Abstract
It has been well accepted that p53 overexpression is associated with advanced stages of cancer. However, the prognostic role of p53 overexpression in esophageal squamous cell carcinoma (ESCC) remains unclear. To investigate the prognostic role of p53 overexpression in patients with ESCC, a retrospective cohort study of 136 ESCC patients was carried out. The expression of p53 protein in tumor tissues was investigated immunohistochemically. Positive expression of p53 protein was detected in 57 ESCC patients (41.9%). The p53 overexpression was associated with smoking (P < 0.001), tumor differentiation (P < 0.001), and tumor size (P < 0.001). In the Kaplan-Meier analysis, patients with p53 overexpression had significantly shorter overall survival than those patients with negative p53 expression (log-rank P < 0.001). Multivariable analysis by Cox regression model further showed that p53 overexpression was a significantly independent predictor of poorer overall survival (hazard ratio [HR] = 1.91; 95% confidence interval [95% CI] 1.03-3.54, P = 0.04). Thus, p53 overexpression is associated with poor prognosis in patients with early stage esophageal squamous cell carcinoma, and it’s a significantly independent predictor of poorer overall survival.
Keywords: Esophageal squamous cell carcinoma, p53, survival
Introduction
Esophageal cancer is one of the commonest and deadliest cancers worldwide, and it ranks sixth among all cancers in mortality worldwide [1,2]. There were an estimated 482,300 new esophageal cancer cases and 406,800 deaths occurred in 2008 worldwide [1]. Esophageal squamous cell carcinoma (ESCC) is one type of esophageal, and it’s the main histological type in East Asian countries, such as China and Japan [3]. Cigarette smoking, red meat consumption and low socioeconomic status have been associated with higher risk of ESCC [3,4]. Though there are several advances in the treatment of esophageal cancer, the overall survival of patients with ESCC is still not improved obviously [5]. It’s no doubt that identification of prognostic factors for those patients is very helpful for clinicians to choose suitable treatments for ESCC patients which may further help to prolong the overall survival of patients.
It has been well known that the tumor suppressor gene p53 is involved in the control of the cell cycle and cell apoptosis, and has vital roles in the protection of cell from DNA-damaging agents [6,7]. The environmental and genetic factors involved in the development of cancer usually lead to the alterations of p53 gene and p53 protein expression [6,7]. It has been well accepted that p53 overexpression is associated with advanced stages of cancer [8-10]. The p53 inactivation in human cancer may result through binding to viral proteins, as a result of MDM2 or indirectly by p53 protein localization in the cytoplasm [11,12]. Besides, it has been proven that mutation in the p53 gene is the most common genetic mutation in cancers, including ESCC [11,12]. These mutations can lead to an increase in expression of p53, which accumulates in nuclei and can be detected by immunohistochemistry (IHC) methods. Considering the important role of p53 in the development of cancer, many studies put forward that p53 expression in the tumor tissues of ESCC patients may have an important prognostic role on the survival of ESCC cancer patients. However, the prognostic role of p53 overexpression in early stage ESCC remained unclear [13-15]. To investigate the prognostic role of p53 overexpression in patients with early stage ESCC, a retrospective cohort study of 136 ESCC patients was carried out. The current study also aimed to evaluate whether p53 expression was a prognostic factor in an independent way.
Materials and methods
Study population
All patients were recruited from First Affiliated Hospital of Xinxiang Medical University, Xinxiang City, China. A total of 256 consecutive patients with histologically-confirmed early stage ESCC were preliminarily included into the retrospective cohort study. 120 ESCC patients were excluded for the lack of usable data of follow-up. Thus, 136 ESCC patients were finally included into the study (69 men and 67 women; mean age 60.3 ± 8.5 years, ranging from 39 to 78 years). All eligible subjects were recruited between January 2005 and June 2007 and written informed consents were obtained. The study was approved by the Ethics Committee of First Affiliated Hospital of Xinxiang Medical University.
