Abstract
Objective: To evaluate the relationship between the subtype of cells/cellular constituents (the density of T lymphocyte subsets, B lymphocyte, macrophages, and FOXP3 positive cells in 93 patients with meningioma, WHO grades I and II) in the tumor microenvironment and clinicopathological parameters (gender, age, tumor location, size, recurrence and pathological type) of meningioma. Methods: Immunohistochemical demonstrations of CD20 and CD4 lymphocytes, CD68, and FOXP3 expression were performed. In order to assess the densities of CD4, CD20, CD68 and FOXP3 positive cells in 93 meningioma patients, the results were derived from independent reviews by two pathologists. Chi-square test was used for independent samples. Results: There were no relationships between the CD4+, CD68+ cell subsets and patients’ age, sex, tumor size, grade and the recurrence of tumor. However, patients with recurrence had a significantly higher density of CD20+ B cells compared to patients with no recurrence (P = 0.003). For the Foxp3+ cell subset, results showed us that more female patients had high density of Foxp3+ cells compared with male patients, while the opposite results were observed in the low density group (P = 0.009). Furthermore, the density of Foxp3+ cells was significantly correlated with the tumor size (P = 0.004) and the pathological types (P = 0.004). Conclusion: Results in this study demonstrate that higher CD20+ B cell density in the tumor is associated with lower tumor recurrence and the density of Foxp3+ cells is significantly correlated with the patients’ sex, tumor size and the pathological types. The results also suggest that understanding of the cellular constituents of tumors and the tumor microenvironment may help investigate the tumor pathogenesis and immunotherapies in meningioma.
Keywords: Meningioma, tumor microenvironment, cell subsets, immunotherapies
Introduction
Meningiomas are neoplasms thought to derive from arachnoidal cap cells in the meningeal coverings of the spinal cord and brain. They are the most common benign intracranial tumors that can cause significant morbidity and in rare cases death [1,2], but the cellular constituents which may be ablated or reprogrammed in the tumor aberrant microenvironment are complexes. Established tumors are complex masses that contain not only neoplastic cells but also nontransformed cellular elements such as myeloid-derived suppressor cells, alternatively activated macrophages, dendritic cells and the full gamut of immune cells which has been confired dysregulated and functionally impaired [3]. The role of the tumor microenvironment in meningioma has attracted little attention. In this study, we investigated the possible functional roles of CD4, CD20, CD68 and FOXP3 in meningioma to evaluate the relationship between the subtype of cells in the tumor microenvironment and parameters in meningioma. And to understand of the cellular constituents of tumors and the tumor microenvironment may help guide the cancer immunotherapies in meningioma.
Materials and methods
Clinical specimens
A total of 93 patients who underwent surgery at Chinese PLA General Hospital for histologically confirmed meningioma during 2010-2012 were selected in this study. The study was carried out with pre-approval from the Hospital’s Ethical Research Committee and all enrolled patients provided written informed consent for tissue donation and analysis, and for publication of findings. There were totally 30 men and 63 women, whose ages range from 27 to 94 years (median: 49 years). Clinicopathological characteristics in our study are presented in Table 1.
Table 1.
Age, sex, size, recurrence and location of meningioma in 93 patients
| Age | Age | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Parameter | <60 (N = 75) | ≥60 (N = 18) | <60 (N = 75) | ≥60 (N = 18) | |
| Sex | Tumor location | ||||
| Male | 28 | 2 | Brain | 41 | 11 |
| Female | 47 | 16 | Frontal sinus | 6 | 0 |
| Size | Skull base | 5 | 2 | ||
| Giant | 11 | 1 | Cerebellum | 5 | 2 |
| Large | 36 | 7 | Cerebellopontine angle | 6 | 2 |
| Small | 28 | 10 | Sphenoid ridge | 3 | 0 |
| Recurrence | Sellar region | 5 | 1 | ||
| Yes | 5 | 1 | Gradenigo | 3 | 0 |
| No | 68 | 19 | Orbital | 1 | 0 |
Immunohistochemistry
Immunohistochemical staining was done on 4-μm thick formalin-fixed, paraffin-embedded tissue sections. Briefly, slides were deparaffinized and pretreated with 1 mmol/L EDTA (pH 8.0) and heat-mediated antigen retrieval solution in a steam pressure cooker. All further steps were done at room temperature in a hydrated chamber. Endogenous peroxidase activity was quenched with 3% Hydrogen Peroxide for 30 min, and slides were preincubated in goat serum. Staining for CD4 antibody (1:50; Abcam), CD20 antibody (1:200; Abcam), CD68 antibody (1:100; Dako), and FOXP3 antibody (1:50; Abcam) antigen were performed by using primary antibodies and dilutions shown in Table 2. Antibodies were applied overnight. The slides were then washed in PBS and detected with the use of an EnVision + DAB System (Dako). Background staining was performed with Mayers hematoxylin and sections then dehydrated through ascending alcohols to xylene and mounted. PBS was used as negative control.
