Abstract
Retroperitoneal leiomyosarcomas (LMSs) are rare gynecological malignancies that display poor prognosis and high mortality. Cell cycle-related and expression-elevated protein in tumor (CREPT) is an oncogene that is involved in the regulation of many cell cycle-related proteins. However, its distribution and clinical significance in retroperitoneal LMS remains poorly understood. This study assessed the histological classifications of postoperative tumor samples from 71 cases of retroperitoneal LMS that were collected at The General Hospital of the People’s Liberation Army from January 1998 to December 2012. We found that more than half of the patients displayed positive expressions of CREPT, Ki-67 and PCNA via immunohistochemical analysis. The expression of CREPT correlated with histological grade (P = 0.044), and the PCNA expression level correlated with the differentiation of tumor cells and histological grade (P < 0.001 and P = 0.009, respectively). Multivariate analysis showed that survival was associated with histological grade and the expression level of CREPT (P = 0.011 and P = 0.012, respectively). Kaplan-Meier analysis showed that the patients lacking CREPT expression exhibited significantly longer overall postoperative survival (median, 60.0 months) than the patients displaying CREPT expression (median, 33.0 months), and CREPT expression correlated with distant recurrence within 5 years after surgery (P = 0.004). Western blot analyses showed that CREPT was more strongly expressed in the retroperitoneal LMS tumor tissue than in paired control tissue. Based on the above data, we concluded that CREPT displays unique immunostaining for retroperitoneal LMS tissue and can be used to supplement other currently available retroperitoneal LMS markers.
Keywords: CREPT, retroperitoneal neoplasms, leiomyosarcoma, prognosis
Introduction
The retroperitoneum can host a wide spectrum of pathologies, including a variety of benign and malignant neoplasms [1]. Retroperitoneal tumors can create a diagnostic dilemma and present several therapeutic challenges because of their rarity, relatively late presentation and anatomical location, which is often proximal to several vital structures in the retroperitoneal space [2]. The prognosis of patients with retroperitoneal sarcoma is poor with a 12-40% overall 5-year survival rate [3]. Leiomyosarcomas (LMSs), a type of retroperitoneal sarcoma, are the 2nd most common primary retroperitoneal neoplasm [4]. The most effective treatment for this disease is generally considered to be surgery, but less than 50% of these patients receive this treatment [5]. Failure to accomplish complete excision is attributed to several factors: the size of the tumor, its location and the number of organs involved [6]. Consequently, the survival of patients diagnosed with retroperitoneal neoplasms is very low, and there is an urgent need to understand the pathogenesis of retroperitoneal neoplasms and to develop new diagnostic markers and treatment modalities.
CREPT (cell-cycle related and expression-elevated protein in tumor, also named RPR1B) is a novel gene that belongs a new family of proteins within the RPR domain and was recently identified to promote tumorigenesis by up-regulating the expression of genes related to the cell cycle. Our group revealed a mechanism through which CREPT promotes cell proliferation by enhancing the transcription of CYCLIN D1 via preventing RNAPII from “reading through” and possibly promoting the recycling of RNAPII to the promoter of genes; this mechanism occurs in a manner similar to that of the chromatin loop. We previously identified that CREPT is highly expressed in several types of gastroenteric tumors based on immunohistochemistry (IHC) analysis. We demonstrated that stomach cancer patients displaying strong CREPT expression exhibited a poor survival rate after surgery [7]. However, in retroperitoneal LMS, the relationship between CREPT expression and prognosis (particularly that relating to survival) remains unclear.
In the present study, we expanded our immunohistochemical analysis of the expression of CREPT, Ki-67 and PCNA in a set of retroperitoneal LMS samples to further explore the diagnostic value of these markers. We also investigated the relationship between the expression of CREPT and clinical prognosis and the clinicopathological characteristics of retroperitoneal LMS. Our data showed that CREPT could be used as a potential marker for the prognosis of retroperitoneal LMS.
Patients and methods
Patients and tissue samples
A set of 71 retroperitoneal LMS patients who underwent curative surgery without preoperative chemotherapy or radiotherapy were selected for this study from January 1998 to December 2012 at The General Hospital of the People’s Liberation Army (PLA) in Beijing, China. The histological diagnosis of retroperitoneal LMS was established and confirmed by two pathologists. Patients displaying GISTs were excluded from the analysis. The baseline clinical and staging data were retrieved from the hospital database for reviewing.
