Abstract
Amyloidosis is a spectrum of diseases characterized by abnormal extracellular accumulation of proteinaceous material; its precise etiology still remains unclear. It may affect multiple organs, of which the commonest sites are larynx, bronchus and kidney. Laryngeal amyloidosis is usually a localized phenomenon rarely associated with systemic involvement, here we report a case of laryngeal amyloidosis with tracheobronchial involved. The patient was 31-years old. He had a history of open surgical operation for laryngeal amyloidosis in the left ventricle 4-years ago. This time he was suffered by amyloid deposition in his right ventricle, the lesion was excised on staged laryngoscopy under general anesthesia. After 20-months follow-up, the post-operative recovery was wonderful. The bronchoscopy examination and computerized tomography scan for chest demonstrated he also had right main bronchus amyloidosis. Being of no dyspnea, he was unwilling to accept an operation on bronchus. Though amyloidosis is a benign lesion, up to date, there are no curable treatments for such a disease, for severe cases, it may be fatal as a result of airway obstruction or respiratory failure. Here we review the pertinent references on this subject, and discuss the main managements for amyloidosis on larynx and bronchus.
Keywords: Larynx, tracheobronchus, amyloidosis, management
Introduction
Amyloidosis is a spectrum of diseases associated with abnormal extracellular deposition of amyloid, and autologus fibrillar protein material. Histochemically, this protein binds with Congo red, revealing green birefringence under polarized light. In 1842, Rokitansky first described such deposits in tissue [1]. Virchow was the first to use the term amyloid because of the starch-like reaction when treated with iodine and sulphuric acid [2]. In 1851, Borow documented the first case of laryngeal amyloidosis in 1973 [3]. The cause of amyloidosis has not been elucidated. The protein of amyloid fibrils may be light chains synthesized by increased plasma cells and secreted extracellularly, cleaved by lysosomal enzymes in tissue macrophages, polymerized into immunoglobulin light chain amyloid fibrils, and then deposited. Amyloidosis is a rare benign process and the larynx is the common site of localized amyloidosis in the head and neck region [4], Laryngeal amyloidosis is usually a localized phenomenon that is rarely associated with systemic involvement, here we report a case of laryngeal amyloidosis with tracheobronchial involved, and discuss the pertinent managements on this subject.
Case report
A 31-year-old man was admitted to our hospital with complaints of a hoarse voice of 3-years duration with chronic cough, and intermittent hemoptysis and a mild dyspnea currently. He was a non-smoker. Laryngoscopic examination revealed a large non-ulcerated, red subepithelial mass arising from the right ventricle (Figure 1A). The epiglottis and the epiglottic vallecula were normal, both vestibule folds were swelling, the right vocal cord could not be visualized, and the left vocal cord was normal. Laboratory data on admission showed no abnormality of complete blood count, serum electrolytes, creatinine, liver tests, albumin, total serum protein, and erythrocyte sedimentation rate. His family history was unremarkable. He had a history of open surgical operation for laryngeal amyloidosis in left ventricle, and a flexible bronchoscopic examination 4-years ago. The bronchoscopy revealed diffuse thickening, mucosal friability, and narrowing of the right main bronchus. Biopsy specimen revealed eosinophilic deposits in the submucosa with apple-green birefringence on Congo red stain, a finding characteristic of amyloid. A computerized tomography (CT) scan for chest was arranged on admission, the result demonstrated circumferential wall thickening with calcifications as well as luminal narrowing of right main bronchus (Figure 2), but were not observed in the pulmonary parenchyma.
Figure 1.
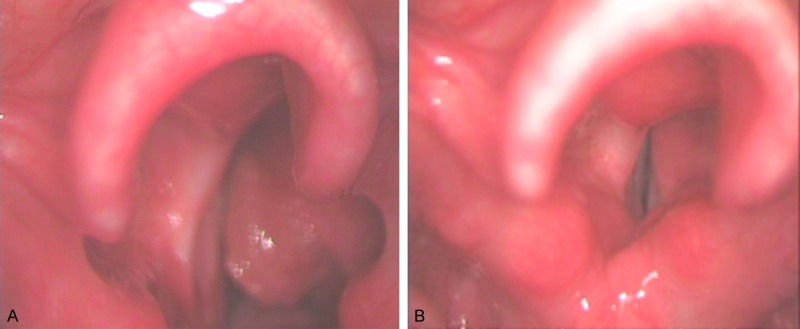
A. Laryngoscopy image showing mass of amyloid on the right ventricle. B. Laryngoscopic examination 18 months postoperatively shows no evidence of recurrence.
