Abstract
It has been reported that miR-19a was up-regulated in gastric cancer (GC), playing an oncogenic role. However, the underlying mechanism is still unknown. Therefore, in our present study, we investigated the role of miR-19a in gastric tissues as well as 2 GC cell lines. In vivo in clinical tissue level, we have detected basal expression level of miR-19a using real-time reversal transcriptional PCR (RT-PCR); in addition, the relevance between expression of miR-19a and clinic-pathological information was also analyzed. In vitro in cell line level, miR-19a was ectopically expressed using over expression and knock-down strategy. It was found that the overexpression of miR-19a was significantly associated with metastasis of GC and inferior overall prognosis on clinical tissue level; that promotes the proliferation, migration and invasion; and that overexpression of miR-19a can promote the epithelial-mesenchymal transition through activating PI3K/AKT pathway. Blocking the PI3K/AKT pathway could cancel the effect of miR-19a. All together, our results suggest that miR-19a could be used as a promising therapeutic target in the treatment of GC.
Keywords: Gastric cancer, miR-19a, PI3K-AKT, EMT
Introduction
Despite a substantially declining incidence of gastric cancer (GC), it still is one of the most frequent malignancies worldwide accounting for 700,000 deaths annually [1]. The carcinogenesis of GC is complicated, and most patients experienced asymptomatic presentation in the early stage, resulting metastases at diagnosis. Surgical intervention, chemotherapy and radiotherapy remain to be the most curative treatment options for metastases, but the results of such trials always lead to incidence of post-operative relapse, metastasis and clinical responses [2,3]. To improve the clinical outcome of GC treatment, it is important to clarify GC pathogenesis and to investigate the genes responsible for the progress, metastasis of gastric cancer [4].
Recently, miRNAs have emerged as a major research focus in the field of tumor suppressors [5,6]. MiRNA (approximately 17-25 nucleotides) is a small non-coding RNAs, which can bind to the 3-untranslated region (3’-UTR) loci of its target gene’s mRNA, leading to target mRNA degradation or translational suppression [7]. Increasing evidence suggests that microRNAs (miRNAs) act as central mediators in networks that establish regulatory circuits in cancers, contribute to the carcinogenesis of GC [8,9]. In animal cells, the binding of target mRNA with the miRNA/RISC only requires partial miRNA nucleotides (usually six to eight miRNA nucleotides), which mean that one target mRNA might interact with hundreds of miRNAs. Volinia et al. first found that 26 miRNAs were overexpressed and 17 miRNAs were downexpressed in six solid cancers including stomach [10], and up to now, many studies have confirmed a variety of miRNAs aberrantly expressed in GC, including miR-15b, miR-16, miR-21, miR-34, miR-131, miR-141 etc. [11]. Among them, miR-19a was observed significantly increased in GC [6,12]. However, the detailed role of miR-19a in GC is still poorly understood.
Epithelial-to-mesenchymal transition (EMT) is a fundamental process in embryonic development and considered as an important step leading to tumor invasion and metastasis [13-15]. The most observed character of EMT is that the cells turn to be spindle-like morphology, and loss of epithelial cell markers, including E-cadherin and vimentin [16]. Recent reports have highlighted the importance of miRNA as a powerful regulator of EMT in cancer cells. In this study, we investigated the relationship between miR-19a and the pathogenesis of gastric cancer, and demonstrated that overexpression of miR-19a promotes the proliferation, migration and invasion in gastric cancer, which are in line with EMT. Additionally, we found that cell signaling pathway PI3K/AKT may be involved in EMT in GC.
Materials and methods
Tissue samples and cell lines
Specimens of GC and corresponding normal tissues were collected from 50 patients who were diagnosed and underwent gastrectomy surgery in Zhangjiagang first people’s hospital, Suzhou University. Non-tumor samples from the macroscopic tumor margin were isolated at the same time and used as the matched adjacent nonneoplastic tissues. After resection, all specimens were snap frozen in liquid nitrogen immediately and then stored in -80°C refrigerator. The written informed consent had been obtained from all the patients, and the protocols used in the study were approved by the Hospital’s Protection of Human Subjects Committee. Human GC cell lines SGC7901, NUGC-3 were obtained from the Academy of Military Medical Science (Beijing, China), and human gastric mucosa cell line GES-1 were obtained from the Chinese Academy of Sciences (Shanghai, China). All of the cells were maintained in DMEM supplemented with 10% fetal bovine serum at 37°C with 5% CO2 in a humidified incubator (Forma Scientific, Marietta, OH, USA).
RNA extraction and real-time RT-PCR
Total RNAs were extracted from frozen tissues or cells using a mirVanaTM miRNA Isolation Kit (Ambion), according to the manufacturer’s instructions. Total RNA yields were determined by A260 measurement using the ND-1000 NanoDrop spectrophotometer (NanoDrop, Wilmington). MiR-19a was quantified by a two-step real-time PCR using the miScript-Reverse Transcription Kit and a standard SYBR Green PCR kit (Toyobo, Osaka, Japan) in Applied Biosystems 7300 Real Time PCR system. All of the primers were purchased from uGCT Inc. (Beijing, China). U6 and β-actin were used as an internal control for miRNAs and RNAs, and each sample was analyzed in triplicate. Data were presented as fold differences based on calculations of 2-ΔΔCT.
