the left of the line indicate an OR < 1. Abbreviations: M-H = Mantel-Haenszel (fixed-effects model); 95% CI: 95% confidential interval.
Effects of the LNG-IUS on endometrial hyperplasia without atypia
There were six cases (3.3%) of endometrial hyperplasia without atypia in the control group, while there were no cases in the treatment group. However, as shown in Figure 6, no significant inhibition of endometrial hyperplasia was found (P = 0.08, OR 0.20, 95% CI 0.04-1.18; heterogeneity chi-squared = 0.40, I-squared = 0%, P = 0.82).
Figure 6.
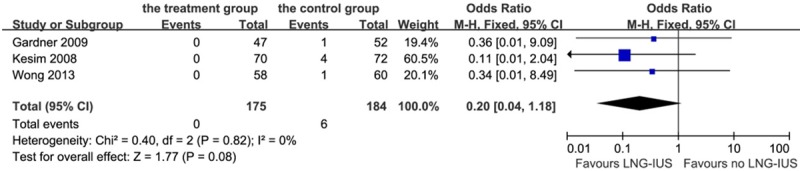
Forest plot of OR for the association between LNG-IUS intervention and endometrial hyperplasia without atypia. The size of the square box is proportional to the weight that each study contributes in the meta-analysis. A diamond marks the overall estimate and confidence interval. Symbols on the right of the solid line indicate an OR > 1 and symbols on the left of the line indicate an OR < 1. Abbreviations: M-H = Mantel-Haenszel (fixed-effects model); 95% CI: 95% confidential interval.
Effects of the LNG-IUS on endometrial thickness
There was no statistical difference among the three studies on the overall effect of the LNG-IUS on repression of endometrial thickness (P = 0.63) using a fixed-effects inverse variance model analysis: OR -0.17, 95% CI: -0.88-0.53; heterogeneity chi-squared = 0.52, I-squared = 0%, P = 0.77 (Figure 7).
Figure 7.
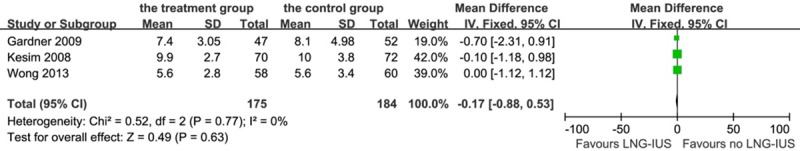
Forest plot of OR for the association between LNG-IUS intervention and endometrial thickness. The size of the square box is proportional to the weight that each study contributes in the meta-analysis. A diamond marks the overall estimate and confidence interval. Symbols on the right of the solid line indicate an OR > 1 and symbols on the left of the line indicate an OR < 1. Abbreviations: IV = inverse variance (fixed-effects model); 95% CI: 95% confidential interval.
Incidence of abnormal vaginal bleeding
In terms of side effects of the LNG-IUS, the most frequent one was abnormal vaginal bleeding. From the study, we found a total of 44 patients (25.1%) with abnormal bleeding after the LNG-IUS intervention within 24 months and 21 patients (11.4%) in the control group: OR 6.20, 95% CI: 2.99-12.85, P < 0.01; heterogeneity chi-squared = 3.42, I-squared = 42%, P = 0.18 (Figure 8).
Figure 8.
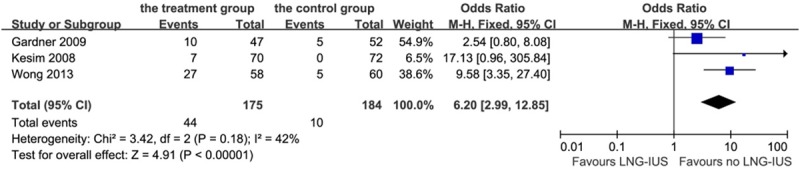
Forest plot of OR for the association between LNG-IUS intervention and abnormal bleeding. The size of the square box is proportional to the weight that each study contributes in the meta-analysis. A diamond marks the overall estimate and confidence interval. Symbols on the right of the solid line indicate an OR > 1 and symbols on the left of the line indicate an OR < 1. Abbreviations: M-H = Mantel-Haenszel (fixed-effects model).