Figure 2.
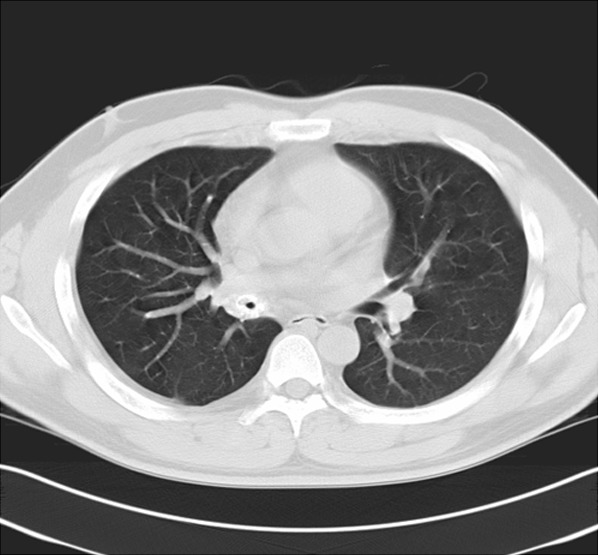
CT scan of the chest revealing circumferential thickening with partial calcification of the right main bronchus.
The patient was scheduled to undergo a local mass excised on staged laryngoscopy under general anesthesia. Histopathological examination revealed amorphous deposits of eosinophilic, acellular and homogenous material within the subepithelial stroma, a finding characteristic of laryngeal amyloidosis. The post-operative recovery was uneventful, and the patient was discharged home the next day after operation. After 20-months follow-up examination, his voice improved greatly, no recurrence was noted although there was some swelling of the right ventricular band (Figure 1B). Sometimes the patient was suffered by recurrent cough, especially when he caught a cold. Being of no dyspnea, he was unwilling to accept an operation on bronchus. Medical treatment modalities including corticosteroids and antibiotics were used to relax his symptom when suffered with respiratory infection.
Discussion
Amyloidosis is a condition characterized by the extracellular accumulation of proteinaceous material in the tissue; its precise etiology still remains unclear. It may affect multiple organs, of which the commonest site is the larynx, bronchus and kidney. Although amyloid protein deposits have a microscopically uniform appearance, it is clear that amyloid is not a single entity. There are about twenty described biochemical forms of amyloidosis [5]; however, the major types are AL (immunoglobulin light chain) and AA (amyloid-associated). AL is derived from plasma cells and contains kappa or lambda immunoglobulin light chains, it is associated with primary systemic amyloidosis, myeloma-associated amyloid, and most localized forms of amyloid. AA is an immunoglobulin protein synthesized by liver; this protein is deposited in secondary amyloidosis.
Amyloidosis can be systemic or localized according to the clinical involvement. When a diagnosis of laryngeal amyloidosis is made, it has been suggested that investigations should be organized to rule out systemic involvement. As to our case, on CT examination, there is an image of esophageal wall slightly thickening circumferentially, an endoscopic examination and biopsy in digestive tract is necessary to make it a clarification, however, the patient had no complainant of dysphagia, and he declined to accept such an invasive investigation. We feel the natural history of the localized amyloidosis is benign and we have no effective treatment to prevent its progression, invasive investigation including endoscopic examination and biopsy may be limited to patients when they have clinical manifestation of the proper organ involved. On the other hand, long-term follow up with routine investigation such as full blood count, serum immunoelectrophoresis, urinalysis for Bence-Jones proteins, CT or MRI scan is requirement.
Though amyloidosis is a benign lesion, up to date, there are no curable treatments for such a disease, especially for systemic amyloidosis, but the patient sometime will be fatal as a result of airway obstruction or respiratory failure. There have been documented cases of fatal upper airway hemorrhage and obstruction due to the laryngeal lesion [6]. We believe that a comprehensive evaluation should be warranted whenever such a disease is encountered. Here we review the pertinent information on this subject.