Transfection assay
The lentiviral system with eGFP-expressing lenti- miR-19a (miR-19a mimic/inhibitor) and the negative control lenti-vector (miR-19a NC) were purchased from Cyagen (Cyagen Biosciences, Guangzhou, China). GC cell lines SGC7901 and NUGC-3 were infected with lenti-miR-19a or lenti-NC according to the manufacturer’s instructions. The stable cells were isolated by flow cytometry to sort eGFP-positive cells.
Cell proliferation assay
For evaluation of cell proliferation rates, 5×104 SGC7901 and NUGC-3 cells were seeded in 96-well plates and incubated overnight. After infected with miR-19a mimic or miR-19a NC, 20 μL MTT (St. Louis, Mo, USA) was added to each well, and plates were incubated for 4 h at 37°C. Then, the reaction was stopped by 150 μL DMSO and optical density was detected with a microplate spectrophotometer (ELx800, Bio-TEK, Winooski, VT, USA) at a wavelength of 490 nm on a microplate reader. Assay was performed in triplicate wells, and each experiment was repeated three times.
Cell migration and invasion assay
Migration and invasion assays were performed as described previous [4]. Cells were starved with serum-free RPMI 1640 medium for 24 h, then 1.5×105 transfected cells were seeded to the upper compartment of transwell chambers (Corning, 24-well plate with 8.0 μm pores) uncoated or coated with Matrigel (BD Biosciences, 0.7 mg/mL). DMEM containing 10% FBS was used as a chemoattractant, and added to the lower chamber. After 24 h incubation, cells on the lower surface of the filter were fixed with methanol and stained with 0.5% crystal violet. Cells which had migrated or invaded to the lower membrane were counted using five-spot-sampling method with a microscope (Olympus, Tokyo, Japan).
Small interfering RNA transfection
Gene silencing by small interfering RNA (siRNA) duplex specific to AKT was used to down-regulate AKT expression. The siRNA was synthesized by Invitrogen, and the siRNA sequences used was as follows: AKT siRNA, 5’-UUCAGGUACUCA AACUCGUUCAUGG-3’ and 5’-CCAUGAACGAGUUUGAGUACCUGAA-3’. Scrambled siRNA duplex was used as a non-specific control siRNA. Transfection was done using Lipofectamine 2000 reagent (Invitrogen), following the manufacturer’s instructions.
Western blotting
Log phase cells were harvested, washed twice and lysed in RIPA lysis buffer. Total proteins of the transfected or control cells were isolated and resolved by 12% SDS-PAGE gel, then blotted onto PVDF membranes (Bio-Rad, Hercules, CA, USA). Membranes were probed with primary antibodies overnight followed by incubation with HRP-conjugated secondary antibodies. Protein expression was assessed by chemi-luminescence kit (ECL-kit, Santa Cruz Biotechnology, Inc.). The densitometry was quantified using Bio-Rad Quantity One software. All experiments were performed in triplicate. Anti-AKT, anti-phospho-AKT, anti-E-cadherin, anti-vimentin antibodies were purchased from Abzoom Biolabs, β-actin (Santa Cruz) was used as an internal control for protein loading. Antibodies diluted as 1:500 with BSA before experiment.
Statistical analysis
All data were presented as the mean ± standard deviation (SD) from at least three separate experiments. Statistical significance was analyzed using One-way ANOVA or two-tail Student’s t-test with SPSS 14.0 software (SPSS Inc., Chicago, IL, USA). Differences were deemed statistically significant when P < 0.05 (*) or P < 0.01 (**).
Results
Expression of miR19a is significantly up-regulated in GC tissues sample and associated with lymph node metastasis and TNM stage
To assess the role of miR-19a in GC, we firstly tested the expression of miR-19a in GC tissues by real-time PCR using RNAs. As shown in Figure 1A, miR-19a was significantly increased more than 2 fold in 50 randomly selected GC patient tissue samples compared with adjacent normal tissues samples. MiR-19a was also remarkably increased in two GC cell lines, SGC7901 and NUGC-3, compared with that of human gastric mucosa cell line, GES-1 (Figure 1B). Then, the relationship between miR-19a expression level and clinicopathologic information of GC was summarized in Table 1. A statistically significant correlation between miR-19a expression levels and metastasis of GC patients was observed in this study. The relative expression of miR-19a increased in patients with lymph node metastasis or with stage I and II were higher than patients without lymph node metastasis or stage III (P < 0.05). However, no significant correlation was found between miR-19a expression and other characteristics such as age, gender, tobacco, alcohol or tumor size.
Figure 1.
