Abstract
To investigate the effect of γ-terpineol on cell proliferation and apoptosis of human hepatoma BEL-7402 cells to elucidate its molecular mechanism. Here, BEL-7402 cells were treated with various concentrations (40, 80, 160, 320 and 640 μg/ml) of γ-terpineol for 48 h, cell proliferation was determined by 3-(4,5-dimethyl-thiazolyl-2)-2,5-diphenyl tetrazolium bromides (MTT) assay. Cell colony inhibition was determined by soft agar assay. Apoptosis and possible molecular mechanisms were evaluated by morphological observation, flow cytometry analysis, and DNA fragmentation assay. The γ-terpineol significantly suppressed BEL-7402 cell proliferation in a dose-dependent manner. Characteristic morphological and biochemical changes associated with apoptosis such as cells shrinkage, deformation and vacuolization of mitochondria, nuclear chromatin condensation and fragmentation, formation of apoptotic bodies were observed after BEL-7402 cells treated with γ-terpineol for 24 h and 48 h. Cell cycle were displayed by flow cytometry analysis, the γ-terpineol treatment resulted in accumulation of cells at G1 or S phase and a blockade of cell proliferation compared to control group. Treating BEL-7402 cells with 320 μg/ml of γ-terpineol for 36 h and 48 h, a typical apoptotic “DNA ladder” was observed using DNA fragmentation assay. The present study demonstrated that possible anti-cancer mechanism of γ-terpineol on human hematomas cells is through inducing cell apoptosis to suppress tumor cell growth.
Keywords: γ-terpineol, human hepatoma, BEL-7402, cell proliferation, apoptosis
Introduction
Plants have played a significant role in maintaining human health and improving the quality of human life for thousands of years, and have served humans well as valuable components of seasonings, beverages, cosmetics, dyes, and medicines. Over the past 10 years, research for new drugs to be used in oncology has refocused on natural products. The rediscovery of natural products has yielded promising compounds such as taxanes and camptothecins [1]. There has been increased interest in the research on natural products, including plant extracts, which might replace synthetic anticancer agents or contribute toward the development of new cancer control agents. During the past 15 years, essential oils have been shown to possess a broad-spectrum of anticancer activity [2,3]. Screening experiments with 13-52 essential oils and major active components against 5-25 tumor have shown crude oil and isolated compounds as potential candidates for inhibition of a variety of cancer types [4,5].
Cinnamomum longepaniculatum (Gamble) N. Chao is an endemic tree in China and grows widely in Sichuan, Hubei, Hunan, Shanxi, and other provinces [6]. The γ-terpineol is an important component of the C. longepaniculatum leaf essential oil, which has been mentioned in the traditional Chinese pharmacopoeia, and has been mainly used to promote hemostasis and detoxification, for the treatment of rheumatic arthralgia, hypertension, chilblain in China and other countries of the Orient [7]. A few studies have demonstrated that the γ-terpineol can broadly inhibit the growth of a variety of different cancer cell lines in vitro [8], However, no study has yet provided definitive proof of the effects of γ-terpineol on the growth and apoptosis of human hepatoma cell line BEL-7402.
Hepatocellular carcinoma (HCC) is the most common primary malignant tumor, in China which occurring in both men and women. Over the last 5 to 8 years evidence has been accumulating in different countries that the incidence of hepatocellular carcinoma is rising [9]. It is reasonable to hypothesize that γ-terpineol may also be helpful in therapies of human hepatoma, possibly through the regulation of cell proliferation and/or cell death. Therefore, this study aimed to assess the processes by which γ-terpineol inhibits cancer cell growth, affects apoptosis of BEL-7402 cells, and the possible molecular mechanism for these actions.
Materials and methods
Preparation of γ-terpineol
The γ-terpineol (γ-terpineol content 90%) from the C. longepaniculatum leaf essential oil was provided by the Yibin Chuanhui company (Sichuan Province, China). A stock solution of 50 mg/mL the γ-terpineol was made in 100% dimethyl sulfoxide (DMSO) and filtered via a 0.22 μm minipore membrane. The stock solution was aliquoted and stored at -20°C, thawed just before testing, and diluted with the culture medium. The final concentration of DMSO for all treatments was less than 0.25%.
Cell culture and treatment
Human hepatoma BEL-7402 cells were purchased from the Cell bank, Chinese Academy of Sciences, China. BEL-7402 cells were incubated in RPMI 1640 medium (Gbico Co., USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 10 mmol/L HEPES, pH 7.4, and kept at 37°C in a humidified 5% CO2 incubator. For this study, cells were either incubated in the absence or presence of different concentrations of the γ-terpineol for 48 h.
