Abstract
Survivin, an inhibitor of apoptosis protein family, appears to be involved in tumor progression and angiogenesis. The current study contains rare reports on the transcriptional regulation of survivin expression in retinal neovascularization (NV). The aim of this study was to investigate hypoxia-inducible factor-1α (HIF-1α) and survivin expression in pathologic ocular angiogenesis and to determine their correlation and cellular location. The model of oxygen-induced retinopathy (OIR) was induced in C57BL/6 mice by exposing 75% oxygen from postnatal day 7 (p7) to p12 and then followed by room air. Fluorescence angiography, immunostaining and HE staining were used to assess the visualization of retinal vascularization and the expression of HIF-1α and survivin protein in retina oxygen-induced retinopathy was characterized by clusters of neovascularization and regions of non-perfusion. HIF-1α and survivin were both highly expressed in OIR and a positive correlation existed between HIF-1α and survivin expression. In OIR, there was more HIF1-α protein expression in the inner nuclear layer (INL), the ganglion cell layer (GCL), and more neovascularization breaking through the inner retina and survivin protein was detected in GCL, and more neovascularization breaking through the inner retina. Our study had shown that both HIF1-α and survivin are involved in the retinal neovaccularization. Meanwhile HIF1-α and survivin have differential cellular location in retinal ischemia, and HIF1-α might be a critical transcription factor involved in the regulation of survivin expression.
Keywords: Hypoxia-inducible factor-1α, survivin, retinal neovascularization
Introduction
Pathological angiogenesis or ocular neovascularization (NV) is a hallmark feature of diabetic retinopathy (DR), retinopathy of prematurity (ROP), exudative age-related macular degeneration and leads to the blindness in developed countries [1]. Despite considerable studies has focused on blockading of some cytokine pathways in the process of NV, whereas effectively prevented the formation of NV does not always occur [2]. It is necessary to search for other factors that involved in the angiogenic process. Hypoxia is a critical etiological factor in retinal NV [3]. Adaptive response to changes in oxygen tension is adjusted by a family of transcription factors.
Hypoxia-inducible factor 1-α (HIF-1α) is the main active subunit of HIF-1, which can activated the transcriptions of many genes that are central for cellular function under hypoxic conditions. Recently reports have indicated that the constitutive expression of HIF-1α is significantly associated with increased angiogenesis, aggressive tumor progression and poor prognosis [4]. Survivin, a member of the inhibitor of apoptosis protein (IAP) family, appears to be involved in a wide range of human tumors and fetal tissues, but minimally detected in terminally differentiated tissues. The over expression of survivin in cancer cells is mainly associated with cancer progression, poor prognosis. Survivin gene expression has been shown to induce by hypoxia exposure [5].
According to previous reports, the down-regulation of HIF1-α could extremely decreases the levels of survivin expression in different types of cancer cells [6]. These data hint that HIF1-α cooperate the regulation of survivin expression. There are few studies on inspecting survivin expression in the retina, although retinal ischemia leads to rapid up-regulation of HIF1-α [7], The novelty of the present study is the cellular localization of HIF1-α and survivin to sites inherent in retinal blood vessels and investigate their correlation in a rodent model of ROP.
Materials and methods
Animals
Pregnant female C57BL/6J mice were prepared by The Laboratory Animal Center of China Medical University (Shenyang, China). All animal experiments were performed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were accepted by the Institutional Animal Care and Use Committee of Medical University Ophthalmic Center.
Oxygen-induced retinopathy (OIR)
The OIR model was produced as previously described method [8]. Briefly, neonatal C57BL/6J mice were randomly assigned to two experimental (n = 8, respectively) and control (n = 8) groups. Postnatal day 7 (P7) mice and their mothers were placed hyperemia (75 ± 3% oxygen) for 5 days (from P7 to P 12) and were then abruptly removed and placed in room air (21% oxygen) for an additional 5 days. The incubator temperature was maintained at 23 ± 2°C, and oxygen levels were checked using a portable oxygen analyzer (Model CY-12C, electrochemical analytical Instruments, Ltd, Meicheng, Zhejiang, China). Control litters were kept in room air from birth to P17.