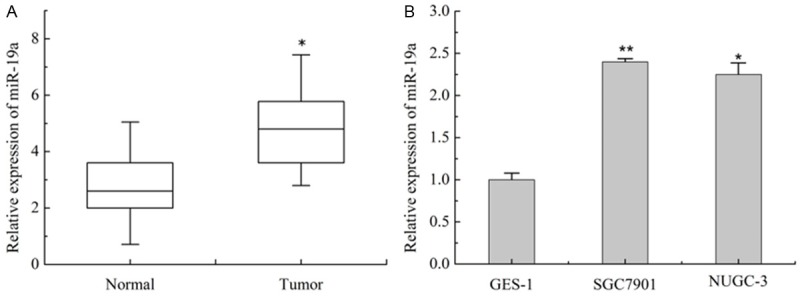
Expression of miR-19a was upregulated in GC tissue samples and cell lines. A. Expression of miR-19a in 50 GC tissue samples and matched non-tumor tissue samples were detected by qRT-PCR. B. Expression of miR-19a in SGC7901, NUGC-3 and GES-1 that detected by qRT-PCR. Values are the average of triple determinations with the SD indicated by error bars. *P < 0.05, **P < 0.01.
Table 1.
Expression of miR-19a and the clinical-pathological characteristics of patients with gastric cancer
| Variables | Total (n = 50) | Expression of miR-19a | |
|---|---|---|---|
|
| |||
| Mean ± S.D. | P-value | ||
| Age | |||
| < 60 | 23 (46%) | 3.58 ± 2.87 | 0.54 |
| ≥ 60 | 27 (54%) | 3.42 ± 3.01 | |
| Gender | |||
| Male | 38 (76%) | 2.90 ± 3.02 | 0.48 |
| Female | 12 (24%) | 3.15 ± 2.96 | |
| TNM stage | |||
| T1, T2 | 19 (38%) | 2.62 ± 2.63 | < 0.05 |
| T3, T4 | 31 (62%) | 5.43 ± 1.89 | |
| Lymph node metastasis | |||
| Positive | 30 (60%) | 5.04 ± 2.30 | < 0.05 |
| Negtive | 20 (40%) | 2.09 ± 2.54 | |
| Tumor size (cm) | |||
| < 5 | 24 (48%) | 3.36 ± 2.65 | 0.66 |
| ≥ 5 | 26 (52%) | 3.23 ± 2.72 | |
| Tobacco | |||
| Yes | 28 (56%) | 3.26 ± 2.43 | 0.18 |
| No | 22 (44%) | 2.97 ± 2.61 | |
| Alcohol | |||
| Yes | 32 (64%) | 3.35 ± 3.12 | 0.31 |
| No | 18 (36%) | 3.08 ± 2.94 | |
MiR-19a promotes the proliferation of GC cells and regulates migration and invasion in GC
Lentivirus infection was used to construct a stable miR-19a mimic or inhibitor cell line. The miR-19a overexpression of SGC7901 and NUGC-3 stably transfected with miR-19a mimic were confirmed by qRT-PCR (P < 0.001) (Figure 2A). qRT-PCR also showed that the SGC7901 and NUGC-3 cell line transfected with the miR-19a inhibitor decreased the expression of miR-19a mRNA (P < 0.001) (Figure 2B).
Figure 2.
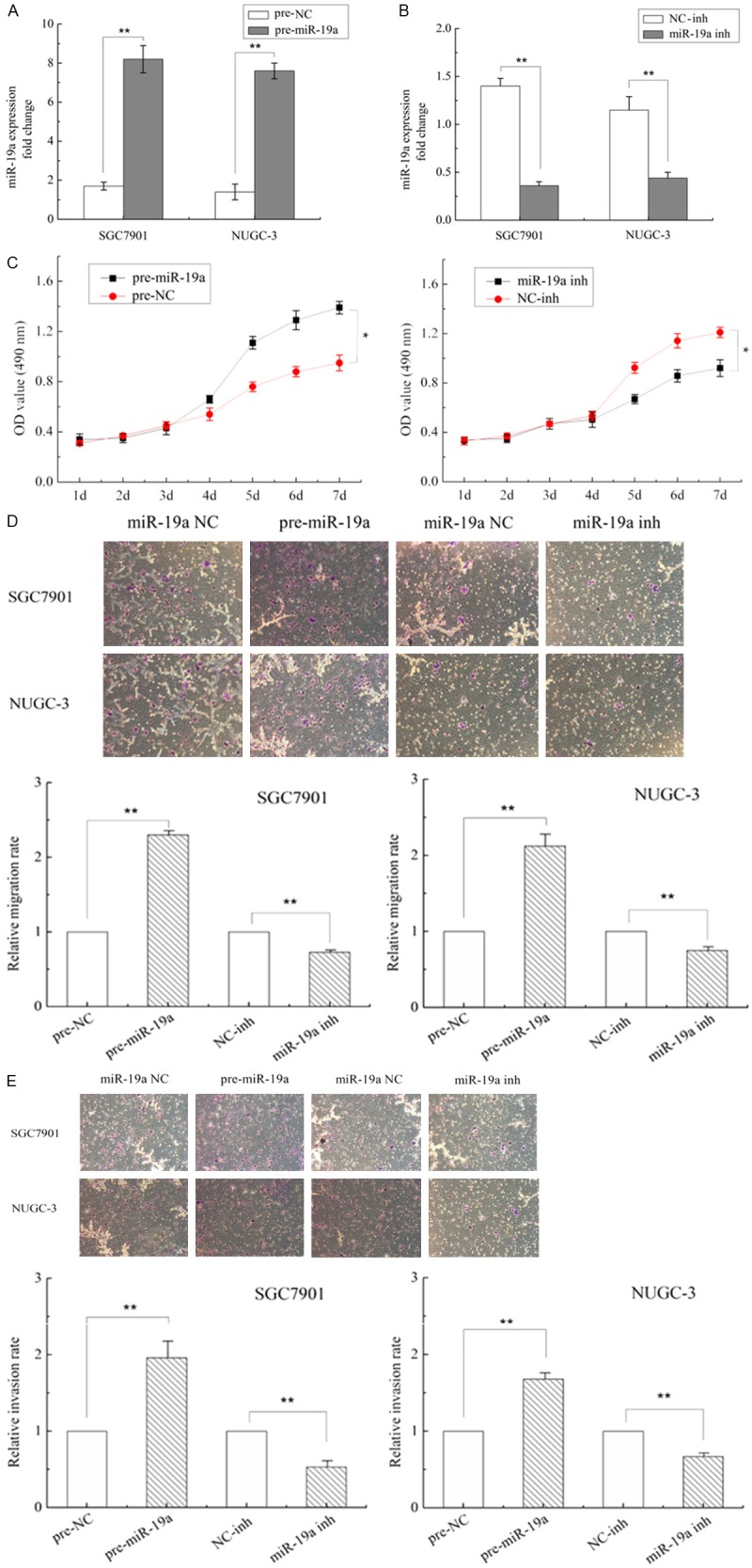
MiR-19a promotes the proliferation of GC cells and regulates migration and invasion in GC. A and B. Expression of miR-19a mRNA in SGC-7901 cells transfected with miR-19a mimic/inhibitor vector (indicated as pre-miR-19a and miR-19a inh) or nagetive control vector (indicated as pre-NC and NC-inh). C. Cell proliferation assay of SGC-7901 cells transfected with miR-19a mimic/inhibitor vector, the OD values were measured each day at the same time point. Data are expressed as mean ± SD from 3 