Abstract
Male breast carcinoma is a relatively rare disease. This study retrospectively investigated the clinicopathological features of 73 cases of male breast carcinoma in Chinese population, and classified the molecular subtype based on surrogate immunohistochemical definitions. The expression of GCDFP15, MGB, AR and FOXP1 were evaluated. Invasive carcinoma of no special type was the most common histological type in the study group (71.2%, 52/73). The luminal A and B subtypes were the major types of male breast carcinoma (60.9%, 34.8% respectively). AR and FOXP1 are expressed in 84.2% (48/57) and 71.9% (41/57) of the studied cases. Carcinoma of the luminal A subtype expressed GCDFP15 (73.5%, 25/34) and MGB (58.8%, 20/34) more frequently than cases of the luminal B subtypes (34.8%, 8/23 and 43.5%, 10/23, respectively; P = 0.004, P = 0.255, respectively). In conclusion, invasive carcinoma of no special type was the most common histological type in male breast carcinoma among Chinese population. Our study revealed that the luminal A and B subtypes were the major types of male breast carcinoma. AR and FOXP1 are highly expressed in male breast cancer. The luminal A subtype tends to express GCDFP15 and MGB more frequently than the luminal B subtype.
Keywords: Breast carcinoma, male, molecular subtype, immunohistochemistry
Introduction
Male breast carcinoma is a relatively rare disease, accounting for < 1% of all breast cancer cases [1]. Research on male breast carcinoma has often been grouped together with its female counterpart. While studies showed that both male and female breast carcinoma share certain characteristics [2], clinicopathological differences like histological type and hormone receptor status also exist within the two groups [2,3].
Hierarchical clustering analyses of gene expression profiles have classified female breast cancer into several intrinsic groups [4] with different clinical outcomes [5]. As high-cost microarray-based studies are not always feasible, immunohistochemical markers have been used as surrogates for classifying breast cancer [6]. Immunohistochemistry defined subtyping of male breast carcinoma exhibited conflicting results in several studies [7-10], due to different IHC-based subtyping algorithms. However, only a few studies have examined male breast carcinoma in Chinese population [11,12], and the molecular subtypes remain understudied.
Immunohistochemical localization of gross cystic disease fluid 15 (GCDFP15) has been reported in 25%-75% of breast carcinomas [13-16] and mammaglobin (MGB) expression has been reported in approximately 55%-80% of breast carcinomas [14,16]. The expression of GCDFP15 and MGB and their correlation with molecular subtypes in female breast cancer has been reported recently [16], but their relationship with the molecular subtypes of male breast carcinoma remains unclear.
In male breast carcinoma, hormone receptors (ER and PR) are more commonly expressed compared with the female patients [2,3]. Androgen receptor (AR), also a steroid hormone nuclear receptor, is highly expressed in ER-positive breast cancer [17], and recent studies showed AR expression was associated with good prognosis [18]. FOXP1 belongs to the family of winged-helix or forkhead transcription factors that play roles in cell proliferation and neoplastic transformation. FOXP1 is correlated with both ER expression and improved survival in breast cancer [19]. In male breast carcinoma, the expression pattern of AR has been described in a few studies [20,21], while the expression of FOXP1 have not been studied in male breast carcinoma.
This study retrospectively investigated the clinicopathological features of male breast carcinoma in a Chinese population, and classified the molecular subtype based on surrogate immunohistochemical definitions. The immunohistochemical expression patterns of GCDFP15, MGB, AR and FOXP1 were also evaluated.
Materials and methods
Patients and specimen
We performed a thorough search for male breast carcinoma in the database of the Department of Pathology at Fudan University Shanghai Cancer Center between Jan 2004 and April 2012. Male patients with complete clinicopathological data were retrospectively collected, including 46 residing patients and 27 consultation patients. HE-stain slides of all 73 patients were retrieved for histological evaluation. Paraffin-embedded tissue blocks of 46 in-house patients and tissue sections of 27 consultant patients were available for molecular subtyping; 57 cases had sufficient paraffin-embedded tissue blocks or tissue sections for further immunohistochemical study.
Clinical information and histological evaluation
Patient age, clinical symptoms, tumor site, tumor size, lymph node status and treatment history were recorded from medical records. The seventh edition of the American Joint Committee on Cancer (AJCC) staging system was used to determine tumor (TNM) stage [22]. Four patients received chemotherapy and hormone therapy without breast surgery due to the advanced stage of the cancer or the patients’ wishes. Of 73 cases, one patient with breast carcinoma on both breasts was identified; in this case, the clinicopathological information with higher stage and grade were recorded for statistical analysis.