Measurement of cell growth inhibition
Growth inhibition of BEL-7402 cells by the γ-terpineol was measured by 3-(4,5-dimethyl-thiazolyl-2)-2,5-diphenyl tetrazolium bromide (MTT) assay [10,11] with minor modifications. Briefly, cancer cells were seeded into 96-well microtiter plates at appropriate densities to maintain the cells in an exponential phase of growth during the experiment. BEL-7402 cells were exposed to γ-terpineol either at 40, 80, 160, 320 or 640 μg/mL for 48 h. Each concentration was tested in quadruplicate. At the end of the treatment, 20 μL of 5 mg/mL MTT (Sigma, St. Louis, MO, USA) was added to each well and the plates were incubated for 4 h at 37°C. DMSO (150 μL) was then added to each well and the plates were rotated for 10-20 min at 200 r/min by a shaker (THZ-C, Shenhua BioTech Co., Shenzhen, China). The optical absorbance was read on a plate reader (BIO-RAD Co., USA) at a wavelength of 570 nm. Media and DMSO controls were included for all experiments. The inhibitory rate of cell proliferation was calculated using the following formula: Growth inhibition (%) = (Acontrol-Atreated)/Acontrol × 100. Results were measured in triplicate.
Measurement of cell colony inhibition
Traditionally, the soft agar colony formation assay is a common method to monitor anchorage-independent growth, which measures proliferation in a semisolid culture media after 3-4 weeks by manual counting of colonies. At first, preparation of base agar. Melt 1% agar in a microwave and cool to 40°C in a water bath. Using falcon tubes, warm 2 × RPMI with 20% FBS and antibiotics to 40°C in the water bath. Allow at least 30 minutes for temperature to equilibrate. Mix equal volumes of the two solutions to give 0.5% Agar + 1 × RPMI + 10% FBS. Add 1.5 ml of mixture from step to each 35 mm Petri dish and set aside for 5 min to allow agar to solidify. The second, preparation of top agarose. Melt 0.7% Agarose in a microwave and cool to 40°C in a water bath. Also warm 2 × RPMI + 20% FBS to the same temperature. Trypsinize adherent cells to release them and count the number of cells per ml. It is helpful to have a positive control for colony formation. Take care that a single cell suspension is obtained. Add 0.1 ml of cell suspension to 10 ml tubes. To plate, add 3 ml of 2 × RPMI + 10% FBS, 3 ml 0.7% Agarose and γ-terpineol of different concentration to a tube of cells from prepared. Mix gently by swirling and add 1.5 ml to each of the four replicate plates. Incubate plates at 37°C in humidified incubator for 10 to 30 days. At last, stain plates with 0.5 ml of 0.005% Crystal Violet for more than 1 hour. Count colonies using a dissecting microscope. The colony inhibitory rate of cell colony was calculated using the following formula: Colony inhibition (%) = (Ncontrol-Ntreated)/Ncontrol × 100.
Light microscopy assay
BEL-7402 cells were seeded in sterile culture dishes on cover slips at a density of 105 cells/mL and cultured in 10% FBS-RPMI 1640 medium at 37°C in a humidified atmosphere of 5% CO2. Cells were grown to 80-90% confluence and synchronized by incubation in 0.5% FBS-RPMI 1640 for 56 h. The experiments were divided into test and control groups. Cells in the test group were incubated in 10% FBS-RPMI 1640 medium containing 320 μg/mL the γ-terpineol for 24 h. Cells in the control group were incubated in 10% FBS-RPMI 1640 alone for 24 h. The cover slips were washed with PBS, and then stained by hematoxylin-eosin staining (HE). Finally, the cells fixed on the cover slips were analyzed, and photographed under light microscope (BH-2, Olympus, Japan).
Transmission electron microscopy assay
BEL-7402 cells were incubated in 10% FBS-RPMI 1640 medium with or without 320 μg/mL the γ-terpineol at 37°C in a humidified atmosphere of 50 mL/L CO2 for 24 h. After treatment with 0.25% trypsin, 5 × 106 cells were collected, centrifuged at 10000 g for 5 min and then washed twice with PBS for 5 min. Cell pellets were fixed in 2.5% glutaraldehyde in PBS at 4°C overnight. They were washed three times, each time for 15 min, in cacodylate-buffer. Specimens were then post-fixed for 2 h in 1% osmium tetroxide (OsO4) dissolved in cacodylate-buffer at room temperature and washed in cacodylate-buffer (three times, 15 min each). Samples were dehydrated in ethanol and embedded in Epon-Araldite resin according to the method described by Musetti et al. Ultrathin sections were stained with uranyl acetate and lead citrate and observed under a PHILIPS CM 10 transmission electron microscope (TEM) (Philips Scientifics, Eindhoven, The Netherlands), operated at 80 kV.