Table 2.
Primary antibodies showing clone and supplier, with optimized dilution used for this study
| Antigen | Clone | Dilution | Supplier |
|---|---|---|---|
| CD4 | BC/1F6 | 1:50 | Abcam |
| CD20 | EP459Y | 1:200 | Abcam |
| CD68 | PG-M1 | 1:100 | Dako |
| FOXP3 | Abcam 22510 | 1:50 | Abcam |
Density of the infiltrated cell subsets
The high or low densities of CD4, CD20, CD68 and FOXP3 positive cells in 93 meningioma patients were assessed by the independent reviews of two pathologists.
Statistical analysis
The SPSS software (version 12.0, SPSS, IL, USA) and GraphPad Prism Software, (version 5.0, San Diego, CA, USA) were used for statistical analysis. Pearson’s Chi-square test was used.
Results
Distribution of cell subsets in tumor tissues
The cell subsets including CD4+, CD20+, CD68+ and Foxp3+ cells were detected in the meningioma tissues by IHC. Results showed that patients could apparently be divided into two groups with high or low infiltrating CD4+, CD20+, CD68+ and Foxp3+ cells subsets (Figure 1). However, in contrast to CD68+ and Foxp3+ cells subsets, there were fewer CD20, a B cell marker, positive cells in the tumor tissues (Figure 1).
Figure 1.
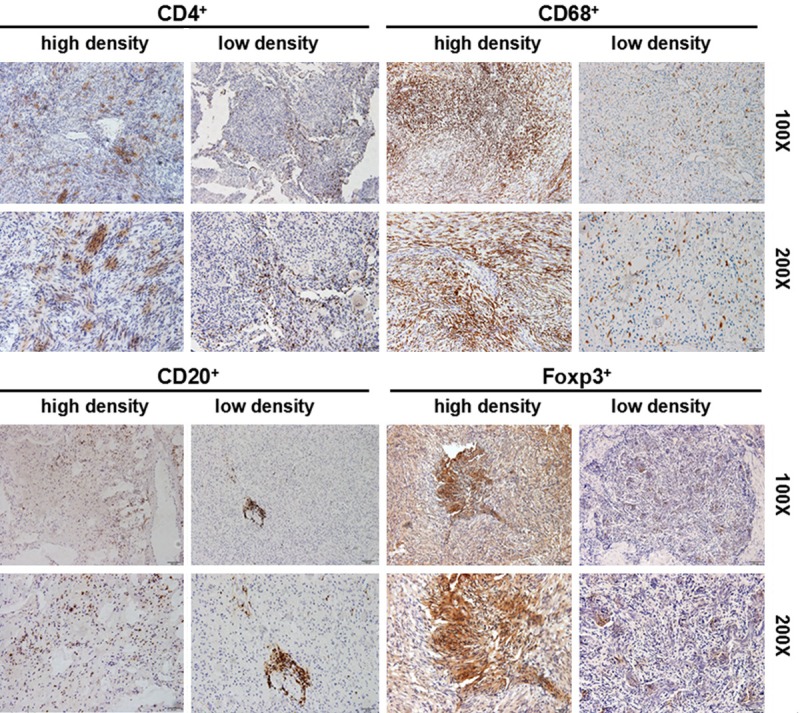
CD4+ T lymphocyte subset, CD20+ B lymphocyte, CD68+ macrophages, and FOXP3+ cells in tumor tissues. Immunohistochemistry was performed by using the anti-CD4, anti-CD20, anti-CD68 and anti-Foxp3 antibodies, respectively. The low density and high density cells in the tumor tissues were shown.