Slides of all the cases were reviewed independently by two pathologists to confirm the diagnosis. Histological type was characterized based on the tumor classification set by the WHO [23]. Histological grade was defined using the modified Bloom and Richardson score scheme [24] for invasive carcinoma. Ductal carcinoma in situ, necrosis, sclerotic stroma, calcification of the tumors and dermis or nipple infiltration were also recorded if present microscopically. The diagnosis of invasive ductal carcinoma with invasive micropapillary carcinoma was rendered when micropapillary components accounted for less than 90% of all the invasive carcinoma present. Mixed invasive ductal and mucinous carcinoma was described when mucinous components accounted for less than 90% of the invasive tumor. Invasive papillary carcinoma, intraductal papillary carcinoma, encapsulated papillary carcinoma and solid papillary carcinoma were categorized as papillary carcinoma in this study.
Subtypes defined by immunohistochemical staining [25]
Immunohistochemical staining for ER, PR, HER2, Ki-67, CK5/6 and EGFR (Table 1) was accomplished using Ventana BenchMark ULTRA automated stainer (Ventana Medical Systems Inc., Roche, Tuscon, AZ, USA) and Ventana Ultra View Universal DAB Detection kit. The American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guideline were followed for scoring ER, PR and HER2 [26,27]. ER and PR were considered positive if greater than 1% of tumor cells exhibited nuclear staining [26]. Immunohistochemistry for HER2 was defined as positive when 3+ cell circumferential staining was observed, while 0 or 1+ was recorded as negative. Five cases with 2+ staining were further confirmed by the Abbott-Vysis HER2 FISH assay. Immunostaining of the whole slide area was evaluated by two experienced pathologists who remained unaware of tumor characteristics and other staining results.
Table 1.
Antibodies characterization for immunostaining
| Antibody | Vendor | Clone | Dilution | Antigen Retrieval Solution |
|---|---|---|---|---|
| ER | Roche | SP1 | Using antibody | Tris-based buffer (pH: 8.0) |
| PR | Roche | 1E2 | Using antibody | Tris-based buffer (pH: 8.0) |
| HER-2/neu | Roche | 4B5 | Using antibody | Tris-based buffer (pH: 8.0) |
| Ki-67 | Roche | 30-9 | Using antibody | Tris-based buffer (pH: 8.0) |
| CK5/6 | DAKO | D5/16B4 | 1:200 | Tris-based buffer (pH: 8.0) |
| EGFR | Changdao, China | 111.6 | 1:100 | Tris-based buffer (pH: 8.0) |
| GCDFP15 | DAKO | 23A3 | 1:50 | Citrate buffer (pH: 6.0) |
| MGB | DAKO | 304-1A5 | 1:50 | Citrate buffer (pH: 6.0) |
| AR | DAKO | AR441 | 1:50 | EDTA (pH: 9.0) |
| FOXP1 | Epitomics | EPR4113 | 1:300 | EDTA (pH: 9.0) |
The IHC-based subtyping criteria used in this study classified the cases into five different categories: luminal A (ER+ and/or PR+, HER2- and Ki-67 low), luminal B HER2 negative (ER+ and/or PR+, HER2-, and Ki-67 high), luminal B HER2 positive (ER+ and/or PR+, and HER2+, and any Ki-67), HER2 positive (HER2+, ER- and PR-), ‘basal like’ (ER-, PR- and HER2-, and/or CK5/6+, and/or EGFR+). For Ki-67, the cut-off point was set to 14% according to a previous study [6,25].
Immunohistochemistry and scoring for GCDFP15, MGB, AR and FOXP1
Antibody staining for AR, GCDFP15, Mammaglobin and FOXP1 were performed manually on 57 cases using EnVision™ Detection Systems (DAKO). Details of primary antibodies and antigen retrieval solutions are listed in Table 1. Both positive and negative controls were used throughout the process.
The expression pattern was analyzed on whole tissue sections. Immunostaining for AR was considered positive when 1% of cells showed nuclear expression [18]. Cytoplasmic immunoreactivity of GCDFP15 and mammaglobin was scored based on the percentage of positive cells: negative (0%), focal positive (1%-90%) and diffuse positive (> 90%). The pattern of FOXP1 expression was scored as: negative = 0; weak/focal staining = 1; strong focal/widespread moderate staining = 2; or strong/widespread staining = 3. Score 2 and 3 tumors were considered positive for FOXP1 statistical analyses based on previous studies [19].
Statistical analysis
Descriptive statistics were calculated. The chi-square test was used for evaluating the relationship between immunohistochemical characterization and IHC-base molecular subtypes of breast cancer cases. The fisher exact test was performed when necessary. All statistical tests were two sided and P values less than 0.05 were considered significant. All analyses were carried using SPSS software (version 17.0, SPSS Company, Chicago, IL).
Results
Patients’ characteristics
The clinical information of the 73 cases of male breast carcinoma is summarized in Table 2. The patient’s median age at diagnosis was 59 (ranging from 10 to 89). The most frequent clinical symptoms were a mass in the breast (97.3%). 10 (13.7%) patients complained of nipple discharge and 12 (16.4%) had changes in skin contour or texture. The breast tumor was unilateral in 98.6% (72/73) of patients and bilateral in only 1 (1.4%) patient. Most tumors were confined to the central subareolar area. Sixty (82.2%) patients had a modified mastectomy, and 4 (5.5%) received a radical mastectomy. While radiation therapy and adjuvant chemotherapy were applied in 17.8% and 39.7% of the patients, respectively, adjuvant hormone therapy was the most commonly applied therapy (in 82.2% of the patients).