There were 71 retroperitoneal LMS patients; 57 females and 14 males (4:1) ranging from 21 to 79 years of age (median, 48 years). At the time of surgical resection, the tumors ranged in size from 3.5 to 45 cm (median, 12.9 cm). Based on the Federation National des Centres de Lutte contre le Cancer (FNCLCC) standards [8], 48 patients (67.6%) were histological grade II, 63 (88.7%) contained moderately differentiated tumors, and 39 (54.9%) displayed low mitotic counts. A total of 64 (90.1%) retroperitoneal LMS patients with available follow-up data were selected to generate the Kaplan-Meier survival curves. Additionally, 5 paired fresh frozen samples that included the retroperitoneal LMS and adjacent noncancerous tissues were collected for Western blot analysis. The specimen collection and study procedures were approved by The Ethics Committee of the Chinese PLA General Hospital.
IHC and staining evaluation
The paraffin-embedded tumor tissues were sliced into 3 μm sections and deparaffinized. The sections were heated in a microwave oven for antigen retrieval, and a standard streptavidin/peroxidase complex method (SP) was used for immunostaining, as previously described [9]. The monoclonal mouse anti-human CREPT (1:60) and monoclonal rabbit anti-human Ki-67 (1:300) and PCNA antibodies (1:1,000) (Santa Cruz, USA) were used as primary antibodies. After counterstaining with Meyer’s hematoxylin, the sections were observed under a light microscope.
All immunohistochemically stained sections were examined in a blinded manner without any knowledge of the clinicopathological parameters or patient outcomes.
The immunoreactivity for CREPT, Ki-67 and PCNA was recorded as strong or weak based on the staining intensity score and the percentage score. The proportion score was assigned according to the percentage of the tumor cells displaying positive nuclear staining (0, < 10%; 1, 11-30%; 2, 31-80%; or 3, > 80%). The intensity score was assigned according to the average intensity of the immunopositive tumor cells (0, none; 1, weak; 2, moderate; or 3, strong). The expression score was calculated using the percentage and intensity scores, which ranged from 0 to 9. The expression levels were categorized as negative (score 0), 1+ (score 1-3), 2+ (score 4-6) and 3+ (score 7-9). Any positive expression level (from 1+ to 3+) was regarded as positive expression.
Western blot analysis
Total protein samples from both tumor and adjacent normal tissues were extracted using RIPA buffer, and the protein concentration of each sample was determined using the Bradford method (Thermo). Equal amounts of protein (40 μg) were separated via SDS-PAGE and transferred to a PVDF membrane (Millipore, Bedford, MA, USA). The membrane was blocked in 8% non-fat dry milk for 1 h at 37°C prior to incubation in the monoclonal anti-human CREPT, Ki-67, PCNA or β-actin antibody (Abcam, Cambridge, MA, USA) at room temperature for 2 h. After washing with Tris-buffered saline, the membrane was incubated in anti-rabbit or anti-mouse IgG (Santa Cruz Biotechnology, USA) for 1 h at room temperature. The blots were visualized via enhanced chemiluminescence (ECL) according to the manufacturer’s protocol. The experiments were independently repeated at least three times.
Statistical analysis
The statistical analyses were performed using Chi-square and Fisher’s exact tests to determine the differences between groups. Overall survival (OS) was defined as the time of surgery until the time of death, and disease-free survival (DFS) was defined as the time of surgery until the appearance of evidence of radiological recurrence or metastasis. Kaplan-Meier analysis followed by the log rank test was performed to estimate the OS of each group. For the univariate and multivariate analyses, independent prognostic factors of patient survival were determined using Cox regression methods. The statistical analyses of the clinical samples were performed using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). A two-tailed P-value < 0.05 was considered to be statistically significant.