Iimmunohistochemical results in meningioma
Immunohistochemistry with CD34, epithelial membrane antigen (EMA), Ki-67, PR, S-100 and vimentin was performed in 93 cases, including totally 30 men (32.3%) and 63 women (67.6%) with a median age of 49 years (range, 27-94 years). Using the WHO grade, 89 and 4 patients had grade I and II tumor respectively. The result revealed that EMA and vimentin staining were with the majority positive, the percentage were 83.87% and 82.80% respectively. The tumor cells stained CD34 positive in the tumor cells of only 29 cases (31.18%), and the Ki-67 positive was 67 cases in grade I and 4 cases in grade II (totally 78.49%). Progestin Receptors (PR) staining were positivity in 72 cases (77.42%) and S-100 positivity in 22 cases (23.65%) (Table 3).
Table 3.
Immunohistochemical results in meningioma of different subtype
| Grade | n | CD34 | EMA | Ki-67 | PR | S-100 | Vimentin |
|---|---|---|---|---|---|---|---|
| WHO grade I | |||||||
| Transitional | 30 | 8 | 28 | 25 | 28 | 9 | 26 |
| Fibrous | 24 | 6 | 20 | 22 | 12 | 7 | 22 |
| Meningothelial | 15 | 1 | 12 | 9 | 12 | 2 | 13 |
| Psammomatous | 8 | 4 | 8 | 5 | 6 | 1 | 4 |
| Angiomatous | 7 | 6 | 1 | 5 | 5 | 1 | 6 |
| Secretory | 5 | 2 | 5 | 3 | 5 | 0 | 4 |
| WHO grade II | |||||||
| Atypical | 4 | 2 | 4 | 4 | 4 | 2 | 2 |
| Total | 93 | 29 | 78 | 73 | 72 | 22 | 77 |
| Positive rate | 31.18% | 83.87% | 78.49% | 77.42% | 23.65% | 82.80% |
Clinical implications of lymphocyte subset density in tumor tissues
According to the independent histopathologic reviews of two pathologists, the CD4+, CD20+, CD68+ and Foxp3+ cell subsets were divided into low and high groups. Statistic analysis demonstrated that there were no relationships between the CD4+, CD68+ cell subsets and patients’ age, sex, tumor size, grade and the recurrence of tumor. However, patients with recurrence had a significantly higher density of CD20+ B cells compared to patients with no recurrence (P = 0.003). But no other significant correlations between the clinical parameters and CD20+ B cell subset in the tumor tissues were observed. For the Foxp3+ cell subset, results showed us that more female patients had high density of Foxp3+ cells compared with male patients, while the opposite results were observed in the low density group (P = 0.009). Furthermore, the density of Foxp3+ cells is significantly correlation with the tumor size (P = 0.004) and the pathological types (P = 0.004) (Table 4).
Table 4.
Correlations between the expression of CD4, CD20, CD68 and FOXp3 in meningioma and clinicopathologic parameters in 93 patients
| Parameter | CD4+ | P value | CD20+ | P value | CD68+ | P value | Foxp3+ | P value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Low Group | High Group | Low Group | High Group | Low Group | High Group | Low Group | High Group | |||||
| Age, y | ||||||||||||
| <60 | 61 | 14 | 0.592 | 68 | 7 | 0.632 | 40 | 35 | 0.128 | 22 | 53 | 0.073 |
| ≥60 | 13 | 5 | 15 | 3 | 6 | 12 | 1 | 17 | ||||
| Recurrence | ||||||||||||
| No | 78 | 9 | 0.147 | 84 | 3 | 0.003 | 59 | 28 | 0.654 | 40 | 47 | 0.33 |
| Yes | 4 | 2 | 3 | 3 | 3 | 3 | 1 | 5 | ||||
| Sex | ||||||||||||
| Male | 21 | 9 | 0.114 | 25 | 5 | 0.362 | 13 | 17 | 0.553 | 13 | 17 | 0.009 |
| Female | 53 | 10 | 58 | 5 | 33 | 30 | 10 | 53 | ||||
| Tumor size, cm | ||||||||||||
| ≥7 cm | 11 | 1 | 0.219 | 12 | 0 | 0.613 | 4 | 8 | 0.331 | 0 | 12 | 0.004 |
| ≥4.5-7 cm | 33 | 10 | 40 | 3 | 22 | 21 | 19 | 24 | ||||
| <4.5 cm | 34 | 4 | 35 | 3 | 22 | 16 | 8 | 30 | ||||
| Grade | ||||||||||||
| WHO Grade I | 69 | 20 | 0.574 | 80 | 9 | 1 | 51 | 38 | 0.854 | 26 | 63 | 0.481 |
| Transitional | 22 | 8 | 0.573 | 26 | 4 | 0.354 | 16 | 14 | 0.083 | 8 | 22 | 0.004 |
| Fibrous | 20 | 4 | 23 | 1 | 10 | 14 | 2 | 22 | ||||
| Meningothelial | 10 | 5 | 13 | 2 | 8 | 7 | 8 | 7 | ||||
| Psammomatous | 7 | 1 | 8 | 0 | 6 | 2 | 1 | 7 | ||||
| Angiomatous | 5 | 2 | 5 | 2 | 6 | 1 | 3 | 4 | ||||
| Secretory | 5 | 0 | 5 | 0 | 5 | 0 | 4 | 1 | ||||
| WHO grade II | ||||||||||||
| Atypical | 4 | 0 | 4 | 0 | 3 | 1 | 0 | 4 | ||||
NOTE: CD4, CD20, CD68 and FOXp3 cells were classified as high density and low density groups based on the independent histopathologic reviews of two pathologists. Data were analyzed by Pearson’s Chi-square test analysis.