Retinal fluorescence angiography and visualization of retinal vascularization
Randomly chosen two pups from each group were anesthetized at the end of the 17-day experimental period and perfused by the left ventricle injection with 1 ml of phosphate buffered saline (PBS) containing 50mg/mL fluorescein-dextran (2×106 molecular weight; Sigma, St. Louis, MO), as described previously [8]. The animals were then killed and the eyes were enucleated and fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min. The retinas were isolated from the eyecup and fixed with 4% paraformaldehyde for 3 h. The retinas were flat-mounted on a gelatin-coated slide and the vasculature was examined under a fluorescence microscope.
Histology of proliferative retinopathy
After the 17-day experimental period, randomly chosen six pups from each group were sacrificed and the eyes were enucleated, immersed in 4% paraformaldehyde in PBS for at least 24 h, and processed through graded ethanol concentrations for paraffin embedding .sections (6 μm) were placed on poly-L-lysine-coated slides and were collected and incubated overnight at 37°C. Hematoxylin and eosin were performed. Approximately 10 serial sections were cut from each eye. Between two and four sections on each side of the optic nerve, and 30 to 90 μm apart, were counted for neovascularization. Cross-sections that included the optic nerve were excluded. Vascular cell nuclei were considered to be associated with new vessels, and were present on the virtual side of the internal limiting membrane (ILM).
Immunohistochemistry for HIF1-α and survivin
Randomly ten sections were selected from each eye. Immunohistochemical staining was performed for HIF1-α and Survivin using the strep avidin-biotin complex method. deparaffinized and treated with 3% hydrogen peroxide for 10 min and then incubated for 30 min at room temperature with blocking serum The sections were then incubated overnight at 4°C with primary antibodies (rabbit polyclonal antibodies; (Santa Cruz, CA): HIF1-α (1:200 in PBS), Survivin (1:200 in PBS), respectively. All sections were then washed with 0.1 M PBS (three 2-min washes) to reduce nonspecific binding. Subsequently sections were incubated with secondary antibody goat anti-rabbit IgG (1:200 in PBS, Santa Cruz, CA)) for 20 min at room temperature. Sections were visualized by 0.05% diaminobenzidine (DAB). Finally, sections were then stained with Mayer hematoxylin, and negative controls were used as Sections incubated in PBS rather than primary antiserum. Cells positive for HIF1-α and Survivin showed light yellow or dark brown coloration in the cytoplasm. The integrated areas of HIF1-α and Survivin staining were determined by using automatic microscope and image analysis system (LUZEX-F, Japan) and detected the integral optical density (IOD) values as an indicator of HIF1-α and Survivin expression.
Statistical analysis
All data are expressed as means ± SD. Comparisons between groups were performed using student’s t-tests. Analysis of correlation between HIF1-α and Survivin expression was performed by linear correlation. Statistical analyses were performed using SPSS 10.0 software and P values < 0.05 were considered to be statistically significant.
Results
Hyperoxia-induced proliferative retinopathy
The vascular development and neovascularization pattern was easily observed in retinal flat-mounts that were prepared after perfusion with fluorescein-dextran. The retina of control mice had basic matured, large vessels emerging from the optic disc and was uniformly distributed in a radial fashion, with many branches till the periphery of the retina (Figure 1A). The retinal vascular patterns in mice exposed to hyperoxia were characterized by clusters of neovascularization, with regions of non-perfusion, significant expansion and dilatation of the retinal vessels, representing a typical pattern of pathological retinal neovascularization (Figure 1B).
Figure 1.