Table 2.
Patient’s general characteristics of 73 cases of male breast carcinoma
| Characteristics | n | % |
|---|---|---|
| Age (years) | ||
| ≤ 50 | 17 | 23.3 |
| > 50 | 56 | 76.7 |
| Clinical Symptoms | ||
| Lump | 71 | 97.3 |
| Pain | 4 | 5.5 |
| Nipple Discharge | 10 | 13.7 |
| Nipple (Retraction, distortion or eczema) | 7 | 9.6 |
| Change in skin contour or texture | 12 | 16.4 |
| Tumor site | ||
| Left | 35 | 47.9 |
| Right | 37 | 50.7 |
| Bilateral | 1 | 1.4 |
| Sub locationa | ||
| Beneath nipple & Subareolar region | 47a | 63.5 |
| Other quadrant | 27a | 36.5 |
| Tumor size | ||
| ≤ 2 | 45 | 61.6 |
| 2-5 | 24 | 32.9 |
| > 5 | 4 | 5.5 |
| Tumor stage | ||
| 0 | 4 | 5.5 |
| I | 29 | 39.7 |
| II | 22 | 30.1 |
| III | 17 | 23.3 |
| IV | 1 | 1.4 |
| Surgical approach | ||
| Modified mastectomy | 60 | 82.2 |
| Radical mastectomy | 4 | 5.5 |
| Simple mastectomy with SLN | 4 | 5.5 |
| Lumpectomy | 1 | 1.4 |
| Castration | 1 | 1.4 |
| No mammal surgery (only biopsy)b | 4 | 5.5 |
In one case, breast carcinoma was identified in both breasts, and the tumors located beneath nipple and within upper outer quadrant respectively.
Biopsy was performed on the four patients who didn’t receive breast surgery.
Distribution of molecular subtypes and histological features
Pathological findings of molecular subtypes based on surrogate immunohistochemical definitions are presented in Table 3. Of 73 patients, 4 cases were classified as carcinoma in situ, and the remaining 69 cases were identified as invasive carcinoma. The 69 cases were then categorized into four groups: 42 (60.9%) were subtype luminal A, 24 (34.8%) were subtype luminal B (HER2 negative), 1 case was (1.4%) was HER2 positive and 2 cases (2.9%) were basal-like.
Table 3.
Classical pathological features and Microscopic findings of 69 cases of invasive beast carcinoma and their distribution over subtypes
| Subtype | |||||
|---|---|---|---|---|---|
|
|
|||||
| Characteristics | Total | Luminal A | Luminal B | HER2 | ‘Basal like’ |
| Histological type | 69 | 42 | 24 | 1 | 2 |
| Invasive carcinoma of no special type | 52 (75.4%) | 31 (73.8%) | 21 (87.5%) | 0 | 0 |
| IDC | 43 (62.3%) | 26 (61.9%) | 17 (70.8%) | 0 | 0 |
| IDC with osteoclasitc giant cell | 1 (1.4%) | 1 (2.4%) | 0 | 0 | 0 |
| IDC with invasive micropapillary | 8 (11.6%) | 4 (9.5%) | 4 (16.7%) | 0 | 0 |
| Invasive carcinoma of special subtypea | 7 (10.1) | 4 (9.5%) | 1 (4.2%) | 0 | 2 (100%) |
| Invasive cribriform | 1 (1.4%) | 1 (2.4%) | 0 | 0 | 0 |
| Metaplastic | 1 (1.4%) | 0 | 1 (4.2%) | 0 | 0 |
| Mucinous | 3 (4.4%) | 3 (7.1%) | 0 | 0 | 0 |
| Secretory | 2 (2.9%) | 0 | 0 | 0 | 2 (100%) |
| Papillary carcinomab | 6 (8.7%) | 5 (11.9%) | 1 (4.2%) | 0 | 0 |
| Invasive papillary | 1 (1.4%) | 1 (2.4%) | 0 | 0 | 0 |
| Encapsulated papillary | 2 (2.9%) | 2 (4.8%) | 0 | 0 | 0 |
| Invasive solid papillary | 3 (4.4%) | 2 (4.8%) | 1 (4.2%) | 0 | 0 |
| Mixed (ductal/mucinous) | 4 (5.8%) | 2 (4.8%) | 1 (4.2%) | 1 (100%) | 0 |
| Histological grade | 69 | 42 | 24 | 1 | 2 |
