Abstract
Objective: To study immunization procedures and preparation methods of specific IgY antibodies (IgY-Hp, IgY-IB) produced by hens immunized with Helicobacter pylori (Hp) bacterial antigen and recombinant Hp specific antigen IB, detect the inhibition effects on Hp growth and Hp urease activity, and study the effects of oral administration for treating Hp infection. Methods: By using recombinant cholera toxin subunit B (rCTB) as an adjuvant, hens received intramuscular injection immunization for continuous 7 times at an interval of 14 days. Then, the eggs were collected; IgY was purified. Results: On day 49 after hens were immunized, levels of two antibodies all reached 1:12800; after they were purified by Ammonium sulfate precipitation, their purity was over 80%. IgY-Hp could inhibit Hp growth and inhibit Hp urease activity; although in vitro, IgY-IB could not inhibit Hp growth but could inhibit Hp urease activity. The experiments in vivo found that when IgY-Hp or IgY-IB with sucralfate dual oral therapy was used to treat Hp infected mouse model, the cure rate all could reach 83.3%. Conclusion: According to immunization procedure, high titer specific IgY antibody (1:12800) can be obtained in 49 days and its titer remains stable. Oral administration of the specific IgY antibodies in Hp infected mice can reach a cure rate of 83.3%, and the antibodies are expected to become new drugs and therapeutic methods of targeted therapy against Hp infection.
Keywords: Helicobacter pylori, specific IgY, antibacterial effect
Introduction
Helicobacter pylori (Hp) is Gram-negative Helicobacter colonizing on the human gastric mucosa and has now been confirmed as the main pathogenic factor of chronic active gastritis, peptic ulcer, gastric mucosa-associated lymphatic tissue lymphoma and gastric cancer. In 1994, world Health Organization/International Agency for Research on Cancer (WHO/IARC) listed Hp as Class I carcinogen. Hp infection rate in the population is above 50% [1]. At present, clinical main treatment of Hp infection is combination therapy of multiple antibiotics and acid suppression drugs [2]. Although a certain treatment effect has been achieved, there are many problems, such as patients have long-term dependence on drugs, complete cure is difficult, cost is expensive, and drug-resistant strains become more and more. Therefore, development of specific targeted drugs for treating Hp infection becomes particularly important.
Egg yolk immunoglobulin (IgY) is the major immunoglobulin of poultry and can be specifically gathered in the yolk through the hen. It has been reported that the specific antibody can be used to prevent dental caries, diarrhea and other infectious diseases [3]. Based on the previous research achievements [4], we prepared the anti-Hp whole bacteria IgY (SS1-IgY) and anti-recombinant Hp specific antigen IB IgY (IB-IgY), and we studied inhibition effects of the two specific antibodies on Hp growth and on Hp urease activity in vitro. After Hp infected BALB/c mouse model orally received the two kinds of specific IgY antibodies, respectively, the antibodies’ treatment effects on Hp were studied in vivo so as to provide a theoretical basis for therapeutic antibody drug development of Hp infection targeted therapy.
Materials and methods
Materials
Helicobacter pylori Sydney strain 1 (Hp SS1) came from Hebei Medical University. Escherichia coli and Staphylococcus aureus were stored in our lab. Six-week-old SPF level BALB/c mice and 25-week-old hens were provided by Huaxi Experimental Animal Center of Sichuan University. Hp whole bacterial antigen, IB antigen and CTB adjuvant were provided by Sichuan Vaccine Technology Co., Ltd. Omeprazole was purchased from Youcare Pharmaceutical Group Co., Ltd. Sucralfate was purchased from Huanan Pharmaceutical Group Co., Ltd., Guangdong. Clarithromycin was purchased from Wang Lin Tang Pharmaceutical Co., Ltd., Sichuan Province. Canine parvovirus IgY (IgY-CPV) was provided by Sichuan Agricultural University.
Immunization
Intramuscular injection of rCTB by 400 μg/hen was used as basic immunization; 7 days later, hens received intramuscular injection of SS1, IB antigens, 300 μg/hen; then, every 14 days the additional immunization was performed up to day 91.
Preparation
SS1-IgY and IB-IgY were purified by water Ammonium sulfate precipitation. Indirect ELISA assay was used to detect titers of specific antibodies, SDS-PAGE electrophoresis was used to detect antibody purity and KD5600 UV spectrophotometer was used to detect IgY protein concentration [5].