Flow cytometric analysis (FCM)
The suspended single cell solutions subjected to treatment with γ-terpineol at 320 μg/ml for different times (12, 24, 36, and 48 h) were harvested. Each group had three culture bottles. Cells were washed with PBS, fixed with 70% ethanol at -20°C for 30 min and stored at 4°C overnight, then washed with PBS again, treated with 100 mg/L PI 100 μl at 4°C for 30 min in darkness. Apoptotic cells were assayed using FACSort Becton Dickinson Flow Cytometer at 488 nm and data were analyzed with CELLQuest Software. For each sample, 20,000 cells were measured.
DNA fragmentation assay
BEL-7402 cells were seeded in two 25-mL flasks at a density of 105 cells/mL and incubated in 10% FBS-RPMI 1640 medium as previously described in the presence or absence of 320 μg/mL the γ-terpineol for 12, 24, 36 and 48 h. After harvesting the cells, samples were washed with PBS and centrifuged at 1,000 r/min. The cell pellets were resuspended in a 40-μL solution of 0.2 mol/L NaH2PO4/0.1 mol/L citric acid (192:8) and incubated at room temperature for 60 min. After centrifugation for 30 min at 480 r/min, the supernatant was transferred to a 1.5 mL microcentrifuge tube. Three microliters of NP-40 (2.5 mg/mL) and 3 μL RNase A (10 mg/mL) were added to the tube and incubated at 37°C for 60 min. After the addition of 3 μL proteinase K (10 mg/mL), the tube was once again incubated at 50°C for 50 min. At the end of the reaction, 5 μL loading buffer was added and samples were electrophoresed on a 10 g/L agarose gel containing 0.5 μg/mL ethidium bromide, at 7.5 V/cm in 1 × TAE buffer for 1.5 h. Analyses and photography were performed with the gel imaging and analysis system (BIO-RAD Co., USA).
Statistical analysis
All data were expressed as mean ± standard deviation. Statistical analysis was performed by t test using software SPSS11.0 for Windows. P < 0.05 was considered significant.
Results
Measurement of cell growth inhibition
Human hepatoma BEL-7402 cells was treated with different concentrations of γ-terpineol for 48h and analyzed by MTT assay. The γ-terpineol was shown to inhibit the proliferation of BEL-7402 cells in a dose-dependent manner. After exposure of BEL-7402 cells to 40, 80, 160, 320, or 640 μg/mL γ-terpineol for 48 h, cell proliferation was inhibited by 8.87%, 11.99%, 26.62%, 49.68%, and 69.16%, respectively (Table 1).
Table 1.
Inhibitory effect of the γ-terpineol on hepatoma cell line BEL-7402
| Concentrations (mg/ml) | A570nm (x ± s) | Inhibitionrate (%) |
|---|---|---|
| 0 | 0.417 ± 0.007a | |
| 40 | 0.380 ± 0.006b | 8.87% |
| 80 | 0.367 ± 0.014b | 11.99% |
| 160 | 0.306 ± 0.008c | 26.62% |
| 320 | 0.203 ± 0.002d | 49.68% |
| 640 | 0.129 ± 0.004e | 69.16% |
Note: Values with different subscript of small letter in the same column are significantly different (p < 0.05).
Measurement of cell colony inhibition
Cells were cultured in the presence or absence of γ-terpineol and cells number was determined after 10 days. The cell colony of BEL-7402 cells was inhibited in a dose-dependent manner (Figure 1). After exposure of BEL-7402 cells to 80, 160, 320, and 640 μg/mL_γ-terpineol for 10 days, the inhibition rate of cell colony was 11.35 ± 1.95%, 21.20 ± 2.51%, 54.70 ± 2.48%, and 89.54 ± 1.92%, respectively.
Figure 1.
