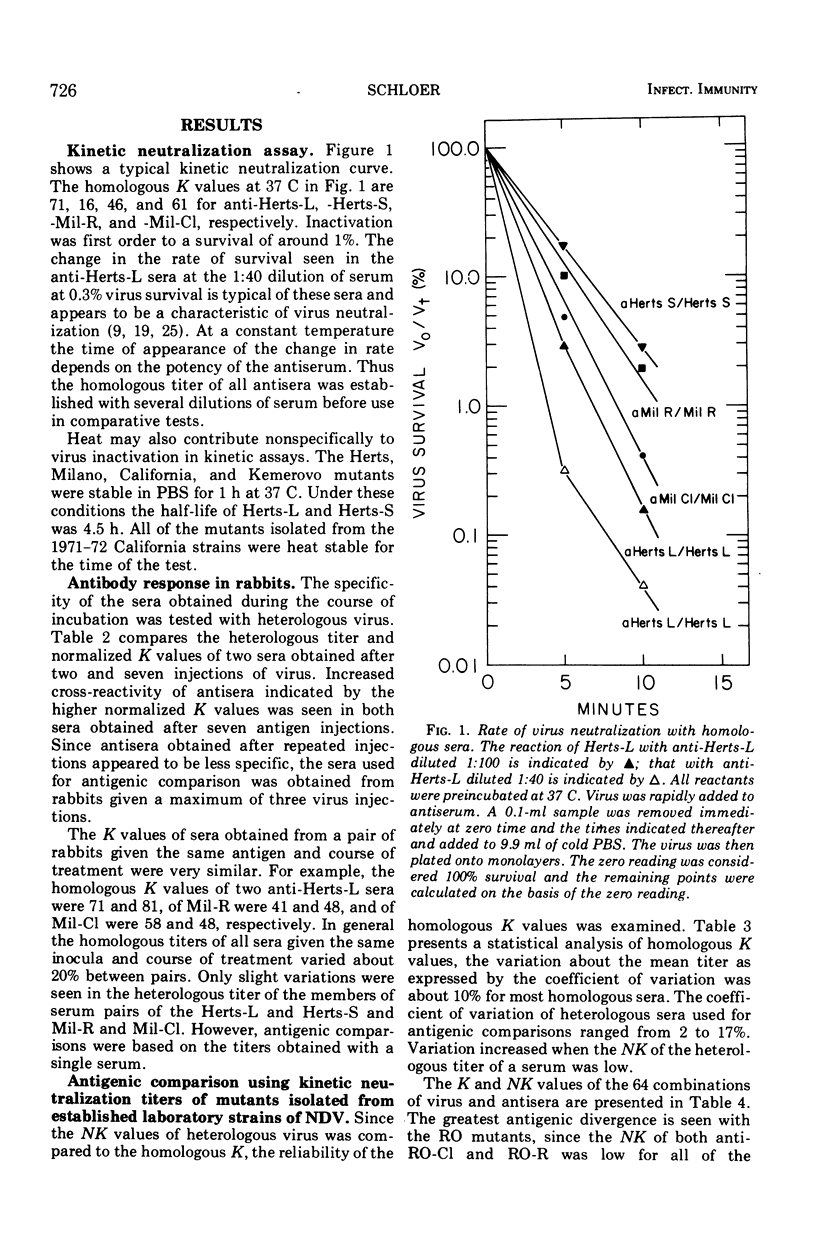
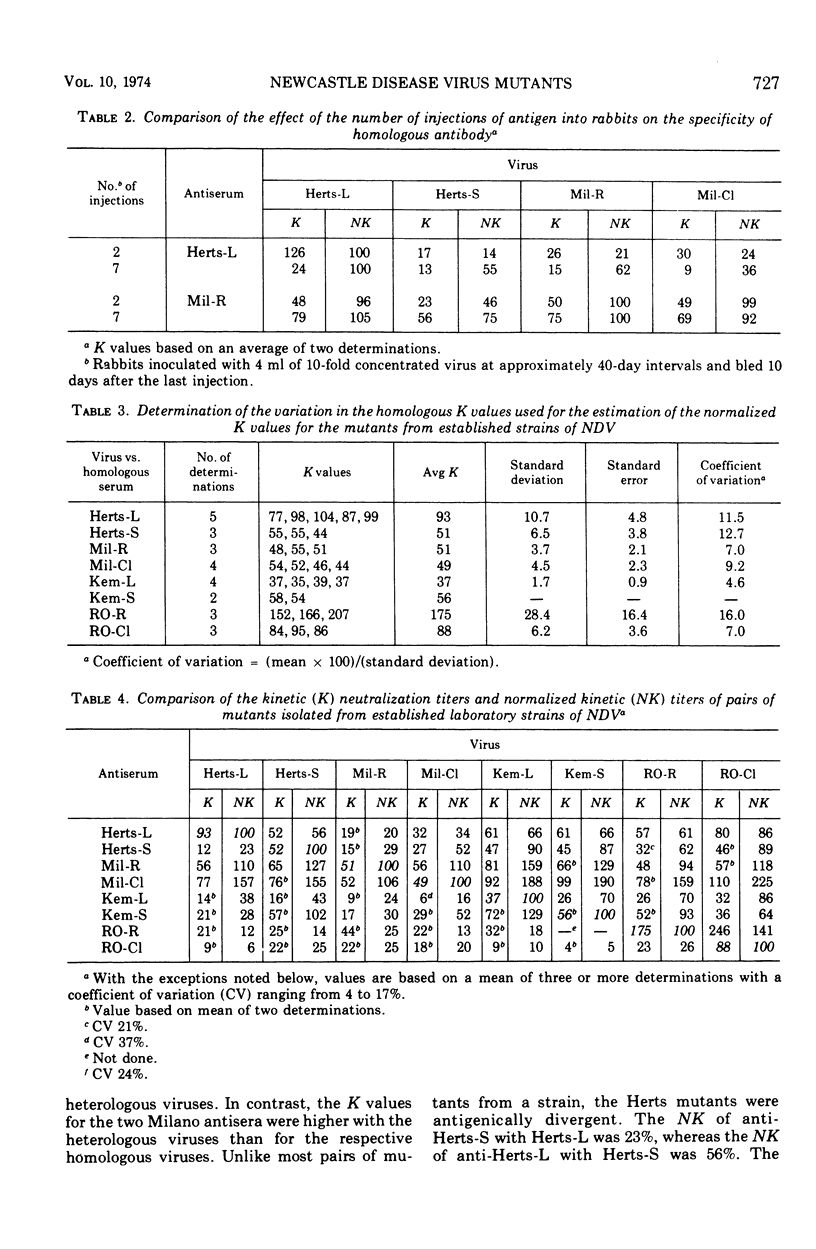
Abstract
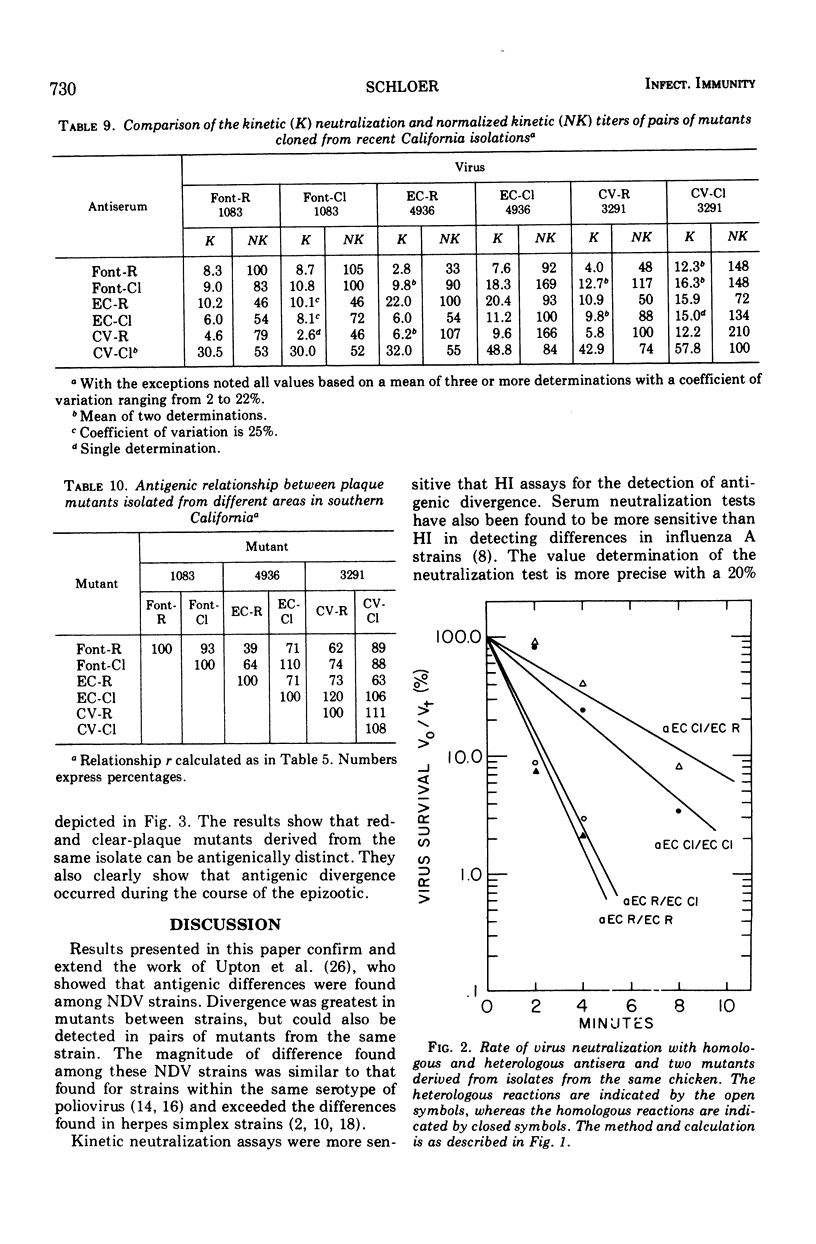
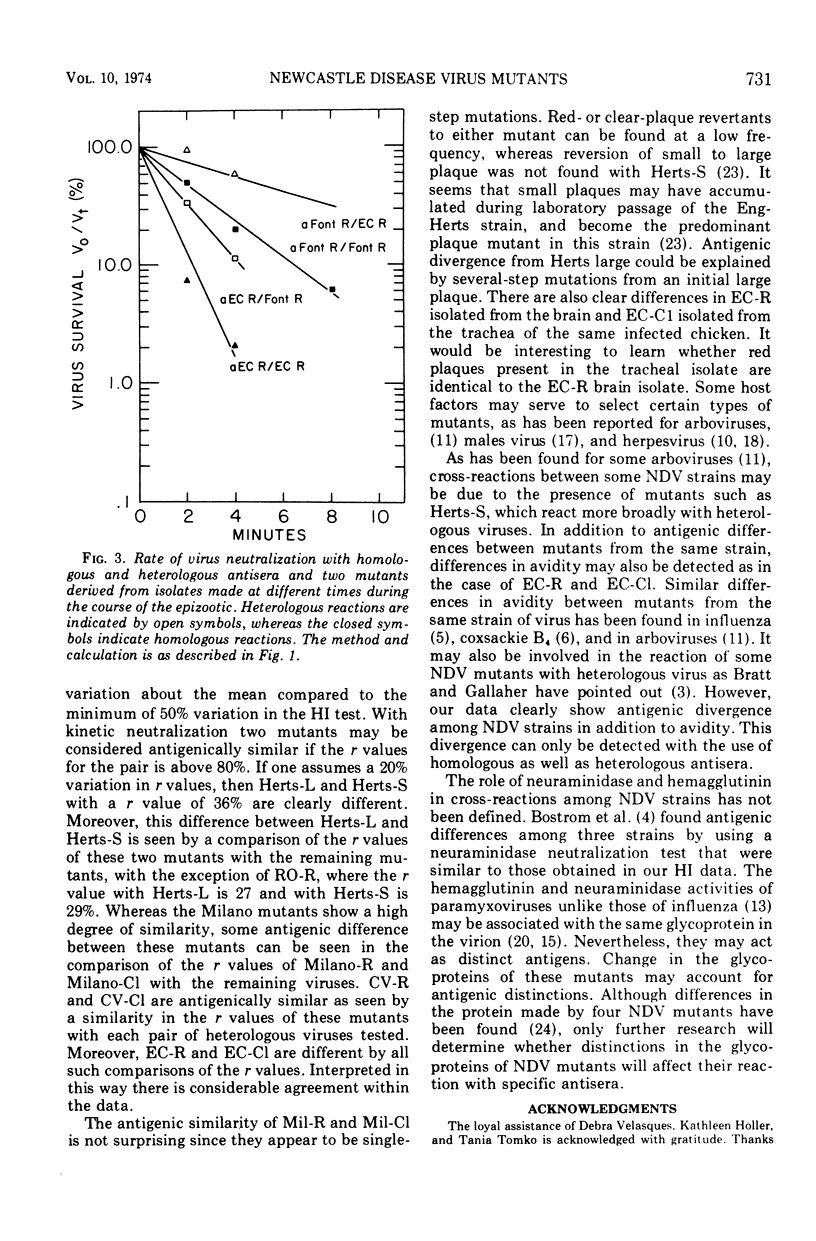
The antigenic relationship between pairs of plaque mutants of Newcastle disease virus (NDV) derived from laboratory strains and from isolates from the 1971-72 California epizootic were examined by kinetic neutralization test. Comparing four sets of mutants from laboratory strains by both kinetic neutralization and hemagglutination inhibition tests, a similarity was found in the antigenic relationship expressed as an r value with both tests. However, kinetic neutralization was the more precise as well as sensitive assay. Antigenic diversity was greatest between pairs of mutants from different strains, but distinctions could also be made between mutants from the same strains such as Herts-L and Herts-S with an r value of 36%. Examination of mutants from the California epizootic isolated from separate locations and at different times showed antigenic divergence which was greatest between two red-plaque mutants with an r value of 39%. Antigenic distinctions were found between a red- and clear-plaque mutant obtained from isolates taken from brain and tracheal swabs of one infected chicken. In addition to antigenic divergence found between pairs of some mutants, two of the clear-plaque mutants reacted more