IgY inhibited Hp urease activity
50 μl 106 CFU/ml Hp bacterial liquid was added into wells on a polyethylene plate, and then 50 μl IgY antibody and negative, positive controls were added. Incubation was performed at 37°C for 24 h. The 50 μl rapid urea reagent was added, and the reaction lasted for 30 min at 37°C. OD values were measured at 578 nm by a microplate reader [6].
Inhibition on IgY’s Hp growth and its bacterial inhibitory specificity
106 CFU/ml Hp was uniformly smeared on the Colombia solid medium; 106 CFU/ml Escherichia coli and Staphylococcus aureus were smeared on the LB solid mediums, respectively. A puncher with diameter of 6 mm was used to punch, and agar in the holes was removed. 50 μl specific IgY antibody and positive and negative controls were added to each well, respectively. After above solutions were added, Hp was placed in an anaerobic jar at 37°C and was cultured for 72 h for observing inhibition ring size; plate mediums with Escherichia coli and Staphylococcus aureus were placed into an incubator at 37°C and were cultured for 24 h for observing inhibition ring sizes [7].
Treatment of oral IgY for Hp infected mouse model
54 SPF level mice with 16 ± 2 g were taken and numbers of males and females were equal. After fasting and water deprivation for 12 h, the mice received oral 0.2 M sodium bicarbonate, 0.2 ml/mouse; 20 min later, the mice received oral administration of 108 CFU/ml (urea reagent reaction OD578 = 2.8) for infection; 3 h after oral administration, water and food were restored. The above oral infection process was repeated two more times, and the time interval between two adjacent times was two days [7-9]. On day 14 after infection, mice received drug therapy according to groups, and then every two days the mice received oral IgY and positive control and negative controls once for continuous four times [7-9]. On day 14 after completion of oral administration for therapy, the mice were sacrificed, and the gastric tissue in the mice was taken for PCR, smearing and Gram staining, immunohistochemistry as well as pathology examination for positive Hp infection identification [7-10].
Results
Changes of IgY antibodies after immunization
On day 14 after immunization by the antigens, the specific antibodies began to appear in the yolks of the hens; on day 49, both of the antibodies all reached peak (1:12800), and the immunization was continued and the titers of specific IgY antibodies became stable, detailed in Figure 1.
Figure 1.
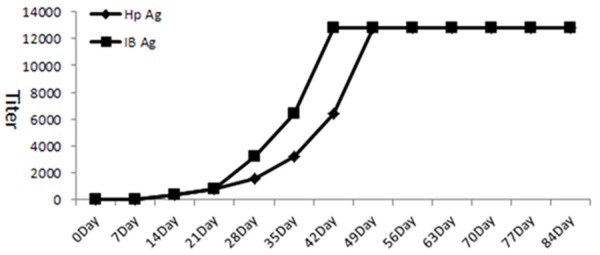
R IgY antibodies were produced by hens which were immunized with recombinant Hp specific antigen IB and Hp natural antigen SS1.
Extraction and purification of IgY antibody
Purity of IgY antibody obtained by Ammonium sulfate precipitation was above 80%, see in Figure 2.
Figure 2.
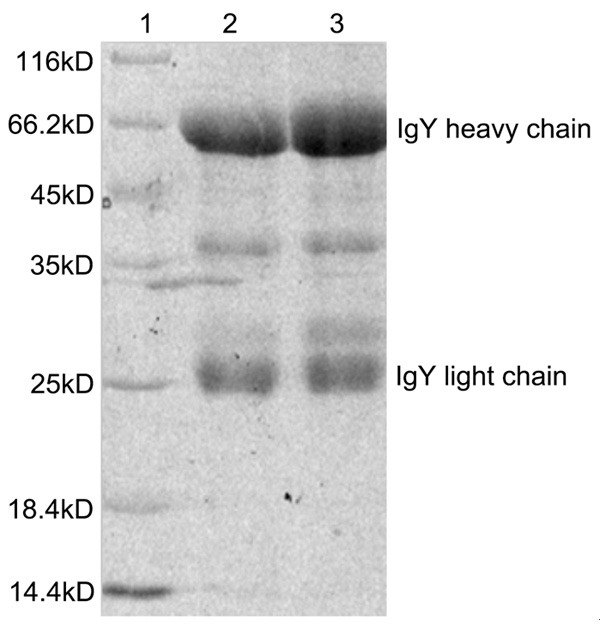
Purification electrophoretogram of specific anti-Hp IgY antibody. 1. Protein marker; 2. Purified SS1-IgY; 3. Purified IB-IgY.