Results
Expression of CREPT, Ki-67, and PCNA in retroperitoneal LMS samples
Histopathological examination showed that the retroperitoneal LMS tumor tissues consisted of spindle cells that formed sheets or fascicles [10]. These cells contained oval nuclei and an elongated hyperchromatic and abundant eosinophilic cytoplasm (H&E, × 400, Figure 1A). To investigate the potential roles of CREPT, Ki-67 and PCNA in retroperitoneal LMS, we examined the expression of CREPT, Ki-67 and PCNA in paraffin-embedded sections from 71 patients via immunohistochemistry. We detected the expression of CREPT, Ki-67 and PCNA only in the nucleus (Figure 1B-D). A total of 56 patients (78.9%) displayed positive CREPT expression. Positive expression of Ki-67 was detected in 49 cases (69.0%), and positive PCNA expression was detected in 63 cases (88.7%). There was a significant association between the expression of CREPT and the mitotic count, the histological grade, Ki-67 expression and PCNA expression (P = 0.046, P = 0.044, P = 0.002 and P < 0 .001, respectively, Table 1).
Figure 1.
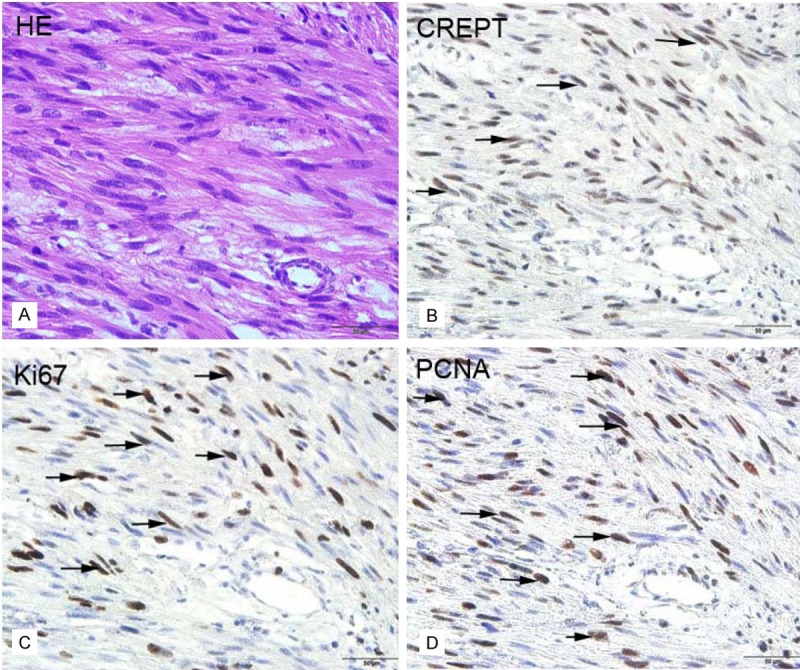
Representative results of the H&E and immunohistochemical staining for CREPT, Ki-67 and PCNA in retroperitoneal LMS tissues. A: H&E staining. B-D: Immunohistochemical staining for CREPT, Ki-67 and PCNA, respectively. The arrows indicate positively stained cells. (Original magnification × 400, bar = 50 μm).
Table 1.
Correlations between Ki-67, CREPT and PCNA expression and clinicopathological characteristics
| Characteristic | Total | CREPTe, n expression (%) Positive, n = 56 | P | Ki-67e, n expression (%) Positive, n = 49 | P | PCNA expression n (%) Positive, n = 63 | P |
|---|---|---|---|---|---|---|---|
| Age, years | |||||||
| < 60 | 63 (88.7) | 49 (49.7) | 1.000 | 43 (43.5) | 1.000 | 50 (48.8) | 0.368 |
| ≥ 60 | 8 (11.3) | 7 (6.30) | 6 (5.50) | 5 (6.20) | |||
| Sex | |||||||
| Female | 57 (80.3) | 44 (45.0) | 0.719 | 38 (39.3) | 0.526 | 42 (44.2) | 0.166 |
| Male | 14 (19.7) | 12 (11.0) | 11(9.70) | 13 (10.8) | |||
| Tumor size, cm | |||||||
| ≤ 10 | 28 (39.4) | 20 (22.1) | 0.146 | 18 (19.3) | 0.608 | 21 (21.7) | 0.781 |
| > 10 | 43 (45.1) | 36 (33.9) | 31 (29.7) | 34 (33.3) | |||
| Tumor differentiation score | |||||||
| 1 score | 7 (9.90) | 4 (5.50) | 0.299 | 3 (4.80) | 0.085 | 1 (5.40) | <0.001 |
| 2 score | 63 (88.7) | 51 (49.7) | 43 (43.5) | 53 (8.8) | |||
| 3 score | 1 (1.40) | 1 (0.80) | 1 (0.70) | 1 (0.80) | |||
| Mitotic count score | |||||||