avidly with antibody than did the corresponding red-plaque partner. Thus, both differences in antigenicity and avidity can be found among these NDV mutants. The antigenic variation found among these mutants is similar to that found within a serotype. This would imply that at the present NDV is a single serological type.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHETTI I., HORSFALL F. L., Jr Persistent antigenic variation of influenza A viruses after incomplete neutralization in ovo with heterologous immune serum. J Exp Med. 1950 Nov 1;92(5):441–462. doi: 10.1084/jem.92.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHE W. K., SCHERP H. W. ANTIGENIC ANALYSIS OF HERPES SIMPLEX VIRUS BY NEUTRALIZATION KINETICS. J Immunol. 1963 Nov;91:658–665. [PubMed] [Google Scholar]
- Brostrom M. A., Bruening G., Bankowski R. A. Comparison of neuraminidases of paramyxoviruses with immunologically dissimilar hemagglutinins. Virology. 1971 Dec;46(3):856–865. doi: 10.1016/0042-6822(71)90086-9. [DOI] [PubMed] [Google Scholar]
- CHOPPIN P. W., EGGERS H. J. Heterogeneity of Coxsackie B4 virus: two kinds of particles which differ in antibody sensitivity, growth rate, and plaque size. Virology. 1962 Nov;18:470–476. doi: 10.1016/0042-6822(62)90037-5. [DOI] [PubMed] [Google Scholar]