Incidence of breast cancer recurrence and cancer-induced death
Eleven (6.3%) patients were found having breast cancer recurrence after LNG-IUS intervention and 7 (3.8%) patients were found in the control group, which had no statistical difference on this aspect (P = 0.28, OR 1.75, 95% CI: 0.64-4.80; heterogeneity chi-squared = 0.12, I-squared = 0%, P = 0.73, Figure 9). Besides, 8 (4.6%) patients were found cancer-induced death in the treatment group and 7 (3.8%) in the control group, with no significant difference (P = 0.71, OR 1.22, 95% CI: 0.42-3.52; heterogeneity chi-squared = 0.01, I-squared = 0%, P = 0.91, Figure 10).
Figure 9.
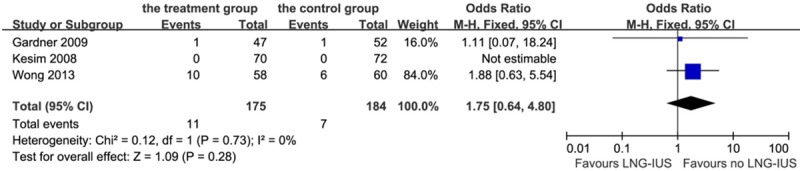
Forest plot of OR for the association between LNG-IUS intervention and breast cancer recurrence. The size of the square box is proportional to the weight that each study contributes in the meta-analysis. A diamond marks the overall estimate and confidence interval. Symbols on the right of the solid line indicate an OR > 1 and symbols on the left of the line indicate an OR < 1. Abbreviations: M-H = Mantel-Haenszel (fixed-effects model).
Figure 10.
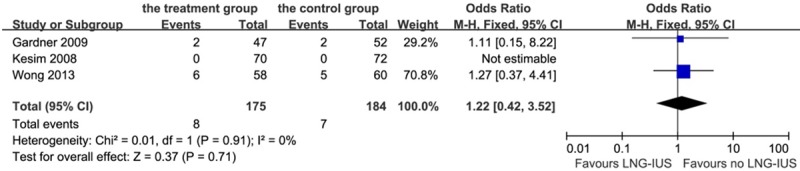
Forest plot of OR for the association between LNG-IUS intervention and cancer-induced death. The size of the square box is proportional to the weight that each study contributes in the meta-analysis. A diamond marks the overall estimate and confidence interval. Symbols on the right of the solid line indicate an OR > 1 and symbols on the left of the line indicate an OR < 1. Abbreviations: M-H = Mantel-Haenszel (fixed-effects model).
Discussion
Tamoxifen is used primarily for adjuvant treatment in pre- and post-menopausal women with estrogen receptor-positive breast cancer. Because of the dual function of tamoxifen, it is prudent to pay attention to its weak estrogenic action on other tissues except for the breast, where it is antagonistic. It can stimulate the endometrium, resulting in an increased incidence of unscheduled uterine bleeding, endometrial polyps, endometrial hyperplasia, and even cancer [14]. It can also cause an increase in bone mineral density [3] and alter the blood lipid pattern [4]. The American College of Obstetricians and Gynecologists (ACOG) considers that polyps are the most common endometrial lesion induced by tamoxifen, followed by hyperplasia and uterine cancer [15]. Daniel et al. [16] reported that patients treated with tamoxifen had a higher risk of developing endometrial carcinomas compared with age-matched controls not using tamoxifen (risk ratio: 1.3-7.5).
Progesterone has been used for endometrial hyperplasia for many years as it has both indirect antiestrogenic and direct antiproliferative effects on the endometrium [17]. It has been proposed that progesterone, given either orally (norethisterone acetate, megestrol acetate and medroxyprogesterone acetate) or locally, can balance the tamoxifen-inducing endometrial changes [15]. However, it has also been reported that high-dose systemic progestogen may blunt the efficacy of tamoxifen to prevent breast cancer recurrence and don’t reverse the development of polyps, endometrial cysts and fibroids associated with tamoxifen [18]. Besides, high doses of systemic progestogens have been associated with some undesirable systemic side effects [19], such as stomach upset, edema, fatigue, acne, insomnia and even breast discomfort.
The LNG-IUS, developed primarily as a contraceptive device, releases a continuous dose (20 μg/24h) of levonorgestrel into the uterine cavity for over five years, inducing endometrial epithelial atrophy, decidualization and vascular changes so that the endometrium loses sensitivity to circulating estrogen [20]. The LNG-IUS has been used to treat several gynecological diseases, such as adenomyosis, fibroids and menorrhagia, as well as to protect the endometrium during hormonal replacement therapy [21]. In a five-year non-comparative prospective clinical trial on 102 postmenopausal women using estrogen substitution therapy (percutaneous 17-beta estradiol, 1.5 mg daily, or an equivalent dose by patch or orally), who also received intrauterine levonorgestrel, Wildemeersch et al. [22] demonstrated that the LNG-IUS effectively opposed the estrogenic effect on the endometrium resulting in strong suppression. Because of its high efficacy and absence of systemic effects on organ tissues (e.g. breasts), target-delivery in the uterine cavity could be a preferred route to administer a progestogen in women using estrogen substitution therapy. The consistent result was also shown in perimenopausal women [23]. For this reason, it has been suggested that the LNG-IUS may be effective in preventing proliferative endometrial pathology in tamoxifen users.