| 1 | 37 (52.1) | 30 (30.8) | 0.109 | 22 (26.9) | 0.050 | 28 (30.2) | 0.856 |
| 2 | 16 (22.5) | 15 (11.8) | 14 (10.4) | 13 (11.6) | |||
| 3 | 18 (25.4) | 11 (13.4) | 13 (11.7) | 14 (13.2) | |||
| Tumor necrosis score | |||||||
| 0 | 22 (31.0) | 18 (20.5) | 0.059 | 15 (17.9) | 0.468 | 18 (20.1) | 0.438 |
| 1 | 25 (35.2) | 19 (16.6) | 16 (14.5) | 17 (16.3) | |||
| 2 | 24 (33.8) | 19 (18.96) | 18 (16.6) | 20 (18.6) | |||
| Histological grade | |||||||
| 1 | 10 (14.1) | 7 (10.3) | 0.044 | 6 (9.0) | 0.064 | 5 (10.1) | 0.009 |
| 2 | 48 (67.6) | 40 (36.3) | 33 (31.7) | 40 (35.6) | |||
| 3 | 13 (18.3) | 9 (9.50) | 10 (8.3) | 10 (9.38) | |||
Statistical analyses were performed using the Pearson χ2 test.
Univariate and multivariate analysis for prognostic factors
A total of 64 retroperitoneal LMS patients with available follow-up data were selected to generate the Kaplan-Meier survival curves. The OS rangeds from 5.0 to 74.0 (mean 34.0) months. The five-year OS rate of the retroperitoneal LMS patients was 28.1%. Univariate analysis revealed that the expression levels of CREPT, Ki-67 and PCNA were significant prognostic factors for OS (Table 2). The patients displaying positive CREPT expression (mean, 33 months) experienced a shorter OS than those lacking CREPT expression (mean, 60 months). The tumor size, histological grade, and expression of CREPT, Ki-67 and PCNA correlated with poor OS (Figure 2; Table 2). Furthermore, a multivariate analysis revealed that CREPT expression and the histological grade are independent prognostic factors of OS (Table 2; P = 0.012 and P = 0.011, respectively). Taken together, these results suggested that CREPT is strongly correlated with the prognosis of retroperitoneal LMS patients.
Table 2.
Univariate and multivariate survival analyses of 71 patients with retroperitoneal LMS
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| RR | P Value | 95.0% CI for Exp (B) | RR | P Value | 95.0% CI for Exp (B) | |
| Age | 1.130 | 0.780 | 0.479-2.669 | |||
| Sex | 1.804 | 0.116 | 0.846-3.766 | |||
| Size | 1.878 | 0.038 | 1.036-3.405 | |||
| Tumor differentiation | 1.330 | 0.540 | 0.535-3.308 | |||
| Mitotic count | 1.293 | 0.127 | 0.930-1.800 | |||
| Tumor necrosis | 1.142 | 0.422 | 0.826-1.579 | |||
| Histological grade | 1.719 | 0.024 | 1.074-2.751 | 1.858 | 0.011 | 1.153-2.995 |
| Ki-67 expression | 2.041 | 0.047 | 1.011-4.123 | |||
| CREPT expression | 2.381 | 0.022 | 1.132-5.006 | 2.606 | 0.012 | 1.236-5.494 |
| PCNA expression | 2.442 | 0.042 | 1.033-5.773 | |||
Figure 2.