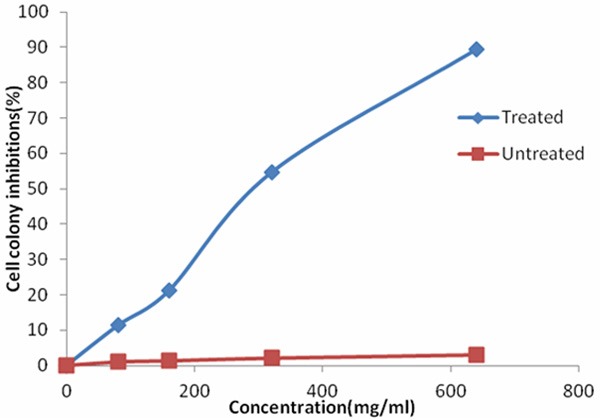
Effects of the γ-terpineol on inhibition of BEL-7402 cell colony.
Light microscopy assay
Under light microscope, untreated BEL-7402 cells were extended, flat cell bodies with uniform chromatin in the nuclei, and appeared obvious mitosis (Figure 2A, 2B). The BEL-7402 cells, treated with the γ-terpineol (320 μg/mL) for 24 h, displayed part of cells morphologic alteration, including cell size reduction, nuclei stained deeply, cytoplasm condensed, nuclear heterochromatin agglutination (Figure 2C). After 48 h treatment of γ-terpineol, most of cells showed the similar morphological changes of apoptosis including cell amount decreased; cells were round, some vacuoles appeared in cytoplasm, cell nucleus have become pyknotic (shrunken and dark) (Figure 2D).
Figure 2.
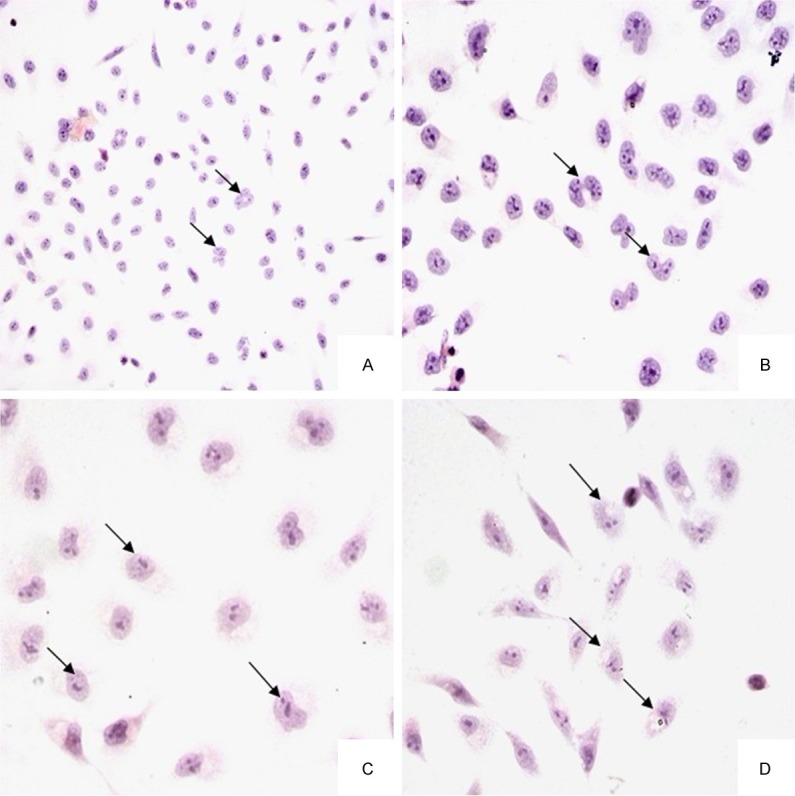
Light micrographs of BEL-7402 cells untreated or treated with γ-terpineol from C. longepaniculatum. Untreated with γ-terpineol for 24 h (A × 200, B × 400), treated with 320 μg/mL for 24 h (C × 400) and 48 h (D × 400).
Transmission electron microscopy assay
Under transmission electron, control cells were big and round, with intact nuclear membrane, electron dense granules in nuclear chromatin (Figure 3A) and normal cell mitosis (Figure 3B). However, the cells treated with the γ-terpineol (320 μg/mL) for 24 h exhibited characteristics of apoptosis including cell membrane shrinkage, low density in nuclear chromatin (Figure 3C), deformation and vacuolization of mitochondria (Figure 3D). After 48 h, cell membrane occur rupture, the deformation and vacuolization of mitochondria become seriously (Figure 3E), nuclear chromatin become condensation and fragmentation, apoptotic bodies appeared (Figure 3F).
Figure 3.