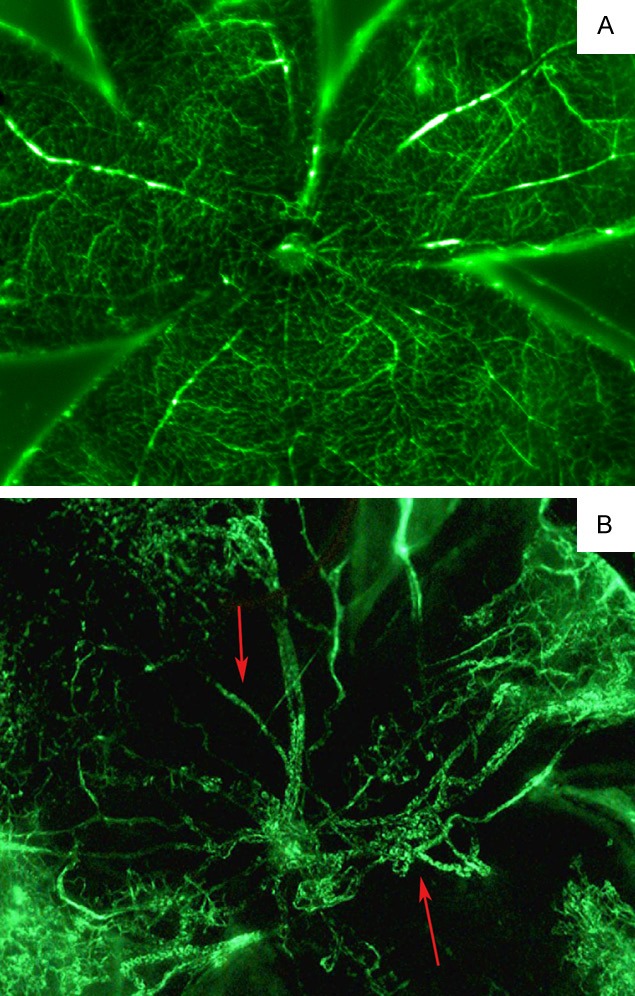
Retinal flat mounts from control and OIR mice at postnatal day 17 (P17). In samples from control mice, intraretinal vascularization was complete at P17 (A), whereas in OIR mice, neovascularization in regions of non-perfusion was observed at P17 (B, arrow).
Assessment of proliferative retinopathy
The degree of hyperoxia-induced neovascularization was quantified in serial paraffin cross-sections by counting the number of vascular cell nuclei on the vitreal side of the internal limiting membrane (ILM). In control mice, there were no nuclei of new vascular endothelial cells breaking through the inner retina than in OIR mice, which had large clusters of blood vessels that were adherent to the ILM (0.27 ± 0.20 vs. 23.38 ± 1.07, P < 0.001) (Figure 2A, 2B).
Figure 2.
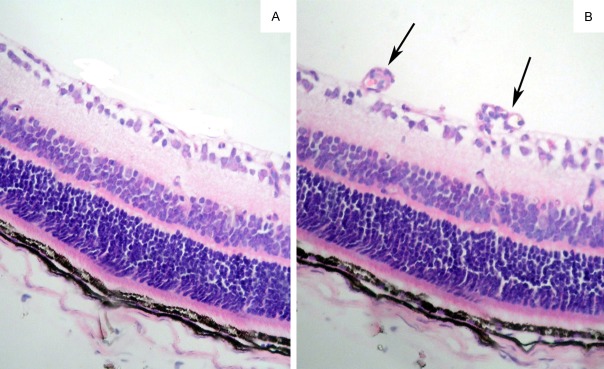
Quantification of proliferative retinopathy in control and OIR mice. In control mice there were few nuclei of new vascular endothelial cells breaking through the inner retinal layer (A). Large clusters of blood vessels were adherent to the internal limiting membrane in ORI mice (B) (arrow). Magnification, ×200.
Immunohistochemical expression of HIF1-α and Survivin
In control mice, there were low levels of HIF1-α protein expression in ganglion cells of the retinal tissue layers and scanty Survivin protein expression in any of the retinal tissue layers. the IOD of HIF1-α and Survivin was 1.81 ± 0.64 vs. 0.47 ± 0.50, respectively (Figure 3A, 3B). In OIR mice, the IOD of HIF1-α and survivin was 4.62 ± 0.19 vs. 3.34 ± 0.25 respectively. There was more HIF1-α protein expression in the inner nuclear layer (INL), the ganglion cell layer (GCL), and more neovascularization breaking through the inner retina (P < 0.05) and Survivin protein was detected in the ganglion cell layer (GCL), and more neovascularization breaking through the inner retina (Figure 3C, 3D) (P < 0.05).