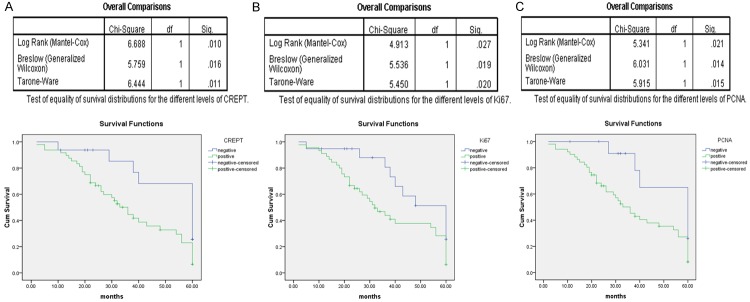
Kaplan-Meier analyses of the overall survival of 64 retroperitoneal LMS patients according to CREPT (A), Ki-67 (B) and PCNA (C) expression.
Comparison of the expression of CREPT, Ki-67, and PCNA in retroperitoneal LMS tumors and adjacent noncancerous tissues via WB
Five paired fresh frozen samples, which included retroperitoneal LMS tumor and adjacent noncancerous tissues, were collected for CREPT, Ki-67 and PCNA protein expression analysis (Figure 3). The results showed that CREPT was highly expressed in retroperitoneal LMS tumor tissues (Figure 3, top panel). In contrast, low levels of CREPT expression were detected in the adjacent normal tissues (Figure 3, top panel). Additionally, we found that the expression levels of Ki-67 and PCNA in the retroperitoneal LMS tumor tissue were significantly higher than those in the adjacent noncancerous tissues (Figure 3, middle panel). These results suggested that CREPT expression is correlated with the tumorigenesis of retroperitoneal LMS.
Figure 3.
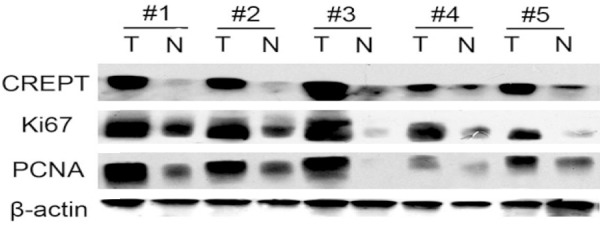
Western blot analysis of CREPT, Ki-67 and PCNA expression in retroperitoneal LMS tumor and normal adjacent tissues. T refers to the tumor tissue, and N refers to the paired normal tissue from the same patient.
Correlation between CREPT expression and initial recurrence
A total of 18 patients survived for the median follow-up duration of 41 (range, 1-127) months. Based on univariate analysis, histological grade, tumor size and CREPT expression were significant prognostic factors (Table 2). Clinical follow-up was performed, and the survival analysis suggested a close correlation between CREPT expression and initial recurrence of retroperitoneal LMS. The 5-year disease-specific survival (DSS) was 34% for primary LMS. Among the 45 patients experiencing recurrence, the pattern of recurrence was predominately distant recurrence (DR), which occurred in 19 patients (42.2%). Local recurrence (LR) occurred in only 16 patients (35.6%); 10 patients (22.2%) exhibited both LR and DR. As shown in Figure 4, CREPT expression was a prognostic factor for distant primary LMS recurrence (P = 0.04).
Figure 4.
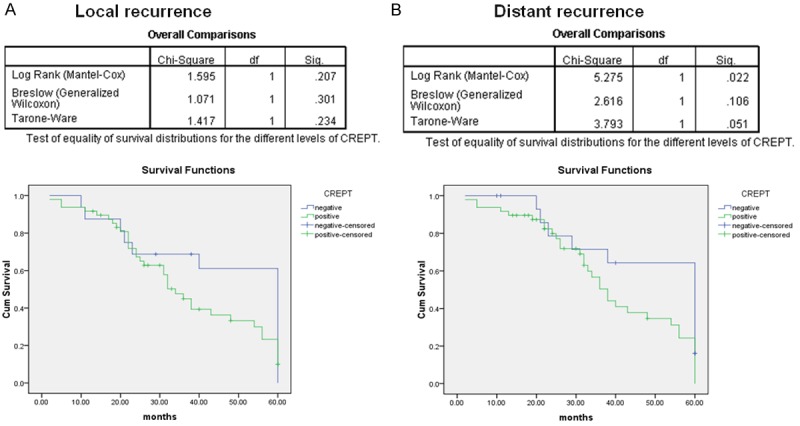
The expression of CREPT is an independent prognostic factor for distant recurrence-free survival (B) but not local recurrence-free survival of primary LMS (A). (P = 0.022 and P = 0.207, respectively).