| 1 | 14 (20.3%) | 11 (26.2%) | 1 (4.2%) | 0 | 2 (100%) |
| 2 | 45 (65.2%) | 28 (66.7%) | 16 (66.7%) | 1 (100%) | 0 |
| 3 | 10 (14.5%) | 3 (7.1%) | 7 (29.2%) | 0 | 0 |
| Microscopic finding of tumor | 69 | 42 | 24 | 1 | 2 |
| Ductal carcinoma in situ | 28 (40.6%) | 17 (40.5%) | 10 (41.7%) | 1 (100%) | 0 |
| Necrosis | 11 (15.9%) | 7 (16.7%) | 4 (16.7%) | 0 | 0 |
| Sclerotic/Hyalined stroma | 22 (31.9%) | 14 (33.3%) | 8 (33.3%) | 0 | 0 |
| Calcification | 7 (10.1%) | 3 (7.1%) | 3 (12.5%) | 1 (100%) | 0 |
| Infiltration | 65 | 40 | 22 | 1 | 2 |
| Dermis | 14 (21.5%) | 10 (25.0%) | 4 (18.2%) | 0 | 0 |
| Nipple | 13 (20.0%) | 6 (15.0%) | 7 (31.8%) | 0 | 0 |
| Lymph node status | 65 | 40 | 22 | 1 | 2 |
| Positive | 26 (40.0%) | 15 (37.5%) | 11 (50.0%) | 0 | 0 |
| Negative | 39 (60.0%) | 25 (62.5%) | 11 (50.0%) | 1 (100%) | 2 (100%) |
Invasive papillary carcinoma was categorized into papillary carcinoma other than invasive carcinoma of special subtype in this study.
Two cases of intraductal papillary carcinoma were excluded in this statistical evaluation.
Histological type variants in this study are listed in Table 3, and representative photomicrographs are shown in Figure 1. Within 73 patients, 52 cases (71.2%) were classified as invasive carcinoma of no special type followed by 8 cases (11.0%) of papillary carcinoma (including 2 cases of intraductal papillary carcinoma and 1 case of invasive papillary carcinoma) (Figure 1A, 1B) and 7 cases (9.6%) of special subtype of invasive carcinoma. Four cases (5.5%) cases exhibited carcinoma in situ including 2 cases (2.7%) of pure ductal carcinoma in situ. Mixed invasive ductal and mucinous carcinoma was also indentified in 4 cases (5.5%). Within the 52 cases of invasive ductal carcinoma, osteoclasitc giant cell, invasive micropapillary (Figure 1C) and mucinous components (Figure 1D) were noticed in various numbers of the patients, as illustrated in Table 3. The 2 cases of secretory carcinoma (Figure 1E), which were described in our previous study [28] made up the only 2 cases of basal-like subtype in this study.
Figure 1.
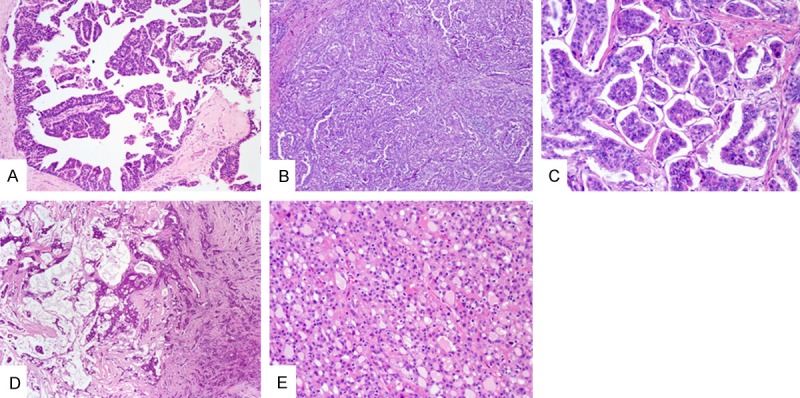
Representative images of the various histologic subtypes in male breast cancer. A: Intraductal papillary carcinoma (Low-magnification, ×100). B: Invasive papillary carcinoma (Low-magnification, ×100). C: Invasive micropapillary carcinoma component exhibited in a case of invasive ductal carcinoma (High-magnification, ×200). D: A mixed type showing both invasive ductal and mucinous carcinoma component (Low-magnification, ×40). E: Secretory carcinoma (High-magnification, ×200).
The majority of the invasive tumors were intermediate histological grade (grade 2), with 28 (66.7%) and 16 (66.7%) classified as subtype luminal A and luminal B respectively. Microscopic evaluation showed that 22 cases (31.9%) presented sclerotic or hyalined stroma. For 65 surgically treated cases, dermis and nipple infiltration were observed in 14 (21.5%) and 13 (20.0%) cases respectively.