The eligibility criteria for the enrolled ESCC patients were: (1) presence of a primary ESCC with no history of concurrent cancer in other organs or history of previous cancer in any organ; (2) recent diagnosis of ESCC in the patients was histologically conformed; (3) Early stage ESCC (IA, IB, or IIA). All ESCC patients were followed up every 6 months by clinical evaluation in hospital or by telephone contact. Deaths caused by ESCC were taken as outcome events, whereas deaths caused by others were considered censored. Survival duration was defined as the time interval from disease diagnosis to either death or the time of the last clinical evaluation of the patients.
Tissue collection
Tumor tissue and corresponding adjacent normal esophageal tissue specimens were obtained from the ESCC patients. All untreated specimen-proven carcinoma of the esophagus in ESCC cases were obtained by esophagectomy or endoscopy procedure. All specimens were fixed and stored in 70% ethanol and embedded in paraffin. Esophageal squamous tumors were comprised of > 70% malignant cells with minimal necrosis. Tumors were histologically verified as ESCC based on the grade of differentiation as well differentiated, moderately differentiated or poorly differentiated. Tumor tissue samples were selected so that all adjacent normal esophageal tissues were obtained from the macroscopically normal esophageal epithelium, distant from the cancerous lesion.
Immunohistochemical staining
Tissue sections 4-μm in thickness were obtained from archival alcohol-fixed paraffin-embedded tissues of the esophageal squamous tumor and mounted on poly-L-lysine-coated slides for immunohistochemistry study. After being dewaxed in xylene and rehydrated in a series of graded alcohols, they were placed in 10 mmol/L citrate buffer pH 6.0 to unmask the epitopes. After microwave antigen retrieval (20 min, 120 W; 3 × 5 min, 450 W), the sections were allowed to cool down to room temperature (approximately 20 min), and then incubated with 3% H2O2 for 10 min to quench the endogenous peroxidase activity. After blocking the nonspecific protein binding with serum-free protein block for 5 min, slides were incubated for 45 min at 37°C with anti-p53 monoclonal antibody DO-7 which was raised against an epitope between amino acids 1 and 45 in the C-terminal domain of human wild-type and mutant p53 recognizing both mutant and wild-type p53 protein, followed by phosphate buffered saline wash. Finally the primary antibody was detected using a secondary antibody. Staining was visualized using diaminobenzidine chromogen for 10 min, followed by acidified hematoxylin counterstaining for 1 min. Thereafter, the sections were mounted with mounting medium. Two expert pathologists who were blinded to the clinical and molecular results evaluated the tissue slides, independently. The final result was obtained through the consensus between the pathologists. Nuclear p53 expression was recorded as no expression, weak expression, moderate expression, or strong expression compared to normal esophageal epithelial cells. The overexpression of p53 was defined as the presence of weak-to-strong expression.
Statistical analysis
The Statistical Package for the Social Sciences software version 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. The associations between p53 expression and clinicopathological parameters were evaluated by the χ2 test and Fisher’s exact test. For the survival analysis, the Kaplan-Meier method was used to assess the survival time distribution, and the log-rank test was used. Prognostic factors were further evaluated in multivariate analysis using the Cox’s proportional hazards model of relevant prognostic variables. The hazard ratio (HR) with 95% confidence interval (95% CI) was used to assess the relationships between those factors and overall survival. A 2-sided P value < 0.05 was considered as significant statistically.
Results
Patient characteristics
136 ESCC patients were finally included into the study. There were a total of 69 men and 64 women. The mean age of those ESCC patients were 60.3 years (ranging from 39 to 78 years). All those 136 patients were followed up at least 3 years, and the mean time of follow-up was 5.3 years (ranging from 3 to 7.5 years). Positive expression of p53 protein was detected in 57 ESCC patients (41.9%). The p53 overexpression was associated with smoking (P < 0.001), tumor differentiation (P < 0.001), and tumor size (P < 0.001) (Table 1). However, p53 overexpression was not associated with other parameters (Table 1).
Table 1.