Inhibition on Hp urease activity by IgY in vitro
Color reaction experiment for urea reagent to detect Hp urease activity showed that two specific IgY antibodies all had inhibitory effect on Hp urease activity in vitro. Compared with blank control (PBS and non-specific IgY-CPV), they significantly inhibited urease activity (P < 0.01), however, there was no significant difference in Hp urease activity inhibition between the two antibodies combined with sucralfate respectively, and each of the two antibodies alone (P > 0.05). The data are shown in Figure 3.
Figure 3.
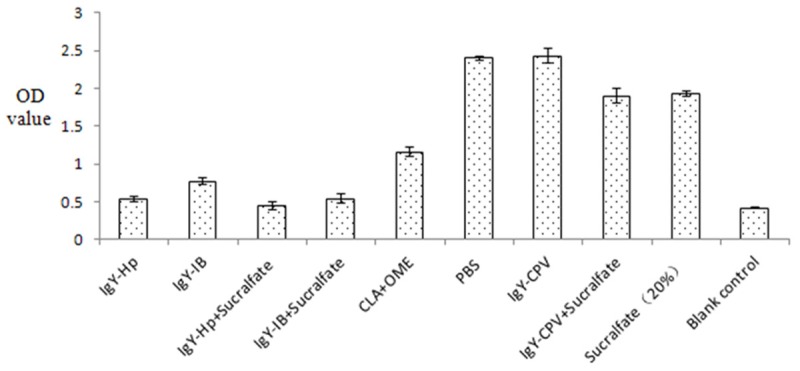
Specific IgY antibody inhibited activity of urease in vitro.
Inhibition on Hp growth by IgY in vitro
Experiments for Hp growth inhibition in vitro showed that 300 μg IgY-Hp had about 6.66 mm Hp inhibition ring and IgY-IB + sucralfate combination had about 3.33 mm Hp inhibition ring, but neither of sucralfate and IgY-IB had bacterial inhibitory effect in vitro. There were significant differences in Hp inhibition ring between specific IgY antibody and blank control PBS as well as between specific IgY antibody and non-specific IgY-CPV antibody (P < 0.01). The detailed bacterial inhibition results in vitro are shown in Figure 4.
Figure 4.
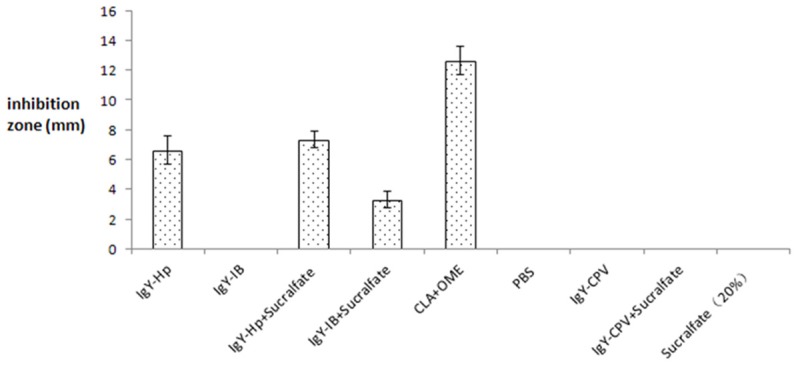
Experiment for Hp growth inhibition by specific IgY antibody in vitro.
Specific inhibition experiment showed that SS1-IgY and IB-IgY had no growth inhibitory effect on Escherichia coli and Staphylococcus aureus.
IgY treatment effect evaluation
Clearance rate of Hp after treating the infected mice
After oral administration of specific IgY in the mice, both of two specific antibodies were found to have clearance effect on Hp infection. Experimental data showed that IgY-SS1 (6 mg) + sucralfate group and IgY-IB (6 mg) + sucralfate group both had 83.3% clearance rate in Hp infected mouse model and the clearance rate in clarithromycin + omeprazole group was 66.7%, with detailed clearance rate data in each group shown in Figure 5. The data showed that Hp infection clearance rates in specific antibody treatment groups were 63.3% higher than sucralfate treatment group and were 21.6% higher than the current popular dual therapy, while control groups (PBS and non-specific IgY-CPV) had not Hp infection clearance effect at all. There were significant differences of Hp infection clearance rates between different treatment groups (P < 0.05).