experiments. *P < 0.05, **P < 0.01. D. Representative images of migrated cells on transwell plates that were originally plated with 105 cells, miR-19a precursors or inhibitors regulate gastric cancer cell SGC7901 and NUGC-3 cells migration relative to NC group (**P < 0.01, t test). E. Representative images show cells invaded through transwell and the numbers of invaded cells. MiR-19a precursors or inhibitors regulate gastric cancer cell SGC7901 and NUGC-3 cells invasion relative to NC group (**P < 0.01, t test). Each experiment was repeated at least 3 times. Error bars correspond to the mean ± SD.
To explore the effect of miR-19a on cell growth, SGC7901 and NUGC-3 infected with miR-19a mimic or inhibitor were used to examine their growth curve. As shown in Figure 2C, MTT assay showed that over-expression of miR-19a promoted SGC7901 and NUGC-3 (data not shown) cell growth compared with their corresponding controls. Accordingly, miR-19a inhibitor treatment suppressed cells growth of SGC7901 and NUGC-3 (data not shown).
To investigate the mechanism which miR-19a promoted metastasis. MiR-19a or control mimics (pre-miR-19a, pre-miR-NC, miR-19a-inh, or miR-inh-NC) were transfected into SGC7901 and NUGC-3 cells, then migration and invasion assays were performed in vitro. It was found that the transient transfection of miR-19a precursors significantly increased the migration and invasion abilities of GC cells compared with negative controls (P < 0.01) (Figure 2C and 2D), while the inhibition of miR-19a expression remarkably impeded cell migration and invasion (Figure 2D and 2E). Such results indicate that miR-19a regulate SGC7901 cells migration and invasion.
MiR-19a induces an EMT phenotype
Because miR-19a were over-expressed in GC tissues, along with the fact that miR-19a promote the migratory and invasive abilities of GC cells, we speculated that the overexpression of miR-19a induced migration and invasion might be associated with EMT. To validate these, we initially evaluated the morphological changes in the 2 cells treated with miR-19a mimics. We found that cells overexpressing miR-19a exhibited a spindle-like or fibroblast-like morphology, and the percentage of such cells was significantly increased compared control cells which often show an epithelial-like appearance (P < 0.01) (Figure 3A).
Figure 3.