- CHOPPIN P. W. MULTIPLICATION OF TWO KINDS OF INFLUENZA A2 VIRUS PARTICLES IN MONKEY KIDNEY CELLS. Virology. 1963 Nov;21:342–352. doi: 10.1016/0042-6822(63)90195-8. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M., STRICKLAND A. G. A study of the basic aspects of neutralization of two animal viruses, western equine encephalitis virus and poliomyelitis virus. Virology. 1956 Apr;2(2):162–205. doi: 10.1016/0042-6822(56)90017-4. [DOI] [PubMed] [Google Scholar]
- Fazekas de Saint Groth The antigenic subunits of influenza viruses. II. The spectrum of cross reactions. J Immunol. 1969 Nov;103(5):1107–1115. [PubMed] [Google Scholar]
- GRANOFF A. THE INTERACTION OF NEWCASTLE DISEASE VIRUS AND NEUTRALIZING ANTIBODY. Virology. 1965 Jan;25:38–47. doi: 10.1016/0042-6822(65)90249-7. [DOI] [PubMed] [Google Scholar]
- HENDERSON J. R., SHAH H. H., WALLIS R. C. ANTIGENIC VARIANTS OF ARBOVIRUSES. I. THE HOST AS A DETERMINANT IN THE EVOLVEMENT OF STRAIN VARIANTS. Virology. 1965 Jun;26:326–332. doi: 10.1016/0042-6822(65)90280-1. [DOI] [PubMed] [Google Scholar]
- Hampar B., Keehn M. A. Cumulative changes in the antigenic properties of herpes simplex virus from persistently infected cell cultures. J Immunol. 1967 Sep;99(3):554–557. [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Kilbourne E. D. Identification in a recombinant influenza virus of structural proteins derived from both parents. Virology. 1966 Nov;30(3):493–501. doi: 10.1016/0042-6822(66)90125-5. [DOI] [PubMed] [Google Scholar]