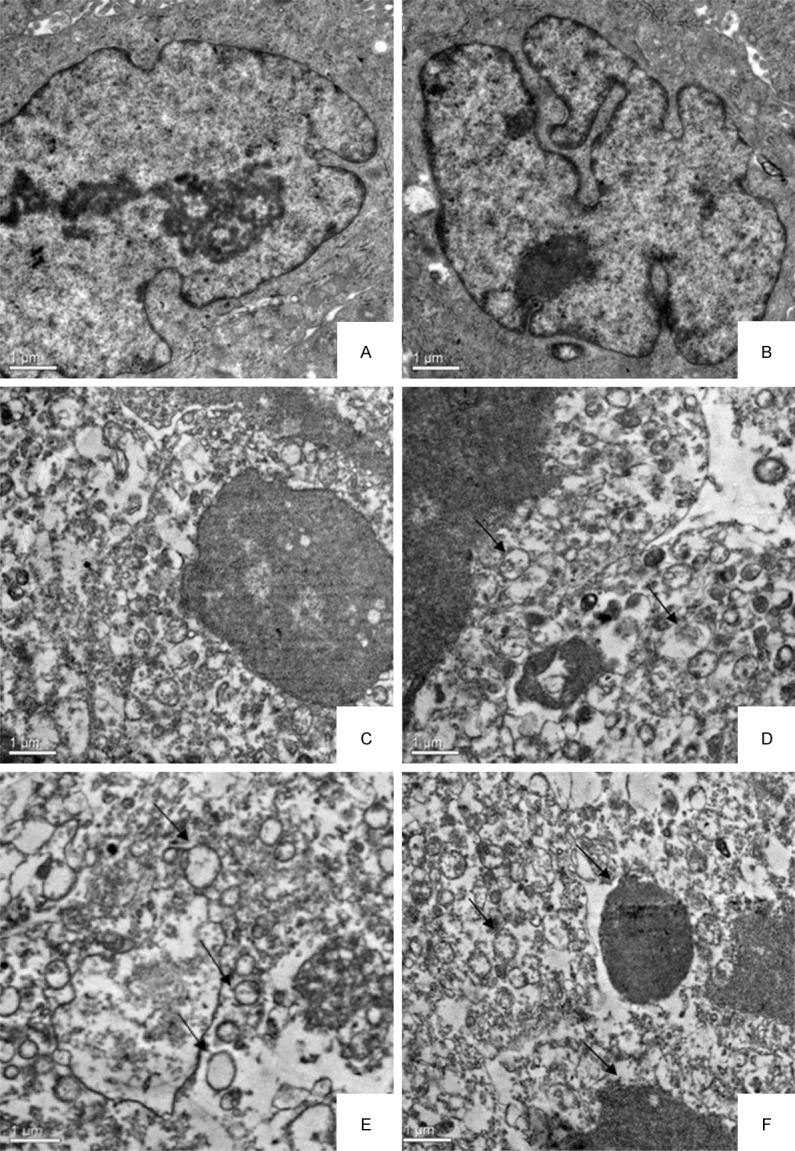
Transmission electron micrographs of the BEL-7402 cell untreated or treated with γ-terpineol from C. longepaniculatum. Untreated with γ-terpineol for 24 h (A, B), treated with 320 μg/mL for 24 h (C, D) and 48 h (E, F).
Flow cytometry analysis
The flow cytometry data showed that γ-terpineol treatment resulted in accumulation of cells at G1 or S phase and a blockade of cell proliferation compared to control group (Figure 4A, 4B). With the treated time prolonging, the number of cells in different cell cycle have changed (Figure 4B). Most of cells were arrested at G1 or S phase eventually led to cell death, as the prolonged incubation response, resulting in the rapid appearance of cells with a DNA content less than that at G1 phase, characteristic of apoptotic cells. In addition, γ-terpineol induced apoptosis of BEL-7402 cells in a time-dependent manner. The number of apoptotic cells, untreated with γ-terpineol, was just 0.45%. The number of apoptotic cells increased from 9.34% to 35.80%, 45.00%, and 71.00% after 12, 24, 36, and 48 h of exposure to γ-terpineol (320 μg/ml), respectively (Figure 4C). Therefore, γ-terpineol induced apoptosis of BEL-7402 cells in a time-dependent manner.
Figure 4.