Discussion
The peak incidence of meningioma is in middle-aged patients, and the female: male ratio is approximately 2:1 [4]. This study presents 93 meningiomas classified according to the latest WHO classification of 2007. In our studies, the ages of patients are from 27 to 94 and the middle age is 52. We also confirmed the higher frequency of meningiomas in females compared to males, and the female: male ratio is approximately 2:1. As all we know, meningiomas may occur anywhere in the central nervous system, however some predilections do exist, and our data from Table 1 support the ideas that the brain is the most tumor location in meningiomas [5].
The recurrence rates in each tumor grade were differences. In meningiomas, reported recurrence rates of grade I, II, and III are 7-25%, 29-52%, 50-94%, respectively [6]. But in our studies, we just had the grade I and grade II meningiomas patients (showed in Table 1), we didn’t statistic analysis recurrence rates in the difference grade. But the result showed us the recurrence rate was relatively high in the patients older than 60 years (6.8%) compared to the patients younger than 60 years (5%) in meningiomas.
In clinical practice, however, the diagnosis of meningiomas is based on light microscopy of routinely stained haematoxylin-eosin sections with criteria given by World Health Organization (WHO). This classification scheme provides guidelines for tumor grading and subtypes [6]. Owing to pathological morphological and organization types diversity in meningiomas, with the aim to investigate the frequency of various histopathological features and different subtypes, immunohistochemical staining is helping in diagnosis and differential diagnose of meninginoma. In our studies, we analyzed 93 patients with meningiomas including histological typing and grade and immunohistochemistry phenotype. Six kinds of meningioma histological subtypes were observed in this study. The histological grade was as follows: grade I in 89 cases, grade II in 4 cases. The immunohistochemical marking was performed in 93 cases, and the result revealed CD34 positivity in 29 (31.18%) cases, EMA positivity in 78 (83.87%) cases, Ki-67 positivity in 73 (78.49%) cases, PR positivity in 72 (77.42%) cases, S-100 positivity in 22 (23.65%) cases and vimentin positivity in 77 (82.80%) cases (Table 3). From the all meningioma histological subset markers, the EMA, vimentim and Ki-67 had high positive rates, those results were in accordance with the many other researchers [7,8]. Therefore, EMA is the key marker for the diagnosis of meningioma, while both vimentim and Ki-67 can be adjective indices for the degree of the risk [9,10].
The immunologic constituents were extremely important to tumor microenvironment, and the cellular constituents of a tumor can include immune cells that are normally found in secondary lymphoid organs. More and more studies have provided clear evidence of in filtrating lymphocytes, natural killer (NK) cells, macrophages, dendritic cells (DC), eosinophils, mast cells, and immature myeloid cells or myeloid-derived suppressor cells (MDSC) in both murine and human tumors [11-13]. Using immunohistochemistry (IHC) to evaluate the presence of T cells in tumor biopsies and their potential impact on prognosis have been studied for decades, and early data suggested that a brisk infiltration of T cells in primary melanoma lesions was a positive prognostic factor [14,15]. More recently similar data have been found in other cancers including ovarian cancer, renal cell carcinoma (RCC), bladder cancer, and several other solid cancers [16-19].