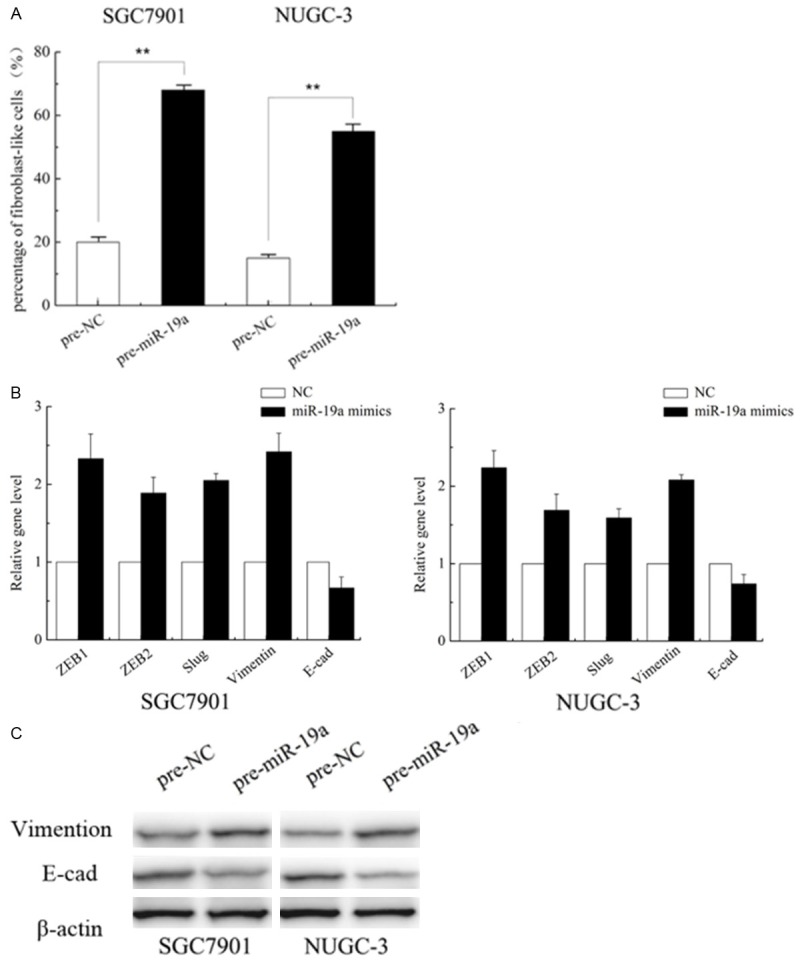
MiR-19a induces an epithelial-to-mesenchymal transition (EMT) phenotype. A. MiR-19a induces an EMT phenotype in SGC7901 and NUGC-3 cells. B. Epithelial and mesenchymal markers ZEB1, ZEB2, Slug, vimentin, and E-cadherin were measured by qRT-PCR from miR-19a mimic or negative control (N.C)-transfected SGC7901 and NUGC-3 cells. The results are shown as fold change compared with negative control. Data are the means ± SD of three independent experiments. *P < 0.05. C. The E-cadherin, vimentin protein levels were measured using western blot analysis. β-actin protein levels served as an internal (loading) control.
EMT is often associated with a decrease or loss of epithelial markers, E-cadherin, and a gain of mesenchymal markers vimentin. Then we detected the transcripts of EMT-associated genes. Concomitant with the change in phenotype, up-regulation of miR-19a level was associated with the increase of ZEB1, ZEB2, Slug, vimentin transcripts (Figure 3B), whereas epithelial marker E-cadherin mRNA showed decrease level in these cells, demonstrating that upregulate miR-19a can promote EMT in GC cells. These differences were further confirmed at the protein levels using western blot analysis (Figure 3C).
miR-19a promotes EMT by activating the PI3K/AKT pathway
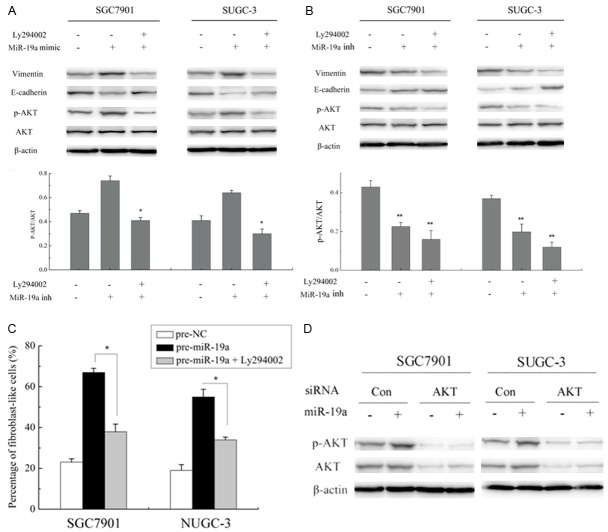
To determine whether miR-19 mediates the activation of PI3K-AKT pathway, PI3K/AKT protein expression levels of control and overexpressing miR-19a SGC7901 and NUGC-3 cells were analyzed by western blotting in the cell lysates. As shown by western blot, overexpressing miR-19a promoted phosphorylation of PI3K-AKT. However, there was no change observed in the expression of total AKT, irrespective of the presence of miR-19a. Densitometry results showed that the p-AKT/AKT ratio of SGC7901 cells transfected with miR-19a mimic was significantly higher than that of normal control cells (P < 0.05) (Figure 4A). To further confirm the effect of miR-19a on the expression or kinase activation of AKT, we transfected cells with miR-19a inhibitor and then performed western blot for p-AKT and AKT. Contrary to the cells transfected with miR-19a mimic, phosphorylation of PI3K-AKT was blocked and the p-AKT/AKT ratio of SGC-7901 and NUGC-3 cells transfected with miR-19a inhibitor was significantly lower than that of normal control group (P < 0.05) (Figure 4B), suggesting that miR-19a plays a critical role in AKT activation in GC.