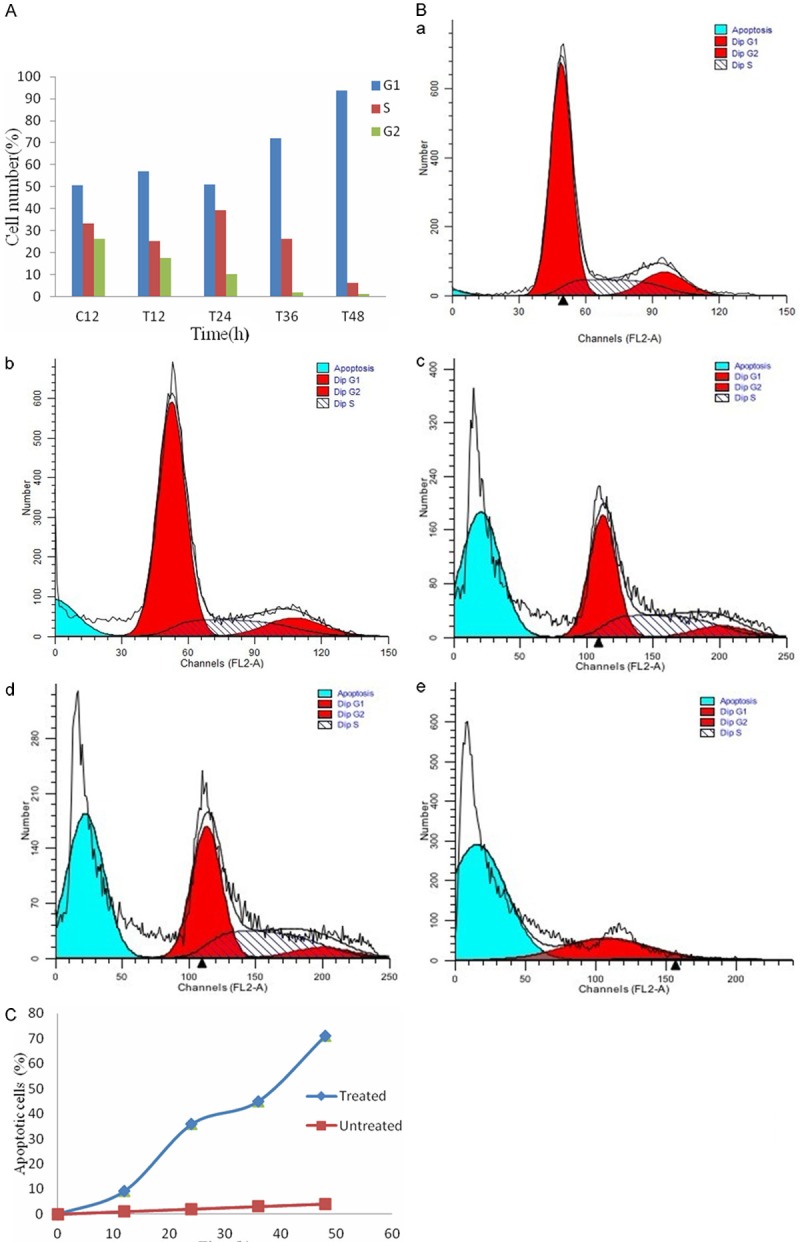
After treated with γ-terpineol for different times, the vary circumstance of cell cycle percentage and the rates of apoptotic cell was remarkable. The histogram of cell cycle percentage with treated time prolong (A). The cell cycle expression after treated with different times (B), (a) treated with 12 h; (b) treated with 24 h; (c) treated with 36 h; (d) treated with 48 h. The curve chart of the rates of apoptotic cell with treated for different times (C).
DNA fragmentation assay
The DNA maintained integrity in the untreated control cells (Figure 5). The γ-terpineol, at a concentration of 320 μg/mL, induced the typical genome DNA fragmentation and a hallmark of apoptosis in multiples of 180-200 bp after 12, 24, 36, 48 h (Figure 5).
Figure 5.
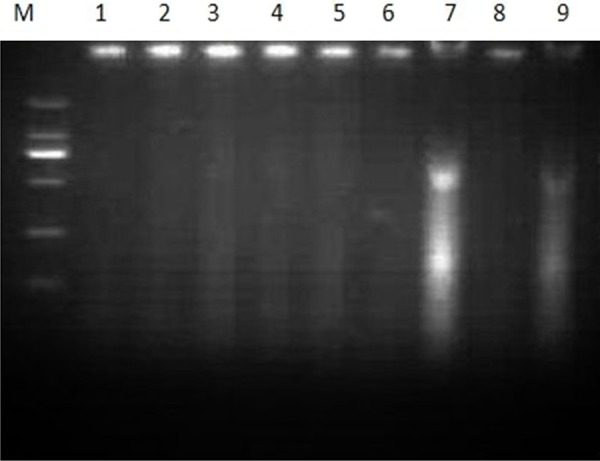
Apoptosis induced by the γ-terpineol. M: DNA marker DL2000; 1: Untreated negative control; 2, 4, 6, 8: Untreated with the γ-terpineol by 12, 24, 36, and 48 h; 3, 5, 7, 9: Treatment with the γ-terpineol by 12, 24, 36, and 48 h.