Two of the three studies had prior published reports. In the study of Gardner, with a 12-month follow-up, they discovered a decreased incidence of endometrial polyps (2% vs. 8%), submucosal fibroids (2% vs. 6%), and complex hyperplasia (0% vs. 2%) in the treatment group when compared with the control group and also found a significant difference in uterine weight [13]. In the study of Chan et al. [12], they demonstrated that women in the treatment group had a much lower incidence of endometrial polyps at 12 months and had a decrease in endometrial proliferative or secretory hyperplasia (0% vs. 15.5%). There was no significant difference in the incidence of submucosal fibroids. At this time, these two studies have updated their final assessments with a follow-up at 4.5 and 5 year respectively.
In this analysis, we suggest that the LNG-IUS has significant benefit in preventing de novo polyps. Besides, LNG-IUS only showed a trend in maintaining endometrial proliferation or secretory status and inhibiting on endometrial hyperplasia without atypia and without any statistical differences in these comparisons. Furthermore, none of the three trials reported on the incidence of endometrial cancer. That might be because of the efficacy of LNG-IUS or the small number of samples, which meant there was insufficient power to ascertain whether the LNG-IUS was beneficial in reducing the incidence of precancerous or cancerous lesions.
When we analyzed the effect of interventions into two groups according to patients’ menstruation, we did not find menopausal status was a significant factor associated with polyp’s prevention. However, in the aspect of maintaining endometrial atrophic or inactive changes, the LNG-IUS had a benefit on the postmenopausal patients. It is possible that this is related to the timing of LNG-IUS insertion. For premenopausal patients, they have their basic endometrial hyperplasia under the role of systemic hormonal level besides the estrogenic effect of tamoxifen on the endometrium.
Abnormal bleeding is regarded as the most common side effect for women who use the LNG-IUS [24]. In our analysis, bleeding events in the treatment group occurred statistically more frequently compared with the control group, but the bleeding appeared mostly as a spotting pattern and stopped within 12 months in all three trials.
The safety of using the LNG-IUS in breast cancer survivors is uncertain. Backman et al. [25] reported that there was no indication of a difference between the 17,360 LNG-IUS-users and an average Finnish female population in any of the 5-year age groups between 30 and 54 years of age, suggesting that use of the LNG-IUS was not associated with an increased risk of breast cancer. The same result was obtained in a retrospective cohort study [26] assessing the recurrence rate among 79 breast cancer Belgian survivors who used the LNG-IUS in comparison with 120 nonusers who were closely matched for age at diagnosis, tumor stage, tumor grade and treatment modality. However, in a subgroup analysis of women who developed breast cancer while using the LNG-IUS and who continued to use the LNG-IUS, a higher risk of recurrence of borderline statistical significance was reported. Among the three trials, two trials reported the outcomes of breast cancer recurrence and cancer-induced death. From the analysis, no statistic difference was found in these two aspects between the treatment and control groups.
This analysis verifies the efficacy of the LNG-IUS in preventing de novo polyps in breast cancer patients treated with tamoxifen. There was insufficient evidence to ascertain whether the LNG-IUS had any benefit in reducing the incidence of precancerous or cancerous lesions. Besides, the LNG-IUS didn’t lead to an increased incidence of breast cancer recurrence and cancer-induced death with a statistical significance. Given the limited data of the LNG-IUS in the recurrence or mortality rate of breast cancer, there is a need for larger and longer-term randomized studies to determine the benefit and risk of the LNG-IUS in tamoxifen-treated breast cancer patients.
Acknowledgements
This analysis was supported by the following grants: Shanghai Health Bureau (20114y155, 20124164), Science and Technology Commission of Shanghai Municipality (124119a4700).
Appendix 1.