Figure 4.
A, B. Expression profiles of p-AKT, AKT, epithelial and mesenchymal markers E-cadherin, vimentin and β-actin in SGC-7901 and SUGC-3 cells of each group by western blot analysis, *P < 0.05. To SGC-7901 and SUGC-3 cells cultures, the PI3K/AKT pathway inhibitor Ly294002 was added, with a final concentration as 10 μM. Normal control groups and groups transfected with miR-19a mimic/inhibitor were established for both SGC-7901 and SUGC-3; C. EMT phenotype in SGC7901 and NUGC-3 cells treated with miR-19a mimic in the presence of Ly294002 (10 μM). The data are expressed as the means of three independent experiments ± SD, *P < 0.05. D. Western blot showed AKT and p-AKT expression levels in cells cotransfected with miR-19a mimic and AKT siRNA.
To clarify the effect of kinase activation of AKT in EMT of GC, the cells were treated with PI3K-AKT pathway inhibitor Ly294002, and we found that spindle-like or fibroblast-like morphology in response to Ly294002 by SGC7901 and NUGC-3 cells treated with miR-19a mimic were blocked (Figure 4C). Concomitant with the change in phenotype, loss of E-cadherin and high expression of vimentin by miR-19a were reverted in SGC7901 and NUGC-3 cells when the PI3K/AKT pathway was blocked by Ly294002 (Figure 4A). To further elucidate the role of AKT activation, AKT siRNA were established and their activity was impaired. The western blot showed that expression of AKT siRNA (Figure 4D) reduced the ability of miR-19a to promote phosphorylation of PI3K-AKT compared with the cells transfected with control siRNA. Taken together, these findings show that the PI3K-AKT signaling pathway modulates the EMT of GC cells through PI3K/AKT signals, especially the activation of AKT.
Discussion
To identify prognostic factors in tumorigenesis was essential for predicting patients’ survival and finding optimal therapeutic strategies. Documented evidence has revealed that several miRNAs participate in regulation of cellular homeostasis such as cancer proliferation, apoptosis and metastasis [18]. The oncogenic miR-17-92 cluster was originally described to be over-expressed in human B-cell lymphoma samples. Later, it was identified as a key oncogenic component in many cancers, highlighting the crucial role of this cluster [19,20]. Recently, genetic dissection of the individual miRNAs of this cluster has demonstrated that miR-19 was the most significant miRNAs of this cluster [21]. Compared with the study of miR-17-92 cluster, little research was involved in miR-19 and gastric cancer. Ueda found that miR-19a is upregulated in GC tissues compared with adjacent normal tissues by miRNA microarray analysis [22]. Wang et al showed that miR-19a/b could promote the multidrug resistance of GC cells by targeting PTEN [23]. When this paper was under preparation, Wu et al reported that miR-19 modulate the metastasis of GC cells by targeting the tumor suppressor MXD1 [6]. However, the exact function and the potential mechanisms of miR-19 in GC have not been fully clarified.
In the present study, we sought to characterize the contribution of miR-19a in the control of EMT driven metastasis in GC. We first observed that miR-19a was over-expressed in the randomly selected GC samples. Further analysis of the relevance between expression of miR-19a and clinic-pathological information indicated that overexpression of miR-19a was closely associated with lymph node metastasis in GC patients. This was supported by in vitro transwell migration and invasion assays with two GC cells SGC7901 and NUGC-3. In addition, the relationships between miR-19a and EMT were performed, and our in vitro experiments strongly demonstrated that miR-19a promote EMT of GC. Furthermore, we found higher phosphorylation of AKT when we test the EMT hallmark E-cadherin and vimentin of GC cells transfected with miR-19a mimic. Interestingly, block the PI3K/AKT pathway cancelled the effect of miR-19a, which may provides a more comprehensive picture of the molecular network that miR-19a promotes EMT through activating PI3K/AKT pathway in metastasis of GC.