Figure 3.
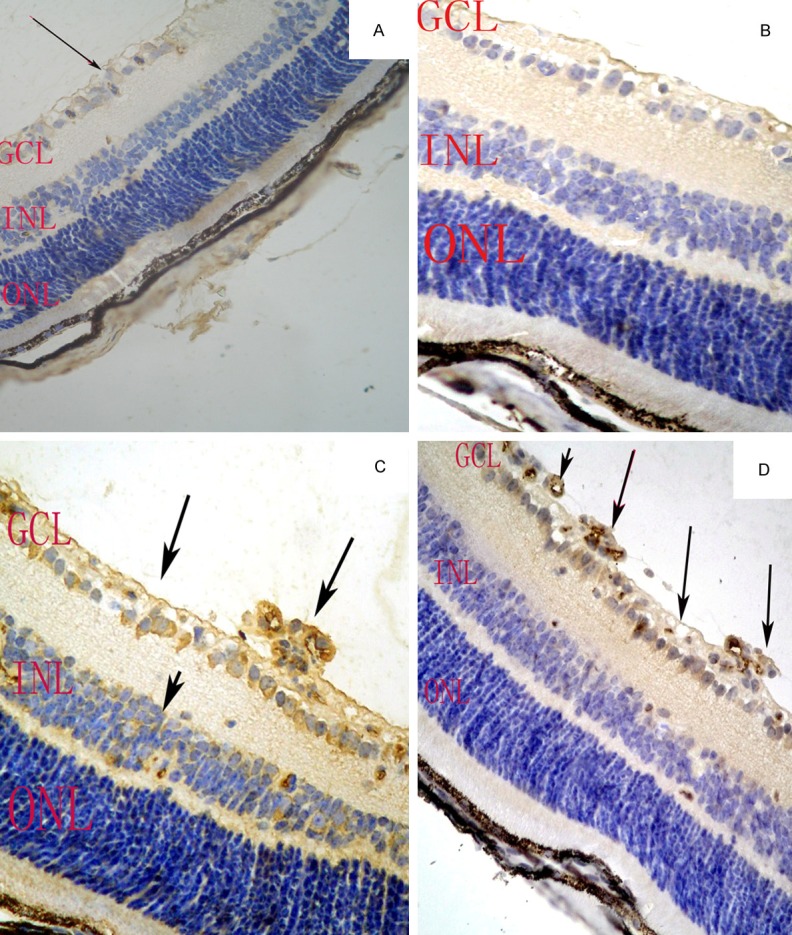
HIF-1α and survivin protein expression in the retinas of control and OIR mice. In control mice, there was low HIF-1α protein expression in GCL of the retina (A, arrow). survivin was scanty expressed in any of the retinal tissue layers (B). In OIR mice, there was HIF-1α protein expression in the inner nuclear layer (INL), the ganglion cell layer (GCL), as well as neovascularization breaking through the inner retinal layer, survivin protein was detected in the ganglion cell layer (GCL) and neovascularization breaking through the inner retinal layer (C, D arrows). Magnification, ×200.
Correlation analysis
There was a significant positive correlation between the protein expression of HIF1-α and survivin (P < 0.01, r = 0.445).