Figure 5.
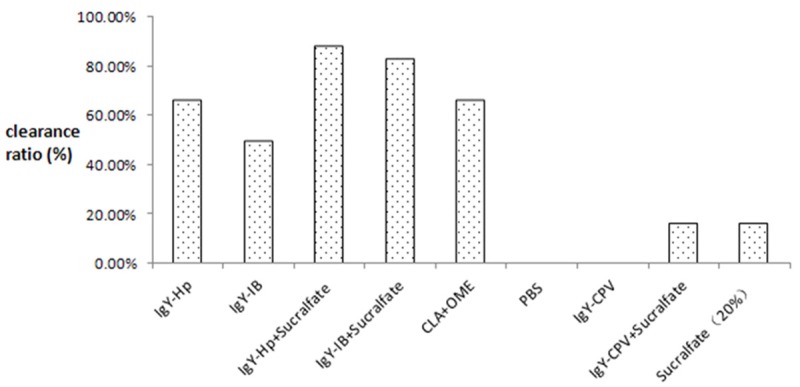
The clearance ratio percentage for H. pylori colonization of different treatment group.
Pathological injury after treating the Hp infected mice
After HE staining of gastric tissue in the mice, pathological observation showed that there were lymphocyte infiltration, necrosis and atrophy in gastric tissue of various mice (n = 6) in non-treatment group (PBS group); immunohistochemistry showed that clusters of Hp were distributed in the gastric tissue. After HE staining of gastric tissue in the mice, pathological observation showed that in gastric tissue in omeprazole + clarithromycin group there were a small amount of lymphocyte and other inflammatory cells; immunohistochemistry showed that Hp were dispersedly distributed in the gastric tissue. While after HE staining of gastric tissue, pathological observation showed no above pathological changes in gastric tissue of IgY-IB + sucralfate treatment group and IgY-SS1 + sucralfate treatment group; immunohistochemistry showed no Hp distribution in the gastric tissue. See in Figures 6 and 7.
Figure 6.
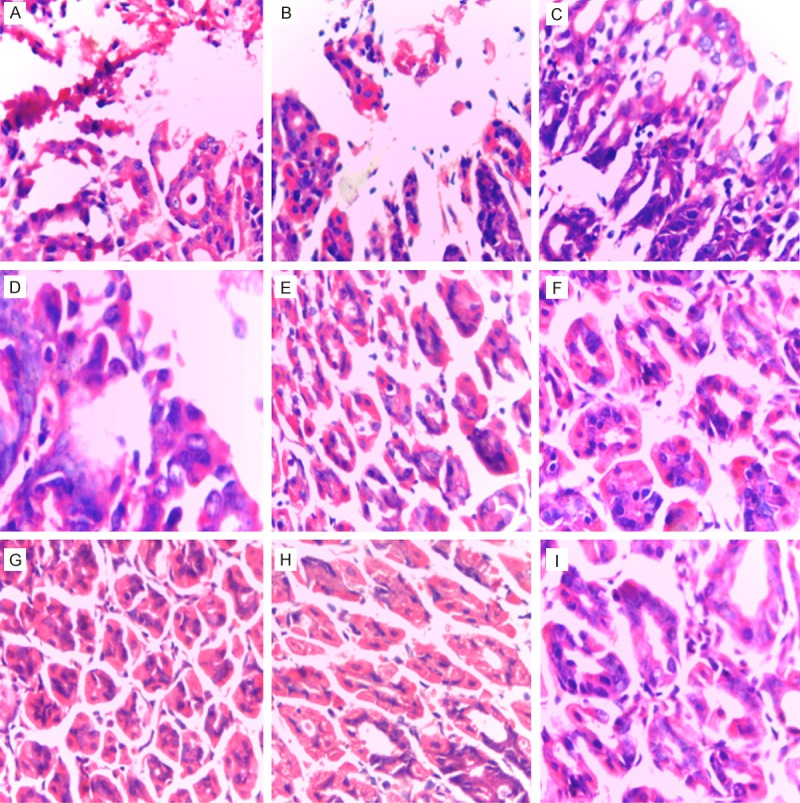
Gastric tissue pathology after specific IgY treated the Hp infected mice. (HE staining, 40 × 10). A: PBS group; B: sucralfate group; C: IgY-CPV group; D: IgY-CPV + sucralfate group; E: IgY-IB group; F: IgY-Hp group; G: omeprazole + clarithromycin group; H: IgY-IB + sucralfate group; I: IgY-Hp + sucralfate group.