The larynx is the most common site of localized amyloidosis in the head and neck region. Localization of the lesion in the larynx is to the ventricle, vestibular folds, vocal folds, epiglottis, aryepiglottic folds, and subglottis in order of frequency [7]. Hoarseness is the most common symptom, progressive dyspnoea; haemoptysis and dysphagia are other presenting symptoms. The male to female ratio is 3:1 and the ages ranging from 11-80 years, but the maximum incidence lies in the fifth decades [7].
A definitive diagnosis of amyloidosis depends on obtaining a tissue specimen biopsy and demonstration of amyloid by appropriate stains. Computed tomography or magnetic resonance imaging may be helpful in mapping lesions, which may be more extensive than they appear during laryngoscopy. In the CT examination, amyloidosis is demonstrated as a marked thickening of the laryngeal soft tissue with high density. On the other hand, the signal characteristics of amyloid on magnetic resonance imaging (MRI) resemble those of skeletal muscles. This may be an important differentiating point because muscle is an easy reference frame, and tumors do not appear in this manner on MRI images [8].
The treatment of extensive benign laryngeal disease can be a challenge problem. The primary goals of the treatment are to excise the pathologic specimen and improve the airway. Because this is a benign disease process, consideration must be given to patient morbidity and ultimate voice outcome. Treatment varies from simple observation of the lesion to partial even total laryngectomy. Surveillance may be indicated in selected instances, because there may be slow progression of disease over many years [9]. As to localized tumor-like lesions, minimal excision technique with serial endoscopies can readily achieve this goal. Recent experience using the CO2 laser to resect amyloid deposits in the larynx and trachea suggests this is the treatment of choice [10,11]. In the case of diffuse submucosal deposits, repeat excision is often necessary to restore the airway and conserve the voice, especially for true vocal cords (TVCs) amyloid. According to the literature, it’s interesting that the voice can be nearly normal or normal with the amyloid visible on the undersurface of even both vocal cords if the upper margin is 2 to 3 mm below the free edge [12], on the other hand, visible residual amyloid on the undersurface of the true vocal cords may regrow very slowly, so in order to conserve voice after operation, a complete removal for TVC amyloid sometimes is not necessary, a second resection on the same or opposite side may be based on voice quality.
Sometimes, when the laryngeal involvement is so severe that an external approach should be given, extended cases require laryngofissure or supraglottic laryngectomy, occasionally, tracheotomy is necessary. Mitrani and Biller [13] performed three thyrotomies with excision and two supraglottic laryngectomies for extensive lesions. However, advances in laser technology and the cumulative experience of treating benign lesions of the larynx have made staged endoscopic excision an effective treatment option in even extensive disease.
The main treatment of the laryngeal amyloidosis is surgical excision. Systemic or intralesional corticosteroid application has not proven to be satisfactory neither has radiotherapy [13]. We believe that management of localized laryngeal amyloidosis should be limited to local excision of the lesions when symptomatic, otherwise observation is the best treatment of asymptomatic or mild symptomatic patients. Extensive lesions that compromise the airway may be treated either endoscopically or by an external approach; however, it should be limited to avoid scarring and laryngeal stenosis. Long-term follow up is essential because of the slowly progressive nature of the disease and the risk of long-term complications such as airway obstruction.
Pulmonary amyloidosis occurs as a localized process restricted to the lung, or as a part of systemic infiltration. They are traditionally divided into tracheobronchial amyloidosis, nodular parenchymal amyloidosis and diffuse parenchymal amyloidosis [14]. Tracheobronchial amyloidosis makes up 25% to 50% of all cases of localized pulmonary amyloidosis, the patient in the present paper correspond to this type. Common presenting symptoms include chronic cough, dyspnea, wheezing, hemoptysis, and recurrent pneumonia. Long-term survival is poor, and it is frequently associated with increased mortality and a reduced life expectancy due to respiratory failure.