Correlation between clinicopathological parameters and p53 overexpression in patients with esophageal squamous cell carcinoma
| Parameters | Number | p53 overexpression | P value | |
|---|---|---|---|---|
|
| ||||
| Yes | No | |||
| Gender | ||||
| Men | 69 | 23 | 46 | 0.06 |
| Women | 67 | 34 | 33 | |
| Age (yr) | ||||
| < 65 | 58 | 24 | 34 | 0.97 |
| ≥ 65 | 78 | 32 | 46 | |
| Smoking | ||||
| Non-smoker | 91 | 23 | 68 | < 0.001 |
| Smoker | 44 | 32 | 12 | |
| Differentiation | ||||
| Well | 50 | 8 | 42 | < 0.001 |
| Moderate | 59 | 29 | 30 | |
| Poor | 27 | 19 | 8 | |
| Tumor site | ||||
| Upper | 43 | 17 | 26 | 0.95 |
| Middle | 56 | 23 | 33 | |
| Lower | 37 | 16 | 21 | |
| Tumor size | ||||
| < 3 | 64 | 9 | 55 | < 0.001 |
| ≥ 3 | 72 | 47 | 25 | |
Prognosis of ESCC patients according to p53 protein expression
In the Kaplan-Meier analysis, patients with p53 overexpression had significantly shorter overall survival than those patients with negative p53 expression (log-rank P < 0.001) (Figure 1). The median survival time for ESCC patients with p53 overexpression was 4.7 years, while median survival time for ESCC patients with negative p53 expression was 5.9 years. Multivariable analysis by Cox regression model further showed that p53 overexpression was a significantly independent predictor of poorer overall survival (HR = 1.91; 95% CI 1.03-3.54, P = 0.04). Multivariable analysis also showed that tumor differentiation was an important predictor of poorer overall survival (HR = 1.98; 95% CI 1.39-2.81, P < 0.001) (Table 2). However, tumor size, age, smoking, tumor site, and gender were not predictors of prognosis in ESCC patients (Table 2).
Figure 1.
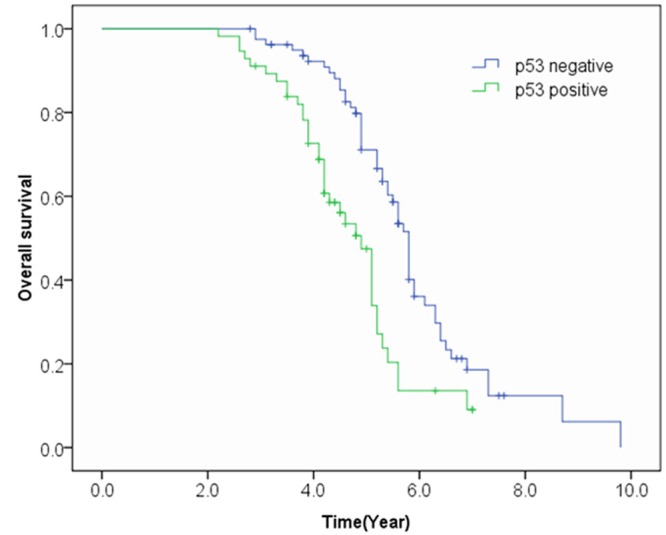
Overall survival curve of patients in relation to p53 expression.
Table 2.
Multivariable analysis of the prognostic roles for clinicopathological parameters in patients with esophageal squamous cell carcinoma
| Parameters | HR | Low limit | Upper limit | P values |
|---|---|---|---|---|
| Tumor size (> 3 cm) | 1.53 | 0.89 | 2.63 | 0.13 |
| Age (> 65) | 0.83 | 0.53 | 1.32 | 0.44 |
| Smoking | 0.63 | 0.37 | 1.07 | 0.09 |
| Differentiation | 1.98 | 1.39 | 2.81 | < 0.001 |
| Tumor site | 1.02 | 0.76 | 1.36 | 0.90 |
| Gender (Women) | 1.03 | 0.65 | 1.64 | 0.89 |
| p53 (Overexpression) | 1.91 | 1.03 | 3.54 | 0.04 |
Discussion
It has been well accepted that p53 overexpression is associated with advanced stages of cancer. However, the prognostic role of p53 overexpression in esophageal squamous cell carcinoma (ESCC) remains unclear. Thus, we performed a retrospective cohort study of 136 ESCC patients to investigate the prognostic role of p53 overexpression in patients with ESCC. The findings from the present cohort study suggested that p53 overexpression was associated with poor prognosis in ESCC patients, and it’s a significantly independent predictor of poorer overall survival (P = 0.04).