In a recent meta-analysis including studies in which clinical significance of tumor infiltrating lymphocytes (TIL) were studied in solid tumors (CD3, CD4, CD8, FoxP3 and rations between these), the presence of CD3, CD8, as well as a high CD8/FoxP3 ration had a positive effect on survival. In addition, the assessment of TIL density and distribution was shown to independently predict sentinel lymph node status and survival in patients with melanoma [20]. More recently, studies showed that the transcription factor fork-head box P3 (Foxp3) is critical for the development of the functional characteristics of T regulatory cells (Tregs). We now have an abundance of data from studies in mice and humans that show an increase in the frequency of Tregs both in the periphery and within tumors from cancers of different histology, including melanomas, lung cancers, esophageal cancers, breast cancers, ovarian cancers, gastric cancers, colorectal cancers, and lymphomas [21-23]. Furthermore, researchers had confirmed that Tregs have the ability to suppress proliferation, cytokine production, and cytolytic activity of CD4+ and CD8+ T cells by mechanisms involving cell-to-cell contact and the release of cytokines such as TGF-b, and Tregs can also induce an immunosuppressive phenotype in other cell types such as macrophages [24,25]. More importantly, Treg depletion leads to tumor regression in vivo, suggesting that the presence of Tregs suppresses effective immune response to tumor [26-28]. To assess the relationships between the Foxp3+ Tregs and the parameters of meningioma patients. We can see more percentage female patients had high density of Foxp3+ cells compared with male patients, while the opposite results were observed in the low density group. Furthermore, the density of Foxp3+ cells is significantly correlation with the tumor size and the pathological types (Table 4).
However, considering the complex composition of the immune system, the role of tumor-infiltrating B cells has directed increasing attention to ovarian cancer, lung cancer, breast cancer and cervical cancer. B cells in the tumor site have many functions, for example it can be served as APCs to facilitate the persistence of CD8+ T cells for long periods, enhance T cell responses by producing Abs, produce cytokines to promote the organization of local lymphoid structure and change the ratio of Th1, Th2 cells [29,30]. The result in our study showed that the density of infiltrating B cells is significance with the tumor recurrence rate. That is patients with higher recurrence had a significantly higher density of CD20+ B cells compared to patients with no recurrence (Table 4). This result will be very helpful for the diagnosis, prognosis, and design of more effective immunotherapies for meningioma.
Macrophages, one of those stromal cells, which are marked by the expression of CD11b and F4/80 in mice and CD11b, CD14, CD33, and CD68 in humans play a crucial role in immune evasion within tumors and they are also have the inherent ability to present antigen to T lymphocytes and provide costimulatory support under the right environment. This ability to cultivate an adaptive immune response is perturbed within the tumor microenvironment and likely plays a role in the shutdown of T-cell-mediated immunity [31-33]. But from our study, the macrophages have no relationships with the parameters in meningioma.
So in our studies, we aimed to identify microenvironment biomarkers (CD4, CD20, CD68 and FOXP3) and parameters (gender, age, tumor location, size, recurrence and pathological type) of meningioma to understand of the cellular constituents of tumors and the tumor microenvironment may help guide the cancer immunotherapies in meningioma. This understanding of the cellular constituents of the tumor microenvironment has helped guide the design of powerful T-cell therapies that are capable of causing the regression of large tumor burdens and recurrence.
Disclosure of conflict of interest
None.