The image of tracheobronchial amyloidosis on CT appears as circumferential wall thickening with calcifications as well as luminal narrowing at various levels of the tracheobronchial tree, as was seen in our patient. Although the CT findings of tracheobronchial amyloidosis are specific of this condition, other diseases should be considered, such as tracheobronchial tuberculosis and relapsing polychondritis [15,16]. Tissue biopsy is necessary for definitive diagnosis with the classic appearance of green birefringence under polarized microscopy.
No established effective treatment exist for pulmonary amyloidosis, management is largely dictated by degree of symptoms. To patient who have no or mild symptom, observation alone is the best choice, just as our patient. To patients who have remarkable symptoms, treatment options ranged from aggressive local and systemic therapy to radiotherapy. (1) Bronchoscopic resection with laser treatment: Bronchoscopic treatment is thought to be safer than open surgical intervention. In a pseudotumor mass or a circumferential wall thickening, for example, mechanical resection and/or dilatation coupled with transmucosal Nd: YAG laser/CO2 laser treatment with low power and high energy density are feasible [17,18]. The use of carbon dioxide laser resection has been shown to yield good results, resection with the Nd: YAG laser has been more widely reported [19,20]. Usually it will likely undergo repeated attempts to control progressive respiratory symptoms. As reported by others [21,22], intraoperative bleeding is the most frequently encountered complication due to the fragility of the blood vessels infiltrated with amyloid proteins. (2) Bronchoscopic dilatation and stenting: The use of silicone stents for treatment of airway narrowing due to amyloidosis has been reported previously [23]. Cazalets and colleagues [24] described 2 patients with TBA that were treated successfully with bronchoscopic dilatation and Yang and colleagues [25] reported 1 patient managed with bronchoscopic resection and stenting with good outcome. In our patient, CT scan showed it was about 2 cm circumferential narrow at the right main bronchus, it will suitable to give him a stenting when necessary. (3) External-beam radiation: External beam radiation therapy has also been used based on the hypothesis that the abnormal secretion of light chains by local plasma cells was the cause of this disorder, and radiation therapy would kill the plasma cells responsible for these secretion of the amyloidogenic protein. Reports indicated external-beam radiation therapy for tracheobronchial amyloidosis with clinical and functional improvement using 60CO [26-28]. Radiation therapy may cause some potential complication, such as: esophagitis, pericarditis, radiation pneumonitis and pulmomary fibrosis, fortunately, the commonly used dose (20 Gy) was lower compared with that used for lung cancer treatment (60-70 Gy). The long-term effect of this treatment on the tracheobronchial amyloidosis is entirely unknown, and the patient must continue to be monitored. (4) Lobectomy: Lobectomy had been described in the literature for treatment of pulmonary amyloidosis in severe cases with extensive airway involvement [29], even though they are rarely performed.
Functional bronchoscopic or surgical resection aimed solely at removal of the symptomatic amyloid deposits should be considered as a general guideline. It’s almost impossible to completely remove all the amyloid tissue, being often difficult to detect, and even more to resect all the submucosal deposits. The main goal in management of such patients is symptoms reduction or resolution.
Currently, there is no specific therapy for systemic amyloidosis. Research is currently underway seeking to prevent amyloid deposition by stabilizing the amyloid precursor proteins in their normal conformation, inhibiting fibril propagation, enhancing fibril degradation. Studies in vitro demonstrated that two ubiquitous chaperone molecules-serum amyloid P (SAP) component and heparan sulfate proteoglycan (HSP)-promote β-pleated sheet formation and prevent proteolytic digestion. Functionally removing these chaperones from fibril complexes inhibited amyloid formation and increased protease susceptibility [30,31]. Pepys and colleagues [32] recently reported SAP binding by Ro 63-8695 could both reduce plasma SAP levels and competitively inhibit SAP-amyloid binding, which might potentially improve the outlook for these patients. However, up to date there is no proved pharmacologic therapy for such a disease, conservative surgical excision with functional preservation remains the treatment of choice.
Disclosure of conflict of interest
None.