Discussion
Soft tissue sarcomas constitute 0.7% of adult malignancies [11]. Ten to twenty percent of soft tissue sarcomas occur in the retroperitoneum, and LMS, liposarcoma and fibrosarcoma are the most common histological types [12-16]. The retroperitoneum provides a broadly expandable anatomic location for tumor growth, and these tumors often attain a large size before symptoms manifest [1,17]. Relevant symptoms are often indistinguishable from those of a mass in the abdominal viscera or reproductive organs. LMSs are relatively insensitive to radiotherapy and chemotherapy, and broad surgical excision is the preferred treatment. Retroperitoneal LMSs display relatively late presentation and are often proximal to several vital structures in the retroperitoneal space [18,19]. Although the standard treatment remains margin-negative surgical resection, the 5-year survival rate after surgical resection remains low, ranging from 29% to 67% [20,21]. However, the exact molecular mechanisms of the progression and recurrence of retroperitoneal LMSs are unclear, and there is a lack of valid and reliable biomarkers to predict retroperitoneal LMS recurrence.
Ki-67, a nuclear antigen present during the G1, S, G2, and M phases of all proliferating human cells, is a biomarker of tumor proliferation [22]. Patients displaying increased Ki-67 expression exhibit a lower cancer-specific survival rate [23]. The level of Ki-67 expression has been used in clinical analyses to predict the survival rate in a scenario with several neoplasms. Previous studies have identified Ki-67 as a tool in determining the malignancy of smooth muscle neoplasms (exemplified by myxoid LMS of the uterus) [24,25]. PCNA is central to many essential cellular processes, such as DNA replication, DNA damage repair, chromatin structure maintenance and cell cycle progression [26]. PCNA has been widely used in studies assessing the growth rate of human malignancies. These previous studies indicated a significant expression level of PCNA in low- and intermediate-grade LMS [27].
CREPT has been reported to be a highly expressed oncogene in a variety of tumors [7]. CREPT expression accelerates malignant cell growth and tumorigenesis. Lu D et al. revealed that CREPT mRNA expression was up-regulated in various human malignant tumors and that the CREPT protein expression level was closely associated with the degree of differentiation and the clinical stage of the tumor [7].
In this report, we first detected the protein expression of CREPT in retroperitoneal LMS tumor tissue. Immunohistochemical staining results showed that CREPT, Ki-67 and PCNA were positively expressed in more than half of the retroperitoneal LMS patients. Further investigation showed that the expression of CREPT correlated with the expression of Ki-67 and PCNA. Our findings were in line with previous results for sarcoma [27]. We observed CREPT protein expression in retroperitoneal LMS samples and paired normal tissues. The expression of CREPT in the retroperitoneal LMS tumor tissue was significantly stronger than that in the adjacent noncancerous tissue. Therefore, we speculated that CREPT may play an important role in retroperitoneal LMS.
Our statistical analysis showed that the expression of CREPT, Ki-67 and PCNA closely correlated with 5-year overall survival and the distant recurrence of retroperitoneal LMS. Patients displaying high CREPT, Ki-67 or PCNA expression exhibited relatively short recurrence-free survival and overall survival; the patients displaying low CREPT expression exhibited relatively longer survival (P = 0.010, P = 0.027 and P = 0.021, respectively).
Univariate analyses identified tumor size, histological grade, and the expression level of CREPT, Ki-67 and PCNA as significant prognostic factors (P = 0.038, P = 0.024, P = 0.022, P = 0.047 and P = 0.042, respectively), and multivariate Cox regression analysis revealed that the histological grade and expression levels of CREPT were independent prognostic factors of poor overall survival (OS) among retroperitoneal LMS patients (P = 0.011 and P = 0.012, respectively). The retroperitoneal LMS patients in our study experienced a higher incidence of DR (42.2% at 5 years) than LR. With respect to DR, we found that CREPT expression is a prognostic factor for the distant recurrence of retroperitoneal LMS (P = 0.04, Figure 4). The above results strongly suggested that CREPT plays an important role in retroperitoneal LMS progression and that CREPT represents a valuable biomarker in predicting the prognosis of patients with retroperitoneal LMS.