Dissemination of tumor cell entails an orderly sequential steps, including tumor cell mobilization, intravasation, and subsequent metastasis, that ultimately lead to the colonization of a secondary site [24]. EMT is believed as a crucial mechanism in initial step of acquisition of migratory and invasive capability. Dysregulation of miRNAs is implicated in EMT modulation [25]. Gregory reported that miRNAs, such as smiR-200 family and miR-205, act as key modulators of EMT and enforcers of the epithelial phenotype targeting ZEB1 and SIP1 [26]. Dong et al. demonstrated miR-194 directly targets BMI-1, and reverses EMT phenotype in endometrial cancer cells [27]. To the best of our knowledge, however, the definitive association of miR-19a with EMT of GC has not been reported, only given the evidences showing that the oncogenic effect of miR-19a was mediated by repression of SOCS1, MXD1 [6,27]. For the first time, we found that up-regulation of miR-19a level is able to induce EMT in GC cells, evidenced by epithelial like morphology, increased expression of E-cadherin and decreased expression of vimentin.
A constitutively active mutant of AKT induces EMT in hepatocellular carcinoma cells and carcinoma cell lines [28]. In this work, we studied the PI3K/AKT-dependent cell signaling pathway in GC cells. It was showed that the AKT is activated in miR-19a induced EMT while the PI3K inhibitor LY294002, blocked this response. The linkage of miR-19a to PI3K/AKT activation provides a rationale for the development of miRNA-based EMT. However, we are not clear whether and how this confirmed PI3K/AKT signaling regulate the EMT by downstream protein such like mTOR, snail, β-catenin [29,30], or some other pathway like NF-κB, RAS/ERK signaling were involved in co regulation of EMT [31]. Further studies are under way to characterize if miR-19a can regulate other signaling as well as miR-19a in vivo role in GC tumor by creating nude mice model.
Collectively, our data showed that miR-19a was increased in GC tissue samples and cell lines, and forced expression of miR-19a promotes GC cell metastasis. Most importantly, we implied that miR-19a modulate EMT by activating the PI3K/AKT pathway. This miR-19a-PI3K/AKT axis sheds new light on the mechanisms of oncogenic roles of miR-19a in GC. As a corroborative evidence of previous study, miR-19a could be used as a promising therapeutic target in the treatment of GC.
Disclosure of conflict of interest
None.
References
- 1.Cheng LL, Itahana Y, Lei ZD, Chia NY, Wu YH, Yu YN, Zhang SL, Thike AA, Pandey A, Rozen S, Voorhoeve PM, Yu Q, Tan PH, Bay BH, Itahana K, Tan P. TP53 genomic status regulates sensitivity of gastric cancer cells to the histone methylation inhibitor 3-deazaneplanocin A (DZNep) Clin Cancer Res. 2012;18:4201–12. doi: 10.1158/1078-0432.CCR-12-0036. [DOI] [PubMed] [Google Scholar]
- 2.Buffart TE, Carvalho B, van Grieken NC, van Wieringen WN, Tijssen M, Kranenbarg EM, Verheul HM, Grabsch HI, Ylstra B, van de Velde CJ, Meijer GA. Losses of chromosome 5q and 14q are associated with favorable clinical outcome of patients with gastric cancer. Oncologist. 2012;17:653–62. doi: 10.1634/theoncologist.2010-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia J, Chen L, Jian W, Wang K, Yang Y, He W, He Y, Chen D, Li W. MicroRNA-362 induces cell proliferation and apoptosis resistance in gastric cancer by activation of NF-κB signaling. J Transl Med. 2014;12:33. doi: 10.1186/1479-5876-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao W, Yang W, Fan R, Li H, Jiang J, Geng M, Jin Y, Wu Y. MiR-34a regulates cisplatin-induce gastric cancer cell death by modulating PI3K/AKT/survivin pathway. Tumor Biol. 2014;35:1287–95. doi: 10.1007/s13277-013-1171-7. [DOI] [PubMed] [Google Scholar]
- 5.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 6.Wu Q, Yang Z, An Y, Hu H, Yin J, Zhang P, Nie Y, Wu K, Shi Y, Fan D. MiR-19a/b modulate the metastasis of gastric cancer cells by targeting the tumour suppressor MXD1. Cell Death Dis. 2014;5:e1144. doi: 10.1038/cddis.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan HW, Li SC, Tsai KW. MicroRNA dysregulation in gastric cancer. Curr Pharm Design. 2013;19:1273–84. doi: 10.2174/138161213804805621. [DOI] [PubMed] [Google Scholar]
- 8.Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S, Alder H, Liu C, Oue N, Yasui W, Yoshida K, Sasaki H, Nomura S, Seto Y, Kaminishi M, Calin GA, Croce CM. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136–46. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F, Sun GP, Zou YF, Hao JQ, Zhong F, Ren WJ. MicroRNAs as promising biomarkers for gastric cancer. Cancer Biomark. 2012;11:259–67. doi: 10.3233/CBM-2012-00284. [DOI] [PubMed] [Google Scholar]