Figure 7.
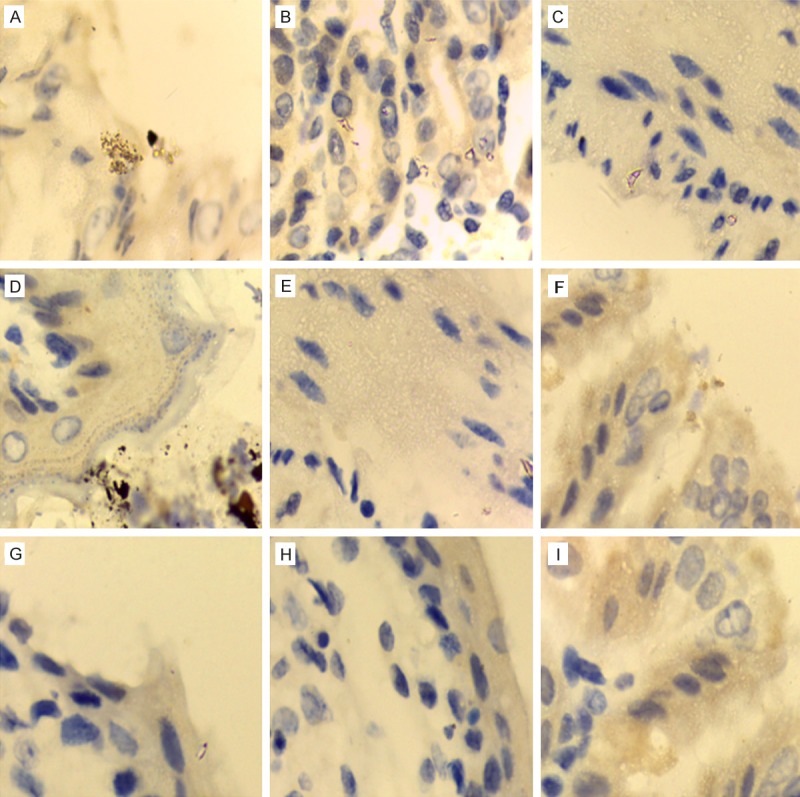
Gastric tissue immunohistochemistry after specific IgY treated the Hp infected mice (100 × 10). A: PBS group; B: sucralfate group; C: IgY-CPV group; D: IgY-CPV + sucralfate group; E: IgY-IB group; F: IgY-Hp group; G: omeprazole + clarithromycin group; H: IgY-IB + sucralfate group; I: IgY-Hp + sucralfate group.
Pathological comprehensive score in each treatment group
Pathological score statistics showed that for the Hp infected mice, gastric pathological injury degrees in IgY-IB + sucralfate treatment group and IgY-SS1 + sucralfate treatment group were the lowest (only 18.8% and 12.5%), nearly 50% lower than non-treatment control groups (PBS group and IgY-CPV group) and about 10% lower than omeprazole and clarithromycin dual therapy group. There was no significant difference in gastric pathological injury reducing effect on the Hp infected mice between sucralfate group and control groups, see in Table 1 and Figure 8.
Table 1.