The p53 inactivation in human cancer may result through binding to viral proteins, as a result of MDM2 or indirectly by p53 protein localization in the cytoplasm [11,12]. Besides, it has been proven that mutation in the p53 gene is the most common genetic mutation in cancers, including ESCC [11,12]. It has been well accepted that p53 overexpression is associated with advanced stages of cancer [8-10]. The prognostic role of p53 overexpression in several types of cancers has been successfully identified, such as head & neck cancer, rectal cancer, and osteosarcom [8,16-18]. The findings from the present study suggested that p53 overexpression was associated with poor prognosis in ESCC patients, which further added new evidence for the prognostic role of p53 overexpression in cancers.
The overexpression of p53 protein were found to be associated with smoking (P < 0.001), tumor differentiation (P < 0.001), and tumor size (P < 0.001) in present study (Table 1). ESCC patients with bigger size of tumors and poor differentiation were more likely to have overexpression of p53 protein in tumor tissues. The findings above indicates that overexpression of p53 protein is possibly associated with advanced stages of ESCC, which may further result in high risk of mortality and poor prognosis. The findings above are consistent with that from previous studies [13,19]. The findings above also suggest that p53 protein has an important role in the development and invasion of cancers, including ESCC.
Though the findings from the study showed that p53 overexpression was associated with poor prognosis in ESCC patients, and it’s a significantly independent predictor of poorer overall survival, several previous studies failed to identify the prognostic role p53 overexpression [20-22]. The negative association between p53 overexpression and prognosis in ESCC patients from those previous studies may result from the small sample size or the shorter time of follow-up. Previous studies usually had follow-up less than 5 years, while the median time of follow-up in our study was more than 5 years which may help us to get a more precise evaluation on the prognostic role of p53 overexpression in ESCC patients. However, since there are conflicting findings on the prognostic role of p53 overexpression in ESCC patients, more studies with large sample size and long time of follow-up are needed to provide a confident conclusion. In addition, a meta-analysis of previous published studies on the prognostic role of p53 overexpression in ESCC patients may also be helpful.
Another important finding in the present study is the identification of prognostic role of p53 overexpression in early-stage ESCC patients. In our present study, all 136 ESCC patients were early-stage, and resectable. The findings from the study may strengthen the value of p53 expression in the prognosis of early-stage ESCC patients, which is obviously different from previous studies.
ESCC is one type of esophageal, and it’s the main histological type in East Asian countries, such as China and Japan [3]. ESCC has caused many deaths and serious damage to human health [1,2]. Though there are several advances in the treatment of esophageal cancer, the overall survival of patients with ESCC is still not improved obviously [5]. It’s no doubt that identification of prognostic factors for those patients is very helpful for clinicians to choose suitable treatments for ESCC patients which may help us to prolong the overall survival of patients. Previous studies have identified several possible prognostic factors for ESCC, such as caspase-3 expression and microRNA (miRNA) expression [23-26]. The findings from present study prove the prognostic role of p53 overexpression in ESCC patients, especially in early-stage patients, which may be helpful for clinicians to choose suitable treatments for early-stage ESCC patients.
In conclusion, this is the first study focused on evaluating the prognostic role of p53 protein expression in patients with early stage ESCC. The results from the present study showed that p53 overexpression was associated with poor prognosis in patients with early stage ESCC. Therefore, p53 overexpression is associated with poor prognosis in ESCC patients, and it’s a significantly independent predictor of poorer overall survival in early stage ESCC. In addition, to get a more precise evaluation on the prognostic role of p53 overexpression in ESCC patients, more studies with large sample size and long time of follow-up are needed.