References
- 1.Backer-Grondahl T, Moen BH, Torp SH. The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol. 2012;5:231–242. [PMC free article] [PubMed] [Google Scholar]
- 2.Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. 1997;21:1455–1465. doi: 10.1097/00000478-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Kerkar SP, Restifo NP. Cellular Constituents of Immune Escape within the Tumor Microenvironment. Cancer Res. 2012;72:3125–3130. doi: 10.1158/0008-5472.CAN-11-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088–1095. doi: 10.1227/01.neu.0000188281.91351.b9. discussion 1088-1095. [DOI] [PubMed] [Google Scholar]
- 5.Pollock BE. Defining the best management for patients with intracranial WHO Grade II Meningiomas. World Neurosurg. 2013;81:712–3. doi: 10.1016/j.wneu.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 6.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passacantilli E, Lapadula G, Caporlingua F, Lenzi J, Antonelli M, Santoro F, Santoro A. Chordoid meningioma: a retrospective series of seven consecutive cases. Neurol Sci. 2013;34:1985–9. doi: 10.1007/s10072-013-1431-8. [DOI] [PubMed] [Google Scholar]
- 8.Wu T, Yang Q, Li Z, Cai F, Zhu S, Zhang F. [Clinicopathological analysis of clear cell meningioma] . Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:131–134. [PubMed] [Google Scholar]
- 9.Sundaram C, Uppin SG, Uppin MS, Rekha JS, Panigrahi MK, Purohit AK, Rammurti S. A clinicopathological and immunohistochemical study of central nervous system hemangiopericytomas. J Clin Neurosci. 2010;17:469–472. doi: 10.1016/j.jocn.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Wu YT, Lin JW, Wang HC, Lee TC, Ho JT, Lin YJ. Clinicopathologic analysis of rhabdoid meningioma. J Clin Neurosci. 2010;17:1271–1275. doi: 10.1016/j.jocn.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 11.van Kempen LC, Ruiter DJ, van Muijen GN, Coussens LM. The tumor microenvironment: a critical determinant of neoplastic evolution. Eur J Cell Biol. 2003;82:539–548. doi: 10.1078/0171-9335-00346. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Yu P, Rowley DA, Fu YX, Schreiber H. The role of stroma in immune recognition and destruction of well-established solid tumors. Curr Opin Immunol. 2006;18:226–231. doi: 10.1016/j.coi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Ladanyi A. [Prognostic value of tumor-infiltrating immune cells in melanoma] . Magy Onkol. 2013;57:85–95. [PubMed] [Google Scholar]
- 15.Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, Wu R, Lizee G, Mahoney S, Alvarado G, Glass M, Johnson VE, McMannis JD, Shpall E, Prieto V, Papadopoulos N, Kim K, Homsi J, Bedikian A, Hwu WJ, Patel S, Ross MI, Lee JE, Gershenwald JE, Lucci A, Royal R, Cormier JN, Davies MA, Mansaray R, Fulbright OJ, Toth C, Ramachandran R, Wardell S, Gonzalez A, Hwu P. Specific Lymphocyte Subsets Predict Response to Adoptive Cell Therapy Using Expanded Autologous Tumor-Infiltrating Lymphocytes in Metastatic Melanoma Patients. Clin Cancer Res. 2012;18:6758–6770. doi: 10.1158/1078-0432.CCR-12-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 17.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, Bajorin DF, Reuter VE, Herr H, Old LJ, Sato E. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A. 2007;104:3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao Y, Ou J, Deng J, Geng P, Zeng R, Tian Y, Liang H, Ni B, Ruan Z. Clinical implications of the tumor-infiltrating lymphocyte subsets in colorectal cancer. Med Oncol. 2013;30:727. doi: 10.1007/s12032-013-0727-0. [DOI] [PubMed] [Google Scholar]
- 20.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 22.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 23.Shevach EM. Fatal attraction: tumors beckon regulatory T cells. Nat Med. 2004;10:900–901. doi: 10.1038/nm0904-900. [DOI] [PubMed] [Google Scholar]
- 24.Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L, Coukos G, Zou W. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67:8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 25.Milne K, Kobel M, Kalloger SE, Barnes RO, Gao DX, Gilks CB, Watson PH, Nelson BH. Systematic Analysis of Immune Infiltrates in High-Grade Serous Ovarian Cancer Reveals CD20, FoxP3 and TIA-1 as Positive Prognostic Factors. PLoS One. 2009;4:e6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, Kaiser LR, June CH. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 27.Greaves P, Clear A, Coutinho R, Wilson A, Matthews J, Owen A, Shanyinde M, Lister TA, Calaminici M, Gribben JG. Expression of FOXP3, CD68, and CD20 at Diagnosis in the Microenvironment of Classical Hodgkin Lymphoma Is Predictive of Outcome. J. Clin. Oncol. 2013;31:256–262. doi: 10.1200/JCO.2011.39.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou WP. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen JS, Nelson BH. Tumor-infiltrating B cells and T cells Working together to promote patient survival. Oncoimmunology. 2012;1:1623–1625. doi: 10.4161/onci.21650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185:4977–4982. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Qian BZ, Pollard JW. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]