Immunohistochemical findings and molecular subtypes
Hormone receptors ER, PR (Figure 2A, 2B) were observed positive in majority of 73 cases (93.2%, 68/73 and 93.2%, 68/73 respectively). 57 cases that were available for further immunohistochemical study. The results of the immunohistochemical study of 57 cases are shown in Table 4. Immunostaining for GCDFP15 (Figure 2D) and mammaglobin (Figure 2E) was positive in 33 (57.9%) and 30 (52.6%) cases, with varied expression pattern from focal positive to diffuse positive. Expression of GCDFP15 was more frequent in the luminal A subtype (25/34, 73.5%) than in the luminal B subtype (8/23, 34.8%) (P = 0.004). A similar tendency was observed in expression of mammaglobin between groups, although no statistical significance was found. The 2 cases of basal-like subtype exhibited mammaglobin positive and GCDFP15 negative (these results were presented in our previous study [28]).
Figure 2.
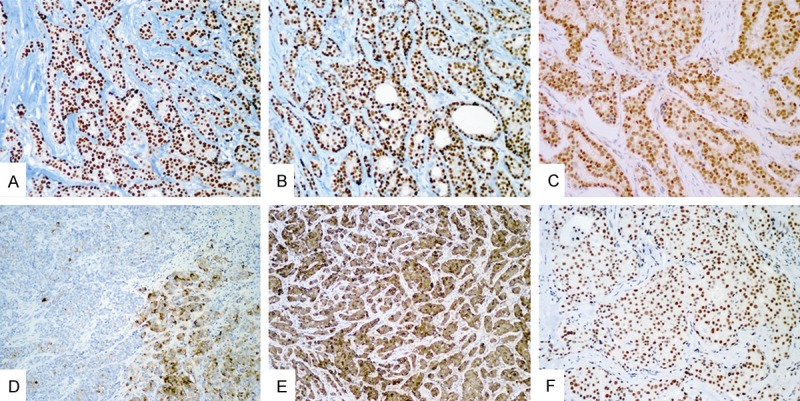
Representative images of immunohistochemical staining (IHC). Hormone reccptor ER, PR and AR exhibit stong positive staining (A-C respectively). A case showing GCDFP15 focal positive (D). Diffuse expresion of mammaglobin in one case (E). FOXP1 exhibit positive nuclear staining in invasive ductal carcinoma (F). (A, B, D, E) (Low-magnification, ×100); (C, F) (High-magnification, ×200).
Table 4.
Association between immunolabling results and cancer subtypes
| Biomarker | Total | Subtype | ||
|---|---|---|---|---|
|
| ||||
| Luminal A | Luminal B | P value | ||
| 57 | 34 | 23 | ||
| GCDFP15 | ||||
| positive | 33 (57.9%) | 25 (73.5%) | 8 (34.8%) | 0.004 |
| negative | 24 (42.1%) | 9 (26.5%) | 15 (65.2%) | |
| MGB | ||||
| positive | 30 (52.6%) | 20 (58.8%) | 10 (43.5%) | 0.255 |
| negative | 27 (47.4%) | 14 (41.2%) | 13 (56.5%) | |
| AR | ||||
| positive | 48 (84.2%) | 28 (82.4%) | 20 (87%) | 0.726 |
| negative | 9 (15.8%) | 6 (17.6%) | 3 (13.0%) | (Fisher exact) |
| FOXP1 | ||||
| positive | 41 (71.9%) | 25 (73.5%) | 16 (69.6%) | 0.744 |
| negative | 16 (28.1%) | 9 (26.5%) | 7 (30.4%) | |
Nuclear immunostaining of AR and FOXP1 were observed in the majority of cases (84.2%, 48/57 and 71.9%, 41/57) (Figure 2C, 2F), and the expression frequency of both AR and FOXP1 differed slightly between subtype luminal A and luminal B.
Discussion
Most research data on male breast carcinoma are drawn from retrospective studies. The median age at diagnosis among male breast cancer patients is 67 years, slightly older than women (61 years) [29]. The reported median age of male breast carcinoma in the Chinese population is lower (57-59 years) [11,12], which is similar to our study cohort (median age 59 years). Male breast cancer mainly presents as a painless mass in the central subareolar region [3], while clinical symptoms, such as nipple discharge and skin changes can also been presented [12]. Male breast cancer is usually unilateral, and rarely involves both breasts [23]. No prospective randomized clinical studies have been performed, and optimal treatment recommendations are undefined. Modified mastectomy was the most common surgical approach, and adjuvant hormone therapy is recommended in hormone receptor positive patients [3].
The predominant histological type in male breast cancer is invasive ductal carcinoma [29,30]. Pure invasive micropapillary carcinoma is rarely observed in both female and male breast cancer [30,31]; it was mostly reported to be mixed with invasive ductal carcinoma in female [31]. Our study defined no cases of pure invasive micropapillary carcinoma but 8 cases of mixed type (IDC with invasive micropapillary), indicating the similarity of invasive micropapillary differentiation in the both genders. Invasive lobular carcinoma is notably rare in men [29,30], and this histological diagnosis was not found in our male patients. Conversely, invasive papillary carcinoma was more common in males compared to females [8,29,30], accounting for approximately 2-4% of the cases. Although only 1 case in our study cohort was diagnosed with invasive papillary carcinoma, papillary carcinoma was observed in 8 patients (11%), suggesting that papillary architecture is commonly presented in male breast carcinoma. Papillary ductal carcinoma in situ has been shown to occur with a higher frequency [32,33]. Burga et al. [30] indicated that the discrepancies in histological type distributions between males and females may provide valuable insight into pathogenesis of breast carcinoma. The poorly developed lobule formation and relative abundance of ducts in male breasts might explain the scarcity of lobular carcinoma and predominance of papillary patterns among in-situ carcinoma [23,33]. The common presence of nipple or dermis infiltration and sclerotic/hyaline stroma observed in our studies might also be a reflection of the lack of development in male breast tissue.