Discussion
Hepatoma is one of the most common malignant tumors worldwide in this study, chosen the human hepatoma cell line BEL-7402_as an in vitro model to explore the effects of γ-terpineol on cancer cell growth and apoptosis to evaluate the value of the γ-terpineol in cancer prevention and therapy.
Effect of γ-terpineol at various concentrations on the growth and cell colony of BEL-7402 cells showed that γ-terpineol significantly suppressed cell proliferation in a dose-dependent manner. After 48 h treatment of γ-terpineol at different concentrations, MTT colorimetric analysis showed that γ-terpineol could observably inhibit the proliferation of BEL-7402 cells with IC50 values of 0.32 mg/ml and exhibit obvious anticancer activity at concentration of 0.64 mg/ml. The inhibitory rates in accordance with the result counted by cell colony. Therefore, the present study demonstrated that the γ-terpineol could suppress human hepatoma cell proliferation in vitro. Manosroi et al. [12] described that essential oil had the IC50 values between 0.125 and 5 mg/ml as moderate possibility to be developed to cancer therapeutic agent. Moreover, the work of Linquan et al. [13] shown that the essential oil from Schizonepeta tenuifo-lia Briq exhibit obvious anti human lung cancer activity in the concentration of 2 mg/ml. Our results showed that γ-terpineol possibility to be developed to cancer therapeutic agent according to those records. Therefore, this subject would provide some experimental basis for its clinical use.
Cytochemical staining and under transmission electron microscopy, characteristic morphological and biochemical changes associated with apoptosis such as cells shrinkage, low density in nuclear chromatin, deformation and vacuolization of mitochondria, nuclear chromatin condensation and fragmentation, formation of apoptotic bodies were observed after BEL-7402 cells treated with γ-terpineol for 24 h and 48 h. TG cotter [14] described that under electron microscopy, early apoptosis cells were seen membrane of integrity, with nuclear morphological changes; later apoptosis cell were seen apoptotic bodies appearance. Wu et al. [15] reported that H2O2 induced BEL-7402 to apoptosis, cells expressed chromatin condensation and fragmentation; apoptotic bodies and vacuoles appeared under electron microscopy. Moreover, under the transmission electron microscopy, γ-terpineol induced seriously deformation and vacuolization of mitochondria. However, mitochondria have a pivotal role in apoptosis, as shown in our study; the exposure of hepatoma cells to γ-terpineol impairs mitochondrial function, leading to a marked deformation and vacuolization of mitochondria.
Flow cytometry could greatly advance the objective elucidation of tumor cell cycle changes and apoptosis rate [16]. Cell cycle would play an important role in cell proliferation. The result shown that the γ-terpineol could induce accumulation of cells arrested at G1 and S phases of the cell cycle. With treated time prolonging, the number of cells at G2 has reduced, even disappeared. Malumbres et al. [17] described that cells apoptosis was a normal physiological activities with morphology alteration and cell cycle alteration. Weinberg et al. [18] reported that the apoptosis cells would arrested at G1 and S phases of the cell cycle, and the number of cells at G2 has reduced, result in less and less cell turn into cell division, even cell appeared death. As shown in our study, quantified by FCM, γ-terpineol induced apoptosis of BEL-7402 cells in a time-dependent manner, the longer treatment of γ-terpineol, the more apoptotic cells there were. Cell cycle changes showed that γ-terpineol could induce accumulation of cells arrested at G1 and S phases of the cell cycle. Moreover, treated with γ-terpineol, there was cell cycle alteration with morphology alteration at the same time.
Kerr et al. [19] described that fragmentation of DNA is the typical characteristic of apoptosis. DNA integrity plays a key role in cell proliferation. Cui et al. [8] describe that PMBE (Pinus massoniana bark extract) induced the typical inter-nucleosomal DNA fragmentation in multiples of 100-200 bp, a hallmark of apoptosis. In this study, the result exhibited that DNA was fragmented after treated with γ-terpineol at different time. Therefore, the γ-terpineol could induce human hepatoma cell apoptosis in vitro.