Pathological injury degree score in different treatment groups
| Group (Samples n = 6) | Evaluation indicator (score) | Pathological injury degree (X/144 × 100%) | Pathological injury improvement degree (1-pathological injury degree) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Inflammation | Hemorrhage | Edema | Atrophy | Necrosis | Bacterial body | |||
| IgY-IB | 18 | 6 | 6 | 0 | 3 | 18 | 35.4% | 64.6% |
| IgY-Hp | 12 | 6 | 9 | 0 | 6 | 9 | 29.2% | 70.8% |
| IgY-IB + sucralfate | 9 | 3 | 6 | 0 | 3 | 6 | 18.8% | 81.2% |
| IgY-Hp + sucralfate | 6 | 0 | 6 | 0 | 3 | 3 | 12.5% | 87.5% |
| Omeprazole + clarithromycin | 9 | 3 | 9 | 3 | 6 | 6 | 25% | 75% |
| PBS | 18 | 12 | 18 | 18 | 18 | 24 | 75% | 25% |
| IgY-CPV | 12 | 12 | 18 | 18 | 18 | 24 | 70.8% | 29.2% |
| IgY-CPV + sucralfate | 12 | 9 | 18 | 18 | 24 | 18 | 68.8% | 31.2% |
| Sucralfate | 18 | 18 | 12 | 18 | 18 | 24 | 75% | 25% |
Notes: Referring to Marlin B. Rogers scoring criteria [9], each of inflammation, hemorrhage, edema, Hp bacterial body colonization, necrosis, atrophy was scored into 1, 2, 3, 4 points according to the lesion severity from mild to severe degree respectively, and the total points were 4 (theoretical total of 6 items was 24 points); in one group, the accumulative total score of all mice with 6 items was 24 × 6 = 144 points; in each group, the actual pathological accumulative score was divided by theoretical total score (144 points) and was multiplied by 100%, and then the gastric pathological injury degree (%) was obtained. The 1-gastric pathological injury degree (%) was the gastric pathological injury improvement degree (%). There was statistically significant difference between the specific antibody treatment groups and the non-treatment group (PBS group) (P < 0.05).
Figure 8.
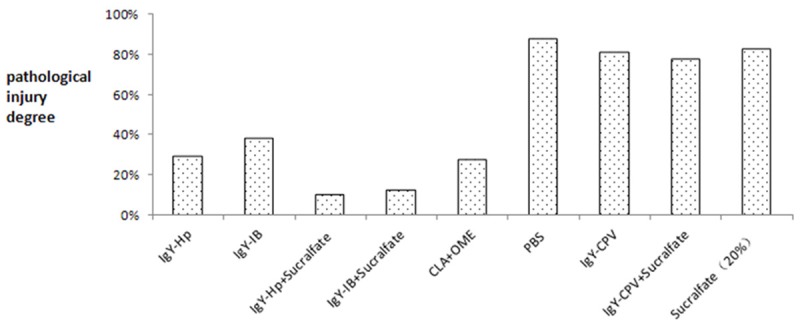
Pathological injury improvement degree in different treatment groups.
Discussion
Hp is the main causative pathogenic bacterium of chronic active gastritis, peptic ulcer, gastric mucosa-associated lymphatic tissue lymphoma and gastric cancer. Antibiotic therapy plays an important role in prevention and treatment of Hp infection, however, the extensive use of broad-spectrum antibiotics causes the body’s microecological flora disturbance and more seriously, it induces a large number of drug-resistant strains [11]. In recent years, studies have found that among several antibiotic drugs recommended for treating Hp, metronidazole resistance rate reaches 60% to 70%, clarithromycin resistance rate reaches 20% to 38% and levofloxacin resistance rate reaches 30% to 38% [12], which seriously affects the treatment efficiency and eradication rate of Hp, resulting in high clinical gastrotherapy recurrence rate and incremental patient burden. In order to completely eradicate Hp infection and reduce the use of antibiotics, specific and targeted drugs of Hp therapy are one of the hotspots of new drug research.
IgY and IgG are very similar in biological function and all have abilities to neutralize viruses, inhibit bacteria and neutralize toxins, but IgY has advantages of acid resistance and alkali resistance, high temperature resistance, high output, easy collection, good stability and no induction of the human body’s autoimmune disease, which are not owned by IgG [3]. Specific IgY has targeted therapy ability and it specifically binds to specific bacteria without killing normal intestinal flora and causing intestinal dysfunction is avoided. In this aspect, antibiotic therapy cannot be compared. Due toits huge potential in treatment of gastric and intestinal infections, IgY is highly emphasized by domestic and foreign scholars. In the pharmaceutical sector, IgY therapeutic drugs for poultry and animals have been already in large-scale use, and IgY used for prevention and treatment of human diseases is under study.