In this study, based on the IHC surrogate definition [6,25], we found that the luminal subtypes were most common; with the luminal A subtype being more common than the luminal B subtype. Our results were consistent with the study carried out by Kornegoor et al. [9], since Ki-67 index was used in molecular subtyping in both studies. Similar results were observed in a recent study [10], in which both luminal A and luminal B subtypes were identified. However, by comparing the IHC-based subtyping criteria, we found that the luminal B subtype cases in their study all shown HER2 over-expressed or amplified, while none of the luminal B subtype cases in our study was identified as HER2 positive. Separation of the luminal A and luminal B subtype in this study was mainly based on the Ki-67 labeling index. This might explain why our study’s results were different from a previous 514-matched cases study [8], which found the luminal A subtype as the most common group, as Ki-67 was not added in classifying the carcinomas. It is noted that only about 30% of luminal B breast carcinomas are HER2 positive, and Ki-67 can help in identifying additional luminal B tumors that would not be identified only by ER, PR and HER2 [6]. A consensus has already been reached on using Ki-67 as a standard biomarker for molecular subtyping [25]. We think that the important role of Ki-67 in discerning different groups in male breast cancer should be emphasized and further studied.
The basal-like subtype is very rare in male breast cancer [7-10]. Only 2 cases were classified as basal-like subtype in our study. Both were diagnosed as secretory carcinomas, which should be distinguished from conventional basal-like breast carcinomas [28]. Basal-like tumors in men breast should be given with caution, taking both immunohistochemical characterization and histological type into consideration.
HER2 expression data in male breast cancer is inconsistent. Several studies have identified HER2 positive carcinomas only by immunostaining [34,35]. In our cohort, immunohistochemistry of HER2 rated as 2+ was only observed in 5 cases. When confirmed by FISH, only one case (1.4%) showed HER2 amplification and thus was classified as HER2 positive subtype (ER and PR negative). Previous studies showed that HER2 positive male breast cancer patients defined both by immunohistochemistry and FISH accounted for 0 to 16% of the cases [10,36], which is lower than female breast cancer. However, more studies are still needed with valid HER2 expression data collected according to recommended guideline.
In our study, positive GCDFP15 and MGB staining was observed in 57.9% and 52.6% out of 57 cases. In female breast cancer, it is reported more than 50% of luminal subtype tumors exhibit GCDFP15 and MGB expression [16,37], which is similar to our results in men, because luminal subtypes were the major groups in our cohort. A recent study showed that GCDFP15 and MGB were more likely to be expressed in luminal and HER2 positive subtypes [16], while in this study we found that luminal A tumors tend to express GCDFP15 more often than luminal B tumors. The same tendency was also observed in MGB expression between the two groups, although no statistical significance was found. The results correlating the expression of GCDFP15 and MGB to clinicopathologic characteristics were variable and inconsistent. A previous study reported that GCDFP15 was associated with axillary lymph node involvement, while Fritzsche et al. found that GCDFP15 positive tumors were associated with longer disease-free survival [15]. Expression of MGB was also associated with well-differentiated, hormone receptor positive breast carcinomas [14]. However, our study failed to show the correlation between GCDFP15 and MGB expression and histological grade or lymph node status. In female breast cancer, luminal B subtype is associated with a poorer prognosis compared with the luminal A subtype [5,6]. In the present study, the prognostic significance of different GCDFP15 expression patterns between subtype luminal A and B remained questionable, due to limited number of cases and short follow-up time.
In female, AR expression was associated with a favorable prognosis in ER-positive breast cancers [18]. Previous studies in male breast carcinoma showed 57 to 90% [8,20,21,34] of cases were AR positive. The predictive role of AR in male breast cancer was demonstrated in a recent case-pair study, where AR positive luminal tumors had better clinical outcomes. A high degree of AR expression was observed in our study, suggesting that anti-androgen therapy should be further explored.
In breast cancer FOXP1 expression is positively correlated with hormone receptor status, and it is suggested that FOXP1 may play a role as ER co-regulator [19]. However, the relationship between FOXP1 and hormone receptors cannot be determined in this study, due to the high frequency of expression of ER and PR. FOXP1 may play an important role in the progression of breast cancer, and its expression was also shown to be associated with favorable clinical survival [19]. Some suggest that FOXP1 might be a potential therapeutic target in cancer [38]. Given that a similar high frequency of FOXP1 expression is observed in both female and male breast carcinoma, the predictive and therapeutic value of FOXP1 could be further studied.