References
- 1.Rokitansky KF. Handbuch der pathologischen Anatomie. Vienna: Braumuller und Seidel; 1842. pp. 209–249. [Google Scholar]
- 2.Virchaw R. Bav and Zussmmersetzying der Corporci Amalacca des Menschen. Vehr Phys Med Geswurzlurg 1851; 2:51–53. [Google Scholar]
- 3.Borow A. Amyloide degenaeration von larynxtumoren: Canule seiber jahre lang Getrager. Arch Klin Chir 1873; 15:242–246. [Google Scholar]
- 4.Kennedy TL, Patel NM. Surgical management of localized amyloidosis. Laryngoscope. 2000;110:918–923. doi: 10.1097/00005537-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Westermark P, Araki S, Benson MD, Cohen AS, Frangione B, Masters CL, Saraiva MJ, Sipe JD, Husby G, Kyle RA, Selkoe D. Nomenclature of amyloid fibril proteins. Report from the meeting of the International Nomenclature Committee on Amyloidosis, August 8-9, 1998, Part 1. Amyloid. 1999;6:63–66. doi: 10.3109/13506129908993290. [DOI] [PubMed] [Google Scholar]
- 6.Chow LT, Chow W, Shum BS. Fatal massive upper respiratory tract haemorrhage: an unusual complication of localized amyloidosis of the larynx. J Laryngol Otol. 1993;107:51–53. doi: 10.1017/s0022215100122145. [DOI] [PubMed] [Google Scholar]
- 7.Aydin O, Ustundag E, Iseri M, Ozkarakas H, Oguz A. Laryngeal amyloidosis with laryngocele. J Laryngol Otol. 1999;113:361–363. [PubMed] [Google Scholar]
- 8.Gean-Marton AD, Kirsch CF, Vezina LG, Weber AL. Focal amyloidosis of the head and neck: evaluation with the CT and MR imaging. Radiology. 1991;181:521–525. doi: 10.1148/radiology.181.2.1924798. [DOI] [PubMed] [Google Scholar]
- 9.O’Halloran LR, Lusk RP. Amyloidosis of the larynx in a child. Ann Otol Rhinol Laryngol. 1994;103:590–594. doi: 10.1177/000348949410300802. [DOI] [PubMed] [Google Scholar]
- 10.Tsai TL, Chu PY, Chang SY. Laryngeal amyloidosis with airway obstruction. Otolaryngol Head Neck Surg. 2002;126:329–330. doi: 10.1067/mhn.2002.123106. [DOI] [PubMed] [Google Scholar]
- 11.Friedman AD, Bhayani R, Memeo L, Kuriloff DB. Localized laryngeal amyloidosis. Otolaryngol Head Neck Surg. 2002;127:487–489. doi: 10.1067/mhn.2002.129820. [DOI] [PubMed] [Google Scholar]
- 12.Dedo HH, Izdebski K. Laryngeal Amyloidosis in 10 Patients. Laryngoscope. 2004;114:1742–1746. doi: 10.1097/00005537-200410000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Mitrani M, Biller HF. Laryngeal amyloidosis. Laryngoscope. 1985;95:1346–1347. doi: 10.1288/00005537-198511000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Spencer H. Spencer’s Pathology of the Lung. 5th edition. New York: McGraw-Hill; 1995. p. 776. [Google Scholar]
- 15.Kim HY, Im JG, Song KS, Lee KS, Kim JS, Lee JS, Lim TH. Localized amyloidosis of the respiratory system: CT features. J Comput Assist Tomogr. 1999;23:627–31. doi: 10.1097/00004728-199907000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Capizzi SA, Betancourt E, Prakash UB. Tracheobronchial amyloidosis. Mayo Clin Proc. 2000;75:1148–52. doi: 10.4065/75.11.1148. [DOI] [PubMed] [Google Scholar]
- 17.Madden BP, Lee M, Paruchuru P. Successful treatment of endobronchial amyloidosis using Nd: YAG laser therapy as an alternative to lobectomy. Monaldi Arch Chest Dis. 2001;56:27–29. [PubMed] [Google Scholar]
- 18.Flemming AF, Fairfax AJ, Arrnold AG, Lane DJ. Treatment of endobronchial amyloidosis by intermittent bronchoscopic resection. Br J Dis Chest. 1980;74:183–188. doi: 10.1016/0007-0971(80)90032-7. [DOI] [PubMed] [Google Scholar]