- McBRIDE W. D. Antigenic analysis of polioviruses by kinetic studies of serum neutralization. Virology. 1959 Jan;7(1):45–58. doi: 10.1016/0042-6822(59)90176-x. [DOI] [PubMed] [Google Scholar]
- Mountcastle W. E., Compans R. W., Choppin P. W. Proteins and glycoproteins of paramyxoviruses: a comparison of simian virus 5, Newcastle disease virus, and Sendai virus. J Virol. 1971 Jan;7(1):47–52. doi: 10.1128/jvi.7.1.47-52.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZAKI Y., DIWAN A. R., McBRIDE W. D., MELNICK J. L. Antigenic characterization of oral poliovaccine progeny by neutralization kinetics. J Immunol. 1963 Feb;90:288–296. [PubMed] [Google Scholar]
- Payne F. E., Baublis J. V. Decreased reactivity of SSPE strains of measles virus with antibody. J Infect Dis. 1973 May;127(5):505–511. doi: 10.1093/infdis/127.5.505. [DOI] [PubMed] [Google Scholar]
- Plummer G., Waner J. L., Phuangsab A., Goodheart C. R. Type 1 and type 2 herpes simplex viruses: serological and biological differences. J Virol. 1970 Jan;5(1):51–59. doi: 10.1128/jvi.5.1.51-59.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBIN H., FRANKLIN R. M. On the mechanism of Newcastle disease virus neutralization by immune serum. Virology. 1957 Feb;3(1):84–95. doi: 10.1016/0042-6822(57)90025-9. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Isolation and purification of the envelope proteins of Newcastle disease virus. J Virol. 