Acknowledgements
This study is granted by the project of Science and Technology Department, Henan Province (No. 112300410163).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Das BC, Sehgal A, Mehrotra R, Kar P, Sardana S, Phukan R, Mahanta J, Purkayastha J, Saxena S, Kapur S, Chatterjee I, Sharma JK. GSTM1 and GSTT1 polymorphism and susceptibility to esophageal cancer in high- and low-risk regions of India. Tumor Biol. 2013;34:3249–3257. doi: 10.1007/s13277-013-0897-6. [DOI] [PubMed] [Google Scholar]
- 3.Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y, Ueda J, Wei W, Inoue M, Tanaka H. Epidemiology of esophageal cancer in japan and china. J Epidemiol. 2013;23:233–242. doi: 10.2188/jea.JE20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu HC, Yang X, Xu LP, Zhao LJ, Tao GZ, Zhang C, Qin Q, Cai J, Ma JX, Mao WD, Zhang XZ, Cheng HY, Sun XC. Meat consumption is associated with esophageal cancer risk in a meat- and cancer-histological-type dependent manner. Dig Dis Sci. 2014;59:664–673. doi: 10.1007/s10620-013-2928-y. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima M, Kato H. Treatment options for esophageal squamous cell carcinoma. Expert Opin Pharmacother. 2013;14:1345–1354. doi: 10.1517/14656566.2013.801454. [DOI] [PubMed] [Google Scholar]
- 6.Case AJ, Domann FE. Absence of manganese superoxide dismutasee delay p53-induced tumor formation. Redox Biol. 2014;2:220–223. doi: 10.1016/j.redox.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Assis LV, Isoldi MC. The function, mechanisms, and role of the genes PTEN and TP53 and the effects of asbestos in the development of malignant mesothelioma: a review focused on the genes’ molecular mechanisms. Tumor Biol. 2014;35:889–901. doi: 10.1007/s13277-013-1210-4. [DOI] [PubMed] [Google Scholar]
- 8.Fu HL, Shao L, Wang Q, Jia T, Li M, Yang DP. A systematic review of p53 as a biomarker of survival in patients with osteosarcoma. Tumour Biol. 2013;34:3817–3821. doi: 10.1007/s13277-013-0966-x. [DOI] [PubMed] [Google Scholar]
- 9.Ku JH, Byun SS, Jeong H, Kwak C, Kim HH, Lee SE. The role of p53 on survival of upper urinary tract urothelial carcinoma: A systematic review and meta-analysis. Clin Genitourin Cancer. 2013;11:221–228. doi: 10.1016/j.clgc.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Inoue K, Kurabayashi A, Shuin T, Ohtsuki Y, Furihata M. Overexpression of p53 protein in human tumors. Med Mol Morphol. 2012;45:115–123. doi: 10.1007/s00795-012-0575-6. [DOI] [PubMed] [Google Scholar]
- 11.Wang X. P53 regulation: Teamwork between ring domains of mdm2 and mdmx. Cell Cycle. 2011;10:4225–4229. doi: 10.4161/cc.10.24.18662. [DOI] [PubMed] [Google Scholar]
- 12.de Moraes E, Dar NA, de Moura Gallo CV, Hainaut P. Cross-talks between cyclooxygenase-2 and tumor suppressor protein p53: Balancing life and death during inflammatory stress and carcinogenesis. Int J Cancer. 2007;121:929–937. doi: 10.1002/ijc.22899. [DOI] [PubMed] [Google Scholar]
- 13.Taghavi N, Biramijamal F, Sotoudeh M, Khademi H, Malekzadeh R, Moaven O, Memar B, A’rabi A, Abbaszadegan MR. P16ink4a hypermethylation and p53, p16 and mdm2 protein expression in esophageal squamous cell carcinoma. BMC Cancer. 2010;10:138. doi: 10.1186/1471-2407-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu WK, Jiang XY, Zhang MP, Zhang ZX. The relationship between hpv16 and expression of cyclooxygenase-2, p53 and their prognostic roles in esophageal squamous cell carcinoma. Eur J Gastroenterol Hepatol. 2010;22:67–74. doi: 10.1097/MEG.0b013e32832c7e76. [DOI] [PubMed] [Google Scholar]