CREPT has been suggested to display unique advantages for predicting the prognosis and distant recurrence of retroperitoneal LMS compared with conventional biomarkers such as Ki-67 and PCNA. Therefore, CREPT serves as a candidate biomarker that could be used in combination with the conventional clinical biomarkers mentioned above. In clinical studies, accurate and reliable prognostic markers are crucial for providing comprehensive prognostic information and an accurate basis for treatment decisions. In this study, we identified the novel potential biomarker CREPT to be a prognostic factor in cancer progression and distant recurrence among patients with retroperitoneal LMS.
Acknowledgements
We would like to thank Lixin Wei of The Department of Pathology at the Chinese PLA General Hospital for providing assistance with the paraffin sections. We thank B. S.M.T. Junzhen Fan for his assistance with sample preparation in this study. This work was supported by grants from The 973 Project (2011CB910502, 2011ZX08011-006), The NSFC (81372167, 30871286, 31071225, 31030040), The Tsinghua Science Foundation (20121080018), and The 863 project (2012AA021703) of China.
Disclosure of conflict of interest
None.
References
- 1.Strauss DC, Hayes AJ, Thomas JM. Retroperitoneal tumours: review of management. Ann R Coll Surg Engl. 2011;93:275–280. doi: 10.1308/003588411X571944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strauss DC, Hayes AJ, Thway K, Moskovic EC, Fisher C, Thomas JM. Surgical management of primary retroperitoneal sarcoma. Br J Surg. 2010;97:698–706. doi: 10.1002/bjs.6994. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins MP, Alvaranga JC, Thomas JM. The management of retroperitoneal soft tissue sarcomas. Eur J Cancer. 1996;32A:622–626. doi: 10.1016/0959-8049(95)00638-9. [DOI] [PubMed] [Google Scholar]
- 4.Neville A, Herts BR. CT characteristics of primary retroperitoneal neoplasms. Crit Rev Comput Tomogr. 2004;45:247–270. [PubMed] [Google Scholar]
- 5.Karakousis CP, Perez RP. Soft tissue sarcomas in adults. CA Cancer J Clin. 1994;44:200–210. doi: 10.3322/canjclin.44.4.200. [DOI] [PubMed] [Google Scholar]
- 6.Alvarenga JC, Ball AB, Fisher C, Fryatt I, Jones L, Thomas JM. Limitations of surgery in the treatment of retroperitoneal sarcoma. Br J Surg. 1991;78:912–916. doi: 10.1002/bjs.1800780806. [DOI] [PubMed] [Google Scholar]
- 7.Lu D, Wu Y, Wang Y, Ren F, Wang D, Su F, Zhang Y, Yang X, Jin G, Hao X, He D, Zhai Y, Irwin DM, Hu J, Sung JJ, Yu J, Jia B, Chang Z. CREPT accelerates tumorigenesis by regulating the transcription of cell-cycle-related genes. Cancer Cell. 2012;21:92–104. doi: 10.1016/j.ccr.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Pautier P, Floquet A, Penel N, Piperno-Neumann S, Isambert N, Rey A, Bompas E, Cioffi A, Delcambre C, Cupissol D, Collin F, Blay JY, Jimenez M, Duffaud F. Randomized multicenter and stratified phase II study of gemcitabine alone versus gemcitabine and docetaxel in patients with metastatic or relapsed leiomyosarcomas: a Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) French Sarcoma Group Study (TAXOGEM study) Oncologist. 2012;17:1213–1220. doi: 10.1634/theoncologist.2011-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esposito V, Baldi A, De Luca A, Tonini G, Vincenzi B, Santini D, Persichetti P, Mancini A, Citro G, Baldi F. Cell cycle related proteins as prognostic parameters in radically resected non-small cell lung cancer. J Clin Pathol. 2005;58:734–739. doi: 10.1136/jcp.2004.023531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss SW, Goldblum JR, Enzinger FM. Enzinger and Weiss’s Soft Tissue Tumors. St. Louis: Mosby; 2001. [Google Scholar]