In conclusion, as shown in our study, γ-terpineol not only induced BEL-7402 cell apoptosis, but also drastically decreased BEL-7402 cell protein expression concurrently. The identification of the genes involved and their related signal transduction pathways critical to tumorigenesis await further study. Therefore, the present study demonstrated that γ-terpineol could induce human hepatoma cell apoptosis in vitro. This might be the first report regarding the anti-cancer mechanism of γ-terpineol on human hepatoma cells through suppress tumor cell growth by inducing cell apoptosis. Therefore, the γ-terpineol may hold promise as an anticancer agent.
Acknowledgements
The research was supported by Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT0848), Sichuan provincial achievement transfor mation cultivation project (11ZZ022), City Technology Bureau of Yibin (200903019), Sichuan youth Science and technology innovation teams supporting project (2011JTD0035), A Project Supported by Scientific Research Fund of SiChuan Provincial Education Department (No. 14TD0031). The authors thank Veterinary Pharmacology Lab, Sichuan Agricultural University (Ya’an, P. R. China) for supplying microorganism bacterium.
Disclosure of conflict of interest
None.
References
- 1.Teng BS, Lu YH, Wang ZT, Tao XY, Wei DZ. In vitro anti-tumor activity of isorhamnetin isolated from Hippophae rhamnoides L. against BEL-7402 cells. Pharmacol Res. 2006;54:186–194. doi: 10.1016/j.phrs.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Ertas ON, Guler T, Simsek UG. The effect of an essential oil mix derived from oregano, clove and anise on broiler performance. Int J Poul Sci. 2005;4:879–884. [Google Scholar]
- 3.Thompson D. Fungitoxic activity of essential oil components on food storage fungi. Mycologia. 1989;81:151–153. [Google Scholar]
- 4.Conner D, Beuchat L, Worthington R, Hitchcock H. Effects of essential oils and oleoresins of plants on ethanol production, respiration and sporulation of yeasts. International Journal of Food Microbiology. 1984;1:63–74. [Google Scholar]
- 5.Dorman H, Deans S. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 6.H Yuanzheng, W Mingzhang, Z Hui, R Weijian. A study on the chemical composition of the essential oil of cinnamomum longepaniculatum leaf. Journal of Wuhan Botanical Research. 1986;1:124–128. [Google Scholar]
- 7.Zhao J, Wang J, Chen Y, Agarwal R. Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation-promotion protocol and identification of procyanidin B5-3’-gallate as the most effective antioxidant constituent. Carcinogenesis. 1999;20:1737. doi: 10.1093/carcin/20.9.1737. [DOI] [PubMed] [Google Scholar]
- 8.Cui YY, Xie H, Qi KB, He YM, Wang JF. Effects of Pinus massoniana bark extract on cell proliferation and apoptosis of human hepatoma BEL-7402 cells. World J Gastroenterol. 2005;11:5277. doi: 10.3748/wjg.v11.i34.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 10.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 11.Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DJ, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589. [PubMed] [Google Scholar]
- 12.Manosroi J, Dhumtanom P, Manosroi A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006;235:114–120. doi: 10.1016/j.canlet.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Zang LQ, Hu F, Wei M, et al. Research on Anti-tumor Effect of Essential Oil in Schizoneprta Tenuifo-lia Briq and Its Inducing Apoptosis. Guangxi Journal of Traditional Chinese Medicine. 2006;29:60–62. [Google Scholar]
- 14.Cotter TG, Lennon SV, Glynn JM, Green DR. Microfilament-disrupting agents prevent the formation of apoptotic bodies in tumor cells undergoing apoptosis. Cancer Res. 1992;52:997. [PubMed] [Google Scholar]
- 15.Wu Y, Chen L, Yu H, Liu H, An W. Transfection of hepatic stimulator substance gene desensitizes hepatoma cells to H2O2-induced cell apoptosis via preservation of mitochondria. Arch Biochem Biophys. 2007;464:48–56. doi: 10.1016/j.abb.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Barlogie B, Raber MN, Schumann J, Johnson TS, Drewinko B, Swartzendruber DE, Meihde WG, Andreeff M, Freireich EJ. Flow cytometry in clinical cancer research. Cancer Res. 1983;43:3982. [PubMed] [Google Scholar]
- 17.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 18.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 19.Kerr J, Wyllie A, Currie A. Apoptosis (Cell Suicide) Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]