In conclusion, this study provides information of molecular subtype and immunohistochemical characterization in male breast cancer of Chinese population, which, to our knowledge, is rare in previous publications. However, there are some limitations about our study including limited cases and incomplete follow-up information. Complete data pooling and more caution are still needed in further studies.
Acknowledgements
This research is supported by the 2011 National Nature Science Foundation of China (81102001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. J. Clin. Oncol. 2010;28:232–39. doi: 10.1200/JCO.2009.23.8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korde LA, Zujewski JA, Kamin L, Giordano S, Domchek S, Anderson WF, Bartlett JM, Gelmon K, Nahleh Z, Bergh J, Cutuli B, Pruneri G, McCaskill-Stevens W, Gralow J, Hortobagyi G, Cardoso F. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J. Clin. Oncol. 2010;28:2114–22. doi: 10.1200/JCO.2009.25.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumors. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 5.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–50. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sánchez-Muñoz A, Román-Jobacho A, Pérez-Villa L, Sánchez-Rovira P, Miramón J, Pérez D, Sáez MI, de Luque V, Medina L, Ramírez-Tortosa CL, Vicioso L, Medina JA, Ribelles N, Alba E. Male breast cancer: immunohistochemical subtypes and clinical outcome characterization. Oncology. 2012;83:228–33. doi: 10.1159/000341537. [DOI] [PubMed] [Google Scholar]
- 8.Shaaban AM, Ball GR, Brannan RA, Cserni G, Di Benedetto A, Dent J, Fulford L, Honarpisheh H, Jordan L, Jones JL, Kanthan R, Maraqa L, Litwiniuk M, Mottolese M, Pollock S, Provenzano E, Quinlan PR, Reall G, Shousha S, Stephens M, Verghese ET, Walker RA, Hanby AM, Speirs V. A comparative biomarker study of 514 matched cases of male and female breast cancer reveals gender-specific biological differences. Breast Cancer Res Treat. 2012;133:949–58. doi: 10.1007/s10549-011-1856-9. [DOI] [PubMed] [Google Scholar]
- 9.Kornegoor R, Verschuur-Maes AH, Buerger H, Hogenes MC, de Bruin PC, Oudejans JJ, van der Groep P, Hinrichs B, van Diest PJ. Molecular subtyping of male breast cancer by immunohistochemistry. Mod Pathol. 2012;25:398–404. doi: 10.1038/modpathol.2011.174. [DOI] [PubMed] [Google Scholar]
- 10.Ge Y, Sneige N, Eltorky MA, Wang Z, Lin E, Gong Y, Guo M. Immunohistochemical characterization of subtypes of male breast carcinoma. Breast Cancer Res. 2009;11:R28. doi: 10.1186/bcr2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu T, Tong Z, He L, Zhang L. Clinicopathological Characteristics and Survival Analysis of 87 Male Breast Cancer Cases. Breast Care (Basel) 2011;6:446–51. doi: 10.1159/000335204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia LP, Zhou FF, Guo GF, Wang F, Wang X, Yuan ZY, Zhang B. Chinese female breast cancer patients show a better overall survival than their male counterparts. Chin Med J (Engl) 2010;123:2347–52. [PubMed] [Google Scholar]
- 13.Mazoujian G, Bodian C, Haagensen DJ, Haagensen CD. Expression of GCDFP-15 in breast carcinomas. Relationship to pathologic and clinical factors. Cancer. 1989;63:2156–61. doi: 10.1002/1097-0142(19890601)63:11<2156::aid-cncr2820631115>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Bhargava R, Beriwal S, Dabbs DJ. Mammaglobin vs. GCDFP-15: an immunohistologic validation survey for sensitivity and specificity. Am J Clin Pathol. 2007;127:103–13. doi: 10.1309/TDP92PQLDE2HLEET. [DOI] [PubMed] [Google Scholar]
- 15.Fritzsche FR, Thomas A, Winzer KJ, Beyer B, Dankof A, Bellach J, Dahl E, Dietel M, Kristiansen G. Co-expression and prognostic value of gross cystic disease fluid protein 15 and mammaglobin in primary breast cancer. Histol Histopathol. 2007;22:1221–30. doi: 10.14670/HH-22.1221. [DOI] [PubMed] [Google Scholar]
- 16.Lewis GH, Subhawong AP, Nassar H, Vang R, Illei PB, Park BH, Argani P. Relationship between molecular subtype of invasive breast carcinoma and expression of gross cystic disease fluid protein 15 and mammaglobin. Am J Clin Pathol. 2011;135:587–91. doi: 10.1309/AJCPMFR6OA8ICHNH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. 2010;23:205–12. doi: 10.1038/modpathol.2009.159. [DOI] [PubMed] [Google Scholar]