In treatment of Hp infection, many scholars’ studies started from the anti-VacA, CagA, HpaA and other specific antigens, and finally succeeded in preparation of specific IgY which achieved good results in the experiments [13]. This study is about specific IgY antibodies (titer of 1:12800, purity of 80%) obtained from immunizing hens. The experiment in vitro showed that IgY-IB inhibited only urease activity, but IgY-Hp could simultaneously inhibit Hp growth and urease activity; the experiment in mice in vivo showed that single use of IgY-IB alone and IgY-Hp had 50%-70% cure rate, while when IgY-IB or IgY-Hp was combined with sucralfate, the cure rate reached 83.3%, which was 18% higher than the cure rate of omeprazole + clarithromycin dual therapy. The data showed that sucralfate had promoting and synergistic effects for oral specific IgY to clear Hp infection, which was similar to the study results of Zhou, et al [7]. Sucralfate can enhance specific IgY’s resistance at low pH and in the presence of pepsin and better inhibit urease activity and kill Hp, thus sucralfate is a relatively ideal IgY protective agent.
This study has proved that both of the two specific IgY antibodies have good effect on Hp metabolism in vitro and in vivo, and they are expected to become new targeted therapeutic drugs of Hp infection by further study. Currently there are no IgY products for human treatment in the market of China, and the major reason is that at present there are still no effective methods to solve the problem of damage of IgY in the human body by gastric acid, pepsin and other factors. Synergistic and protective effect of IgY and sucralfate proved by this project group provides an effective solution idea for targeted gastrointestinal administration of Hp.
Disclosure of conflict of interest
None.
References
- 1.Salih BA. Helicobacter pylori infection in developing countries: the burden for how long? Saudi J Gastroenterol. 2009;15:201–207. doi: 10.4103/1319-3767.54743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang HF. Drug therapy of Helicobacter pylori infection. Guid Chin Med. 2012;30:62–63. [Google Scholar]
- 3.Mine Y, Kovacs-Nolan J. Chicken egg yolk antibodies as therapeutics in enteric infectious disease: a review. J Med Food. 2002;5:159–169. doi: 10.1089/10966200260398198. [DOI] [PubMed] [Google Scholar]
- 4.Wang BN, Yang Y, Kuang Y, Cao K, Li MY, Li WY. [Construction eukaryotic plasmids of the ureI and ctB-ureI and profile its’ expression in cell] . Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2010;26:764–766. [PubMed] [Google Scholar]
- 5.Zhang ZQ, Li ZT. Extraction, Purification and Antibacterial Activity of Immunoglobulin in Yolk. J Henan Agri Scie. 2012;41:152–154. [Google Scholar]
- 6.Wu YN, Zou MQ, Luo P, Guo G, Wang XQ. Preparation of anti-Helicobacter pylori UreB monoclonal antibody and the detection of the ability of inhibition the urease activity. Immuno J. 2005;21:393–396. [Google Scholar]
- 7.Zhou X, Guo LY, Yang Z, Chen X. Protective effects of sucralfate on anti-H. pylori VacA IgY in vivo and in vitro. African J Microbiol Res. 2010;4:1091–1099. [Google Scholar]
- 8.Liou JF, Chang CW, Tailiu JJ, Yu CK, Lei HY, Chen LR, Tai C. Passive protection effect of chicken egg yolk immunoglobulins on enterovirus 71 infected mice. Vaccine. 2010;28:8189–8196. doi: 10.1016/j.vaccine.2010.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang YH, Park D, Yang G, Lee SH, Bae DK, Kyung J, Kim D, Choi EK, Son JC, Hwang SY, Kim YB. Anti-Helicobacter pylori effects of IgY from egg york of immunized hens. Lab Anim Res. 2012;28:55–60. doi: 10.5625/lar.2012.28.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shao JL, Wu F. Development of detection methods for Helicobacter pylori. Chin J Gastroentero Hepato. 2012;21:691–694. [Google Scholar]
- 11.Rogers AB. Histologic scoring of gastritis and gastric cancer in mouse models. Methods Mol Biol. 2012;921:189–203. doi: 10.1007/978-1-62703-005-2_22. [DOI] [PubMed] [Google Scholar]
- 12.Hu FL. The actuality and expectation on Helicobacter pylori infection treatment. Chin J Gastroentero Hepato. 2012;21:687–690. [Google Scholar]
- 13.Guo LY, Wu JY, Huang W, Yang ZB, Wu M, Ma XJ. Evaluation on protective effects of sucralfate on H. pylori-VacA-IgY in animal experiments. Chin J Microeco. 2010;22:988–992. [Google Scholar]