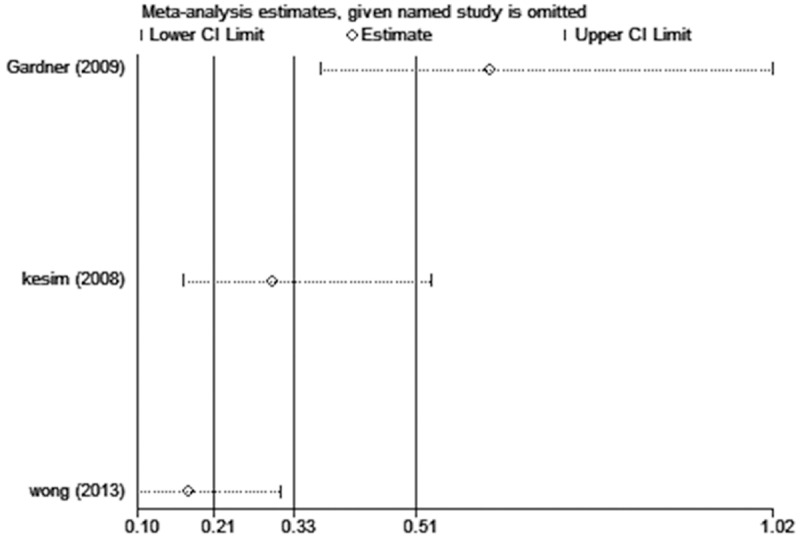
Influence analysis shows the influence of individual studies on the pooled OR. The vertical axis indicates the overall OR and the two vertical axes indicate its 95% CI. Every hollow round indicates the pooled OR when the left study was omitted from this meta-analysis. The two ends of every broken line represent the respective 95% CI.
Disclosure of conflict of interest
None.
References
- 1.Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M, Coombes C, Snowdon C, Gnant M, Jakesz R, Kaufmann M, Boccardo F, Godwin J, Davies C, Peto R. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J. Clin. Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 2.Gnant M, Harbeck N, Thomssen C. St. Gallen 2011: Summary of the Consensus Discussion. Breast Care (Basel) 2011;6:136–141. doi: 10.1159/000328054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santen RJ. Clinical review: Effect of endocrine therapies on bone in breast cancer patients. J Clin Endocrinol Metab. 2011;96:308–319. doi: 10.1210/jc.2010-1679. [DOI] [PubMed] [Google Scholar]
- 4.Cole LK, Jacobs RL, Vance DE. Tamoxifen induces triacylglycerol accumulation in the mouse liver by activation of fatty acid synthesis. Hepatology. 2010;52:1258–1265. doi: 10.1002/hep.23813. [DOI] [PubMed] [Google Scholar]
- 5.Otsuka I, Takahashi S, O’Uchi K, Akimoto N, Hanari K, Ogaki Y, Enatsu YH, Takigawa A, Takaya H, Tanaka A Kaseki H, Yamada T. [Clinicopathological features of endometrial carcinoma in tamoxifen- and toremifene-treated breast cancer patients] . Gan Kagaku Ryoho. 2010;37:279–283. [PubMed] [Google Scholar]
- 6.Leung F, Terzibachian JJ, Govyadovskiy A, Bourtembourg A, Maillet R, Riethmuller D. [Tamoxifen in the adjuvant setting for breast cancer: Reflexions about the risk of uterine carcinosarcoma] . Gynecol Obstet Fertil. 2009;37:447–451. doi: 10.1016/j.gyobfe.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner FJ, Konje JC, Bell SC, Abrams KR, Brown LJ, Taylor DJ, Habiba M. Prevention of tamoxifen induced endometrial polyps using a levonorgestrel releasing intrauterine system long-term follow-up of a randomised control trial. Gynecol Oncol. 2009;114:452–456. doi: 10.1016/j.ygyno.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Wong AW, Chan SS, Yeo W, Yu MY, Tam WH. Prophylactic use of levonorgestrel-releasing intrauterine system in women with breast cancer treated with tamoxifen: a randomized controlled trial. Obstet Gynecol. 2013;121:943–950. doi: 10.1097/AOG.0b013e31828bf80c. [DOI] [PubMed] [Google Scholar]
- 11.Kesim MD, Aydin Y, Atis A, Mandiraci G. Long-term effects of the levonorgestrel-releasing intrauterine system on serum lipids and the endometrium in breast cancer patients taking tamoxifen. Climacteric. 2008;11:252–257. doi: 10.1080/13697130802163168. [DOI] [PubMed] [Google Scholar]
- 12.Chan SS, Tam WH, Yeo W, Yu MM, Ng DP, Wong AW, Kwan WH, Yuen PM. A randomised controlled trial of prophylactic levonorgestrel intrauterine system in tamoxifen-treated women. BJOG. 2007;114:1510–1515. doi: 10.1111/j.1471-0528.2007.01545.x. [DOI] [PubMed] [Google Scholar]
- 13.Gardner FJ, Konje JC, Abrams KR, Brown LJ, Khanna S, Al-Azzawi F, Bell SC, Taylor DJ. Endometrial protection from tamoxifen-stimulated changes by a levonorgestrel-releasing intrauterine system: a randomised controlled trial. Lancet. 2000;356:1711–1717. doi: 10.1016/s0140-6736(00)03204-9. [DOI] [PubMed] [Google Scholar]