1973 Feb;11(2):263–271. doi: 10.1128/jvi.11.2.263-271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloer G. M., Hanson R. P. Relationship of plaque size and virulence for chickens of 14 representative Newcastle disease virus strains. J Virol. 1968 Jan;2(1):40–47. doi: 10.1128/jvi.2.1.40-47.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloer G. M., Hanson R. P. Virulence and in-vitro characteristics of four mutants of Newcastle disease virus. J Infect Dis. 1971 Sep;124(3):289–296. doi: 10.1093/infdis/124.3.289. [DOI] [PubMed] [Google Scholar]
- Schloer G., Hanson R. P. Plaque morphology of Newcastle disease virus as influenced by cell type and environmental factors. Am J Vet Res. 1968 Apr;29(4):883–895. [PubMed] [Google Scholar]
- Shapiro S. C., Bratt M. S. Proteins of four biologically distinct strains of Newcastle disease virus. Proc Soc Exp Biol Med. 1971 Mar;136(3):834–838. doi: 10.3181/00379727-136-35375. [DOI] [PubMed] [Google Scholar]
- Svehag S. E. Formation and dissociation of virus-antibody complexes with special reference to the neutralization process. Prog Med Virol. 1968;10:1–63. [PubMed] [Google Scholar]
- UPTON E., HANSON R. P., BRANDLY C. A. Antigenic differences among strains of Newcastle disease virus. Proc Soc Exp Biol Med. 1953 Dec;84(3):691–693. doi: 10.3181/00379727-84-20754. [DOI] [PubMed] [Google Scholar]