- 11.Todd CS, Michael H, Sutton G. Retroperitoneal leiomyosarcoma: eight cases and a literature review. Gynecol Oncol. 1995;59:333–337. doi: 10.1006/gyno.1995.9967. [DOI] [PubMed] [Google Scholar]
- 12.Adam YG, Oland J, Halevy A, Reif R. Primary retroperitoneal soft-tissue sarcomas. J Surg Oncol. 1984;25:8–11. doi: 10.1002/jso.2930250103. [DOI] [PubMed] [Google Scholar]
- 13.Cody HS 3rd, Turnbull AD, Fortner JG, Hajdu SI. The continuing challenge of retroperitoneal sarcomas. Cancer. 1981;47:2147–2152. doi: 10.1002/1097-0142(19810501)47:9<2147::aid-cncr2820470907>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.McGrath PC, Neifeld JP, Lawrence W Jr, DeMay RM, Kay S, Horsley JS 3rd, Parker GA. Improved survival following complete excision of retroperitoneal sarcomas. Ann Surg. 1984;200:200–204. doi: 10.1097/00000658-198408000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catton CN, O’Sullivan B, Kotwall C, Cummings B, Hao Y, Fornasier V. Outcome and prognosis in retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 1994;29:1005–1010. doi: 10.1016/0360-3016(94)90395-6. [DOI] [PubMed] [Google Scholar]
- 16.Hassan I, Park SZ, Donohue JH, Nagorney DM, Kay PA, Nasciemento AG, Schleck CD, Ilstrup DM. Operative management of primary retroperitoneal sarcomas: a reappraisal of an institutional experience. Ann Surg. 2004;239:244–250. doi: 10.1097/01.sla.0000108670.31446.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;5:845–856. doi: 10.1038/nrc1739. [DOI] [PubMed] [Google Scholar]
- 18.Wang LD, Hong JY, Qiu SL, Gao H, Yang CS. Accumulation of p53 protein in human esophageal precancerous lesions: a possible early biomarker for carcinogenesis. Cancer Res. 1993;53:1783–1787. [PubMed] [Google Scholar]
- 19.Liles JS, Tzeng CW, Short JJ, Kulesza P, Heslin MJ. Retroperitoneal and intra-abdominal sarcoma. Curr Probl Surg. 2009;46:445–503. doi: 10.1067/j.cpsurg.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Clary BM, DeMatteo RP, Lewis JJ, Leung D, Brennan MF. Gastrointestinal stromal tumors and leiomyosarcoma of the abdomen and retroperitoneum: a clinical comparison. Ann Surg Oncol. 2001;8:290–299. doi: 10.1007/s10434-001-0290-3. [DOI] [PubMed] [Google Scholar]
- 21.Gladdy RA, Qin LX, Moraco N, Agaram NP, Brennan MF, Singer S. Predictors of survival and recurrence in primary leiomyosarcoma. Ann Surg Oncol. 2013;20:1851–1857. doi: 10.1245/s10434-013-2876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 23.Rioux-Leclercq N, Turlin B, Bansard JY, Patard JJ, Manunta A, Moulinoux JP, Guillé F, Ramée MP, Lobel B. Value of immunohistochemical Ki-67 and p53 determinations as predictive factors of outcome in renal cell carcinoma. Urology. 2000;55:501–505. doi: 10.1016/s0090-4295(99)00550-6. [DOI] [PubMed] [Google Scholar]
- 24.Mittal K, Demopoulos RI. MIB-1 (Ki-67), p53, estrogen receptor, and progesterone receptor expression in uterine smooth muscle tumors. Hum Pathol. 2001;32:984–987. doi: 10.1053/hupa.2001.27113. [DOI] [PubMed] [Google Scholar]
- 25.Sprogoe-Jakobsen S, Holund B. Immunohistochemistry (Ki-67 and p53) as a tool in determining malignancy in smooth muscle neoplasms (exemplified by a myxoid leiomyosarcoma of the uterus) APMIS. 1996;104:705–708. doi: 10.1111/j.1699-0463.1996.tb04932.x. [DOI] [PubMed] [Google Scholar]
- 26.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Steck K, el-Naggar AK. Comparative flow cytometric analysis of Ki-67 and proliferating cell nuclear antigen (PCNA) in solid neoplasms. Cytometry. 1994;17:258–265. doi: 10.1002/cyto.990170309. [DOI] [PubMed] [Google Scholar]