- 14.Perlman S, Vaisbuch E, Ben-Arie A. [Tamoxifen treatment and malignant endometrial tumors--what’s new?] . Harefuah. 2006;145:219–222, 244. [PubMed] [Google Scholar]
- 15.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion. No. 336: Tamoxifen and uterine cancer. Obstet Gynecol. 2006;107:1475–1478. doi: 10.1097/00006250-200606000-00057. [DOI] [PubMed] [Google Scholar]
- 16.Daniel Y, Inbar M, Bar-Am A, Peyser MR, Lessing JB. The effects of tamoxifen treatment on the endometrium. Fertil Steril. 1996;65:1083–1089. doi: 10.1016/s0015-0282(16)58318-6. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metabo. 2011;22:145–152. doi: 10.1016/j.tem.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powles TJ, Bourne T, Athanasiou S, Chang J, Grubock K, Ashley S, Oakes L, Tidy A, Davey J, Viggers J, Humphries S, Collins W. The effects of norethisterone on endometrial abnormalities identified by transvaginal ultrasound screening of healthy post-menopausal women on tamoxifen or placebo. Br J Cancer. 1998;78:272–275. doi: 10.1038/bjc.1998.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane G, Siddle NC, Ryder TA, Pryse-Davies J, King RJ, Whitehead MI. Dose dependent effects of oral progesterone on the oestrogenised postmenopausal endometrium. Br Med J (Clin Res Ed) 1983;287:1241–1245. doi: 10.1136/bmj.287.6401.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haimovich S, Checa MA, Mancebo G, Fuste P, Carreras R. Treatment of endometrial hyperplasia without atypia in peri- and postmenopausal women with a levonorgestrel intrauterine device. Menopause. 2008;15:1002–1004. doi: 10.1097/gme.0b013e3181659837. [DOI] [PubMed] [Google Scholar]
- 21.Heikinheimo O, Gemzell-Danielsson K. Emerging indications for the levonorgestrel-releasing intrauterine system (LNG-IUS) Acta Obstet Gynecol Scand. 2012;91:3–9. doi: 10.1111/j.1600-0412.2011.01303.x. [DOI] [PubMed] [Google Scholar]
- 22.Wildemeersch D, Pylyser K, De Wever N, Pauwels P, Tjalma W. Endometrial safety after 5 years of continuous combined transdermal estrogen and intrauterine levonorgestrel delivery for postmenopausal hormone substitution. Maturitas. 2007;57:205–209. doi: 10.1016/j.maturitas.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Hampton NR, Rees MC, Lowe DG, Rauramo I, Barlow D, Guillebaud J. Levonorgestrel intrauterine system (LNG-IUS) with conjugated oral equine estrogen: a successful regimen for HRT in perimenopausal women. Hum Reprod. 2005;20:2653–2660. doi: 10.1093/humrep/dei085. [DOI] [PubMed] [Google Scholar]
- 24.Wan YL, Holland C. The efficacy of levonorgestrel intrauterine systems for endometrial protection: a systematic review. Climacteric. 2011;14:622–632. doi: 10.3109/13697137.2011.579650. [DOI] [PubMed] [Google Scholar]
- 25.Backman T, Rauramo I, Jaakkola K, Inki P, Vaahtera K, Launonen A, Koskenvuo M. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol. 2005;106:813–817. doi: 10.1097/01.AOG.0000178754.88912.b9. [DOI] [PubMed] [Google Scholar]
- 26.Trinh XB, Tjalma WA, Makar AP, Buytaert G, Weyler J, van Dam PA. Use of the levonorgestrel-releasing intrauterine system in breast cancer patients. Fertil Steril. 2008;90:17–22. doi: 10.1016/j.fertnstert.2007.05.033. [DOI] [PubMed] [Google Scholar]