Discussion
Wound healing and many other tissues implicates neovascularization (NV), sprouting of new capillaries from pre-existent vessels, to repair or recover damaged vessels. In the eye, retinal NV can be disordered and commonly results in vitreous hemorrhage and retinal detachment that adversely affect severe visual loss [9]. Hypoxia plays a critical role in retinal vascular development and pathologic retinal neovascularization [10]. Hypoxia-inducible factor-1α (HIF-1α), an important transcription factor of oxygen-regulated genes, mediates an extensive scope of cellular and physiological adaptive responses to changes in oxygen tension. HIF-1 is composed of α and β subunits, HIF-1α is the key active subunit, and its protein expression increases with decreasing cellular oxygen levels, which can activate a large panel of gene products that are involved in glucose metabolism, neovascularization, cell survival and migration and is a stable promoter of tumor growth. In recent studies have found the clinical significance of HIF-1α over expression is usually detected in different types of tumors, related with increased angiogenesis, aggressive tumor growth, and poor prognosis [11]. Consistent with these researches, our data approved that HIF-1α expression was obviously higher in hypoxic retina than that in nonischemic retinas of control mice. Further explicating that HIF-1α may take part in the progression of retinal NV. At the same time, our study also showed that there was low levels of HIF-1α protein in control mice under normoxia, whereas, it was prominent expressed in the INL, GCL and neovascularization breaking through the inner retina under hypoxia. It is consistent with the theory that HIF-1α protein expression is always unregulated by cellular hypoxia despite being invisible in most kinds of normoxic cells due to its rapid degradation by the ubiquitin-proteasome system [12].
Recently, many reports have presented a strong correlation between HIF1-α and Survivin in laryngeal carcinoma and non-small-cell lung cancer [13,14]. Survivin, the smallest protein of the IAP family is overexpressed in a variety of human tumors. This overexpression was also found to be correlated with poor patient prognosis in adenosquamous lung and breast cancer [15-17]. Our study first proved the expression of survivin was positively correlated with HIF-1α in retinal NV. In vivo, we also found that the expressions of HIF-1α and survivin in retina were significantly increased under hypoxic conditions.
Interestingly, the increase in HIF-1α was prominent in the inner nuclear layer and ganglion cells layer. In contrast, survivin was greatly confined to a discrete layer of cells within the ganglion layer. The constant occurrence of the commencement of hypoxia, increased levels of HIF-1α, increased expression of surivin, and the spatial correlation, were consistent with the hypothesis that HIF-1α play a role in the hypoxia mediated increase in survivn in tumor [18].
In conclusion, the present study indicate that both HIF1-α and survivin are activated in distinct correlation with retinal hypoxia but have contrasting cell specificities within the inner retina. The findings propose that HIF1-α and survivin have differential roles in retinal ischemia, and HIF1-α might be a critical transcription factor involved in the regulation of survivin expression.
Acknowledgements
This research was supported by Doctoral Scientific Research Foundation of China (No. 20091115).
Disclosure of conflict of interest
None.
References
- 1.Rahmani B, Tielsch J, Gottsch J, Quigley H, Javitt J, Sommer A. The cause-specific prevalence of visual impairment in an urban population: The Baltimore eye survey. Ophthalmology. 1999;103:1721–1726. doi: 10.1016/s0161-6420(96)30435-1. [DOI] [PubMed] [Google Scholar]
- 2.Oliner JD, Bready J, Nguyen L, Estrada J, Hurh E, Ma H, Pretorius J, Fanslow W, Nork TM, Leedle RA, Kaufman S, Coxon A. AMG 386, a selective angiopoietin 1/2-neutralizing peptibody, inhibits angiogenesis in models of ocularneovascular diseases. Invest Ophthalmol Vis Sci. 2012;53:2170–80. doi: 10.1167/iovs.11-7381. [DOI] [PubMed] [Google Scholar]