- 10.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen B, Huang T, Jiang J, Lv L, Li H, Xia S. MiR-141 suppresses proliferation and motility of gastric cancer cells by targeting HDGF. Mol Cell Biochem. 2014;388:211–18. doi: 10.1007/s11010-013-1912-3. [DOI] [PubMed] [Google Scholar]
- 12.Mendell JT. MiRiad roles for the miR-17-92 cluster in development and disease. Cell. 2008;133:217–22. doi: 10.1016/j.cell.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial- mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 14.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Zhang H, Liu J, Tu X, Zang Y, Zhu J, Chen J, Dong L, Zhang J. MiR-30 inhibits TGF-β1-induced epithelial-to-mesenchymal transition in hepatocyte by targeting Snail1. Biochem Biophys Res Commun. 2012;417:1100–05. doi: 10.1016/j.bbrc.2011.12.121. [DOI] [PubMed] [Google Scholar]
- 16.Yan W, Fu Y, Tian D, Liao J, Liu M, Wang B, Xia L, Zhu Q, Luo M. PI3 kinase/Akt signaling mediates epithelial-mesenchymal transition in hypoxic hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2009;382:631–36. doi: 10.1016/j.bbrc.2009.03.088. [DOI] [PubMed] [Google Scholar]
- 17.Rao E, Jiang C, Ji M, Huang X, Iqbal J, Lenz G, Wright G, Staudt LM, Zhao Y, Mckeithan TW, Chan WC, Fu K. The miRNA-17~92 cluster mediates chemoresistance and enhances tumor growth in mantle cell lymphoma via PI3K/AKT pathway activation. Leukemia. 2012;26:1064–72. doi: 10.1038/leu.2011.305. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Yu H, Cai H, Wang Y. The expression and clinical significance of miR-132 in gastric cancer patients. Diagn Pathol. 2014;9:57. doi: 10.1186/1746-1596-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–33. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy BL, Obad S, Bihannic L, Ayrault O, Zindy F, Kauppinen S, Roussel MF. Silencing of the miR-17~92 cluster family inhibits medulloblastoma progression. Cancer Res. 2013;73:7068–78. doi: 10.1158/0008-5472.CAN-13-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olive V, Bennett MJ, Walker JC, Ma C, Jiang I, Cordon-Cardo C, Li QJ, Lowe SW, Hannon GJ, He L. MiR-19 is a key oncogenic component of mir-17-92. Gene Dev. 2009;23:2839–49. doi: 10.1101/gad.1861409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair VS, Maeda LS, Ioannidis JP. Clinical outcome prediction by microRNAs in human cancer: a systematic review. J Natl Cancer Inst. 2012;104:528–40. doi: 10.1093/jnci/djs027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang F, Li T, Zhang B, Li H, Wu Q, Yang L, Nie Y, Wu K, Shi Y, Fan D. MicroRNA-19a/b regulates multidrug resistance in human gastric cancer cells by targeting PTEN. Biochem Biophys Res Commun. 2013;434:688–94. doi: 10.1016/j.bbrc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Zijlstra A, Lewis J, DeGryse B, Stuhlmann H, Quigley JP. The inhibition of tumor cell intravasation and subsequent metastasis via regulation of in vivo tumor cell motility by the tetraspanin CD151. Cancer Cell. 2008;13:221–34. doi: 10.1016/j.ccr.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davalos V, Moutinho C, Villanueva A, Boque R, Silva P, Carneiro F, Esteller M. Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis. Oncogene. 2012;31:2062–74. doi: 10.1038/onc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong M, Jiang L, Zhou Y, Qiu W, Fang L, Tan R, Wen P, Yang J. The miR-200 family regulates TGF-β1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression. Am J Physiol Renal Physiol. 2012;302:F369–F79. doi: 10.1152/ajprenal.00268.2011. [DOI] [PubMed] [Google Scholar]
- 27.Qin S, Ai F, Ji WF, Rao W, Zhang HC, Yao WJ. MiR-19a promotes cell growth and tumorigenesis through targeting SOCS1 in gastric cancer. Asian Pac J Cancer Prev. 2013;14:835–40. doi: 10.7314/apjcp.2013.14.2.835. [DOI] [PubMed] [Google Scholar]
- 28.Ding W, You H, Dang H, LeBlanc F, Galicia V, Lu SC, Stiles B, Rountree CB. Epithelial-to-mesenchymal transition of murine liver tumor cells promotes invasion. Hepatology. 2010;52:945–53. doi: 10.1002/hep.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, Lee EY, Weiss HL, O’Connor KL, Gao T, Evers BM. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–56. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen KC, Chen CY, Lin CJ, Yang TY, Chen TH, Wu LC, Wu CC. Luteolin attenuates TGF-β1-induced epithelial-mesenchymal transition of lung cancer cells by interfering in the PI3K/Akt-NF-κB-Snail pathway. Life Sci. 2013;93:924–33. doi: 10.1016/j.lfs.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Yoo YA, Kang MH, Lee HJ, Kim B, Park JK, Kim HK, Kim JS, Oh SC. Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res. 2011;71:7061–7070. doi: 10.1158/0008-5472.CAN-11-1338. [DOI] [PubMed] [Google Scholar]