- 19.Diaz-Jimenez JP, Rodriquez A, Martinez Ballarin JI. Diffuse tracheobronchial amyloidosis. J Bronchol. 1999;6:13–17. [Google Scholar]
- 20.Shah H, Garbe L, Nussbaum E, Dumon JF, Chiodera PL, Cavaliere S. Benign tumors of the tracheobronchial tree: endoscopic characteristics and role of laser resection. Chest. 1995;107:1744–1751. doi: 10.1378/chest.107.6.1744. [DOI] [PubMed] [Google Scholar]
- 21.Merlini G. Treatment of primary amyloidosis. Semin Hematol. 1995;32:60–79. [PubMed] [Google Scholar]
- 22.O’Regan A, Fenlon HM, Beamis JF Jr, Steele MP, Skinner M, Berk JL. Tracheobronchial amyloidosis. The Boston University experience from 1984 to 1999. Medicine. 2000;79:69–79. doi: 10.1097/00005792-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Dumon JF. A dedicated tracheobronchial stent. Chest. 1990;97:328–332. doi: 10.1378/chest.97.2.328. [DOI] [PubMed] [Google Scholar]
- 24.Cazalets C, Belleguic C, Sost G, Caulet-Maugendre S, Kernec J, Droz D, Grosbois B. Tracheobronchial amyloidosis: a propos of 2 cases. Rev Med Interne. 2002;23:317–21. doi: 10.1016/s0248-8663(01)00557-4. [DOI] [PubMed] [Google Scholar]
- 25.Yang S, Chia SY, Chuah KL, Eng P. Tracheobronchial amyloidosis treated with rigid bronchoscopy and stenting. Surg Endosc. 2003;17:658–659. doi: 10.1007/s00464-002-4260-z. [DOI] [PubMed] [Google Scholar]
- 26.Monroe AT, Walia R, Zlotecki RA, Jantz MA. Tracheobronchial amyloidosis: a case report of successful treatment with external beam radiation therapy. Chest. 2004;125:784–9. doi: 10.1378/chest.125.2.784. [DOI] [PubMed] [Google Scholar]
- 27.Kurrus JA, Hayes JK, Hoidal JR, Menendez MM, Elstad MR. Radiation therapy for tracheobronchial amyloidosis. Chest. 1998;114:1489–92. doi: 10.1378/chest.114.5.1489. [DOI] [PubMed] [Google Scholar]
- 28.Kalra S, Utz JP, Edell ES, Foote RL. External-beam radiation therapy in the treatment of diffuse tracheobronchial amyloidosis. Mayo Clin Proc. 2001;76:853–856. doi: 10.1016/S0025-6196(11)63233-3. [DOI] [PubMed] [Google Scholar]
- 29.Dahl KA, Kernstine KH, Vannatta TL, karwal MW, Thomas KW, Schraith DF. Tracheobronchial amyloidosis: A surgical disease with long-term consequences. J Thorac Cardiovasc Surg. 2004;128:789–792. doi: 10.1016/j.jtcvs.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 30.McDonald DR, Brunden KR, Landreth GE. Amyloid fibrils activate tyrosine kinase-dependent signaling and superoxide production in microglia. J Neurosci. 1997;17:2284–2294. doi: 10.1523/JNEUROSCI.17-07-02284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tennent GA, Lovat LB, Pepys MB. Serum amyloid P component prevents proteolysis of the amyloid fibrils of Alzheimer disease and systemic amyloidosis. Proc Natl Acad Sci U S A. 1995;92:4299–4303. doi: 10.1073/pnas.92.10.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pepys MB, Herbert J, Hutchinson WL, Tennent GA, Lachmann HJ, Gallimore JR, Lovat LB, Bartfai T, Alanine A, Hertel C, Hoffmann T, Jakob-Roetne R, Norcross RD, Kemp JA, Yamamura K, Suzuki M, Taylor GW, Murray S, Thompson D, Purvis A, Kolstoe S, Wood SP, Hawkins PN. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature. 2002;417:254–9. doi: 10.1038/417254a. [DOI] [PubMed] [Google Scholar]