- 15.Kimura S, Kitadai Y, Kuwai T, Tanaka S, Hihara J, Yoshida K, Toge T, Chayama K. Expression of p53 protein in esophageal squamous cell carcinoma: Relation to hypoxia-inducible factor-1alpha, angiogenesis and apoptosis. Pathobiology. 2005;72:179–185. doi: 10.1159/000086787. [DOI] [PubMed] [Google Scholar]
- 16.Smith RA, Tang J, Tudur-Smith C, Neoptolemos JP, Ghaneh P. Meta-analysis of immunohistochemical prognostic markers in resected pancreatic cancer. Br J Cancer. 2011;104:1440–1451. doi: 10.1038/bjc.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tandon S, Tudur-Smith C, Riley RD, Boyd MT, Jones TM. A systematic review of p53 as a prognostic factor of survival in squamous cell carcinoma of the four main anatomical subsites of the head and neck. Cancer Epidemiol Biomarkers Prev. 2010;19:574–587. doi: 10.1158/1055-9965.EPI-09-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen MB, Wu XY, Yu R, Li C, Wang LQ, Shen W, Lu PH. P53 status as a predictive biomarker for patients receiving neoadjuvant radiation-based treatment: A meta-analysis in rectal cancer. PLoS One. 2012;7:e45388. doi: 10.1371/journal.pone.0045388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng TH, Hsu PK, Li AF, Hung IC, Huang MH, Hsu HS. Correlation of p53, mdm2 and p14(arf) protein expression in human esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2009;135:1577–1582. doi: 10.1007/s00432-009-0605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egashira A, Morita M, Yoshida R, Saeki H, Oki E, Sadanaga N, Kakeji Y, Tsujitani S, Maehara Y. Loss of p53 in esophageal squamous cell carcinoma and the correlation with survival: Analyses of gene mutations, protein expression, and loss of heterozygosity in japanese patients. J Surg Oncol. 2011;104:169–175. doi: 10.1002/jso.21920. [DOI] [PubMed] [Google Scholar]
- 21.Luo KJ, Hu Y, Wen J, Fu JH. Cyclind1, p53, e-cadherin, and vegf discordant expression in paired regional metastatic lymph nodes of esophageal squamous cell carcinoma: A tissue array analysis. J Surg Oncol. 2011;104:236–243. doi: 10.1002/jso.21921. [DOI] [PubMed] [Google Scholar]
- 22.Murata A, Baba Y, Watanabe M, Shigaki H, Miyake K, Karashima R, Imamura Y, Ida S, Ishimoto T, Iwagami S, Sakamoto Y, Miyamoto Y, Yoshida N, Baba H. P53 immunohistochemical expression and patient prognosis in esophageal squamous cell carcinoma. Med Oncol. 2013;30:728. doi: 10.1007/s12032-013-0728-z. [DOI] [PubMed] [Google Scholar]
- 23.Wang XS, Luo KJ, Bella AE, Bu SS, Wen J, Zhang SS, Hu Y. Caspase-3 expression in metastatic lymph nodes of esophageal squamous cell carcinoma is prognostic of survival. World J Gastroenterol. 2014;20:4414–4420. doi: 10.3748/wjg.v20.i15.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma RL, Shen LY, Chen KN. Coexpression of anxa2, sod2 and hoxa13 predicts poor prognosis of esophageal squamous cell carcinoma. Oncol Rep. 2014;31:2157–2164. doi: 10.3892/or.2014.3088. [DOI] [PubMed] [Google Scholar]
- 25.Zang W, Wang Y, Du Y, Xuan X, Wang T, Li M, Ma Y, Li P, Chen X, Dong Z, Zhao G. Differential expression profiling of micro RNAs and their potential involvement in esophageal squamous cell carcinoma. Tumor Biol. 2014;35:3295–3304. doi: 10.1007/s13277-013-1432-5. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Sun QK, He YF, Ma DC, Xie MR, Ji CS, Hu B. Overexpression of periostin is significantly correlated to the tumor angiogenesis and poor prognosis in patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:593–601. [PMC free article] [PubMed] [Google Scholar]