- 3.Campochiaro PA. Ocular neovascularization. J Mol Med (Berl) 2013;91:311–21. doi: 10.1007/s00109-013-0993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang N, Dong CR, Jiang R, Tang C, Yang L, Jiang QF, Chen GG, Liu ZM. Overexpression of HIF-1α, metallothionein and SLUG is associated with high TNM stage and lymph node metastasis in papillary thyroid carcinoma. Int J Clin Exp Pathol. 2013;7:322–30. [PMC free article] [PubMed] [Google Scholar]
- 5.Aune G, Stunes AK, Tingulstad S, Salvesen O, Syversen U, Torp SH. The proliferation markers Ki-67/MIB-1, phosphohistone H3, and survivin may contribute in the identification of aggressive ovarian carcinomas. Int J Clin Exp Pathol. 2011;4:444–53. [PMC free article] [PubMed] [Google Scholar]
- 6.Li G, Xie B, Li X, Chen Y, Wang Q, Xu Y, Xu-Welliver M, Zou L. Down-regulation of survivin and hypoxia-inducible factor-1α by β-elemene enhances the radiosensitivity of lung adenocarcinoma xenograft. Cancer Biother Radiopharm. 2012;27:56–64. doi: 10.1089/cbr.2011.1003. [DOI] [PubMed] [Google Scholar]
- 7.Ozaki H, Yu AY, Della N, Ozaki K, Luna JD, Yamada H, Hackett SF, Okamoto N, Zack DJ, Semenza GL, Campochiaro PA. Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci. 1999;40:182–9. [PubMed] [Google Scholar]
- 8.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 9.Talat L, Lightman S, Tomkins-Netzer O. Ischemic retinal vasculitis and its management. J Ophthalmol. 2014;2014:197675. doi: 10.1155/2014/197675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh NK, Hansen DE 3rd, Kundumani-Sridharan V, Rao GN. Both Kdr and Flt1 play a vital role in hypoxia-induced Src-PLD1-PKCγ-cPLA(2) activation and retinal neovascularization. Blood. 2013;121:1911–23. doi: 10.1182/blood-2012-03-419234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu QL, Liu J, Zhu XL, Xu WJ. Expression of nerve growth factor and hypoxia inducible factor-1α and its correlation with angiogenesis in non-small cell lung cancer. J Huazhong Univ Sci Technolog Med Sci. 2014;34:359–62. doi: 10.1007/s11596-014-1283-3. [DOI] [PubMed] [Google Scholar]
- 12.Marioni G, Bertolin A, Giacomelli L. Expression of the apoptosis inhibitor protein survivin in primary laryngeal carcinoma and cervical lymph node metastasis. Anticancer Res. 2006;26:3813–381. [PubMed] [Google Scholar]
- 13.Li DW, Zhou L, Jin B, Xie J, Dong P. Expression and significance of hypoxia-inducible factor-1α and survivin in laryngeal carcinoma tissue and cells. Otolaryngol Head Neck Surg. 2013;48:75–81. doi: 10.1177/0194599812464759. [DOI] [PubMed] [Google Scholar]
- 14.Chen YQ, Zhao CL, Li W. Effect of hypoxia-inducible factor-1alpha on transcription of survivin in non-small cell lung cancer. J Exp Clin Cancer Res. 2009;28:29–37. doi: 10.1186/1756-9966-28-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faversani A, Vaira V, Moro GP, Tosi D, Lopergolo A, Schultz DC, Rivadeneira D, Altieri DC, Bosari S. Survivin family proteins as novel molecular determinants of doxorubicin resistance in organotypic human breast tumors. Breast Cancer Res. 2014;16:R55. doi: 10.1186/bcr3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu S, Zhang Z, Zhang B, Shu Y, Wu H, Huang X, Yu Q, Guo R. Clinical significance of PIK3CA and survivin in primary adenosquamous lung carcinoma. Med Oncol. 2014;31:983–992. doi: 10.1007/s12032-014-0983-7. [DOI] [PubMed] [Google Scholar]
- 17.Damitri TD, Shamsuddin SH, Idris FM, Ariffin WN, Abdul Jalal MI, Jaafar H. Rapamycin and PF4 Induce Apoptosis by Upregulating Bax and Down-Regulating Survivin in MNU-Induced Breast Cancer. Asian Pac J Cancer Prev. 2014;15:3939–44. doi: 10.7314/apjcp.2014.15.9.3939. [DOI] [PubMed] [Google Scholar]
- 18.Bai H, Ge S, Lu J, Qian G, Xu R. Hypoxia inducible factor-1α-mediated activation of survivin in cervical cancer cells. J Obstet Gynaecol Res. 2013;39:555–63. doi: 10.1111/j.1447-0756.2012.01995.x. [DOI] [PubMed] [Google Scholar]