- 18.Castellano I, Allia E, Accortanzo V, Vandone AM, Chiusa L, Arisio R, Durando A, Donadio M, Bussolati G, Coates AS, Viale G, Sapino A. Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res Treat. 2010;124:607–17. doi: 10.1007/s10549-010-0761-y. [DOI] [PubMed] [Google Scholar]
- 19.Fox SB, Brown P, Han C, Ashe S, Leek RD, Harris AL, Banham AH. Expression of the forkhead transcription factor FOXP1 is associated with estrogen receptor alpha and improved survival in primary human breast carcinomas. Clin Cancer Res. 2004;10:3521–27. doi: 10.1158/1078-0432.CCR-03-0461. [DOI] [PubMed] [Google Scholar]
- 20.Kidwai N, Gong Y, Sun X, Deshpande CG, Yeldandi AV, Rao MS, Badve S. Expression of androgen receptor and prostate-specific antigen in male breast carcinoma. Breast Cancer Res. 2004;6:R18–23. doi: 10.1186/bcr733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy CE, Carder PJ, Lansdown MR, Speirs V. Steroid hormone receptor expression in male breast cancer. Eur J Surg Oncol. 2006;32:44–47. doi: 10.1016/j.ejso.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–74. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 23.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO Classification of Tumours of the Breast. 4th edition. Lyon: IARC; 2012. [Google Scholar]
- 24.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 25.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010;28:2784–95. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF American Society of Clinical Oncology; College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 28.Li D, Xiao X, Yang W, Shui R, Tu X, Lu H, Shi D. Secretory breast carcinoma: a clinicopathological and immunophenotypic study of 15 cases with a review of the literature. Mod Pathol. 2012;25:567–75. doi: 10.1038/modpathol.2011.190. [DOI] [PubMed] [Google Scholar]
- 29.Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101:51–57. doi: 10.1002/cncr.20312. [DOI] [PubMed] [Google Scholar]
- 30.Burga AM, Fadare O, Lininger RA, Tavassoli FA. Invasive carcinomas of the male breast: a morphologic study of the distribution of histologic subtypes and metastatic patterns in 778 cases. Virchows Arch. 2006;449:507–12. doi: 10.1007/s00428-006-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nassar H, Wallis T, Andea A, Dey J, Adsay V, Visscher D. Clinicopathologic analysis of invasive micropapillary differentiation in breast carcinoma. Mod Pathol. 2001;14:836–41. doi: 10.1038/modpathol.3880399. [DOI] [PubMed] [Google Scholar]
- 32.Anderson WF, Devesa SS. In situ male breast carcinoma in the surveillance, epidemiology, and end results database of the National Cancer Institute. Cancer. 2005;104:1733–41. doi: 10.1002/cncr.21353. [DOI] [PubMed] [Google Scholar]
- 33.Hittmair AP, Lininger RA, Tavassoli FA. Ductal carcinoma in situ (DCIS) in the male breast - A morphologic study of 84 cases of pure DCIS and 30 cases of DCIS associated with invasive carcinoma - A preliminary report. Cancer. 1998;83:2139–49. doi: 10.1002/(sici)1097-0142(19981115)83:10<2139::aid-cncr12>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 34.Rayson D, Erlichman C, Suman VJ, Roche PC, Wold LE, Ingle JN, Donohue JH. Molecular markers in male breast carcinoma. Cancer. 1998;83:1947–55. doi: 10.1002/(sici)1097-0142(19981101)83:9<1947::aid-cncr10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 35.Curigliano G, Colleoni M, Renne G, Mazzarol G, Gennari R, Peruzzotti G, de Braud E, Robertson C, Maiorano E, Veronesi P, Nolè F, Mandalà M, Ferretti G, Viale G, Goldhirsch A. Recognizing features that are dissimilar in male and female breast cancer: expression of p21(Waf1) and p27(Kip1) using an immunohistochemical assay. Ann Oncol. 2002;13:895–902. doi: 10.1093/annonc/mdf166. [DOI] [PubMed] [Google Scholar]
- 36.Bloom KJ, Govil H, Gattuso P, Reddy V, Francescatti D. Status of HER-2 in male and female breast carcinoma. Am J Surg. 2001;182:389–92. doi: 10.1016/s0002-9610(01)00733-4. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki E, Tsunoda N, Hatanaka Y, Mori N, Iwata H, Yatabe Y. Breast-specific expression of MGB1/mammaglobin: an examination of 480 tumors from various organs and clinicopathological analysis of MGB1-positive breast cancers. Mod Pathol. 2007;20:208–14. doi: 10.1038/modpathol.3800731. [DOI] [PubMed] [Google Scholar]
- 38.Koon HB, Ippolito GC, Banham AH, Tucker PW. FOXP1: a potential therapeutic target in cancer. Expert Opin Ther Targets. 2007;11:955–65. doi: 10.1517/14728222.11.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]


