Abstract
Objective: To investigated the influence of H. pylori on TLR4 and TLR9 in gastric mucosa during gastric carcinogenesis. Methods: Gastric biopsy specimens were taken from 148 patients and divided into five groups, including normal group (n = 10), chronic superficial gastritis group (n = 35), atrophy/intestinal metaplasia group (n = 35), dysplasia group (n = 34) and gastric carcinoma group (n = 34). Immunohistochemistry was used to detect the expression of TLR4 and TLR9. Geimsa staining and rapid urea test were used for determine H. pylori infection. Results: TLR4 was detected in gastric epithelium and monocytes/macrophages in superficial gastritis, atrophy/intestinal metaplasia, dysplasia or carcinoma. TLR9 was mainly accentuated in monocytes/macrophages. TLR4 positive cells in epithelium and in monocytes/macrophages with H. pylori infection were much more than those without H. pylori infection. Similar results were also found in TLR9. When gastric epithelium was accompanied with H. pylori infection, TLR4 was significant higher in superficial gastritis and atrophy/intestinal metaplasia groups compared with dysplasia and carcinoma groups. When gastric epithelium was infected by H. pylori, TLR9 was significant higher in carcinoma group compared with superficial gastritis, atrophy/intestinal metaplasia and dysplasia. TLR4 and TLR9 show significant correlation with the severity of inflammation. Conclusions: H. pylori infection was associated with increased expression of TLR4 and TLR9 in gastric mucosa. In superficial gastritis and atrophy/intestinal metaplasia the inflammation was predominately mediated by TLR4, while in gastric cancer the inflammation was mainly mediated by TLR9.
Keywords: Expression of Toll-like receptor, H. pylori, gastric carcinogenesis
Introduction
Infection of the gastric mucosa by Helicobacter pylori (H. pylori) is one of the most common human bacterial infections. H. pylori cause chronic active gastritis, peptic ulceration, and are the most recognized etiologic risk factor for gastric carcinoma [1]. The majority of H. pylori do not invade the gastric mucosa, while the inflammatory response is triggered by the contact of H. pylori with the gastric epithelium and subsequent secretion of bacterial products into host cells [2]. Toll-like receptors (TLRs) are found to play an important role in the first line of host defense by recognition of microbial components [3].
TLRs are membrane surface receptors consisting of a distinct leucine-rich repeat (LRR) extracellular domain that confers specificity to the receptor, and a conserved toll/interleukin 1 (IL1) receptor (TIR) intracellular domain [4]. These receptors recognize conserved molecular patterns expressed by infectious agents. Through this mechanism, TLRs mediate the production of proinflammatory cytokines and chemokines [5,6]. To date, 13 related TLR genes have been identified and characterized (TLR1-TLR13) [7]. All TLRs activate a common signaling pathway that culminates into the activation of NF-κB and mitogen activated protein kinases (MAPKs) [8]. TLR4 and TLR9 are known to be expressed by gastric epithelial cells in the human stomach [9]. TLR4, the lipopolysaccharide (LPS) receptor, has been proved that its conjunction with CD14 and MD-2 is involved in the response to H. pylori lipopolysaccharides in the stomach [10,11]. The complex transducts signals through MyD88, Toll/IL-1 receptor domain and TRAF6, which promotes transcription of genes involved in immune activation such as NF-кB and MAPKs [12]. TLR9 recognizes unmethylated CpG oligonucleotides that are abundant in bacterial DNA, which triggers alterations in cellular redox balance and the activation of MAPKs and NF-Кb [13,14].
Despite the importance of TLR in the inflammatory activation in response to H. pylori infection, its role in the progression of the lesions associated with gastric carcinogenesis remains largely unknown [15]. In this study, TLR4 and TLR9 expression was evaluated in normal mucus, chronic superficial gastritis, atrophy/intestinal metaplasia, dysplasia and the gastric carcinoma in an attempt to better understand the potential role of these receptors in the process of gastric carcinogenesis.
Material and methods
Participants and histological samples
Data for this study was acquired from Renji hospital, School of Medicine, Shanghai Jiao Tong University. This study consisted of 148 patients (Male: 79, Female: 69, Age: 18-80) who underwent endoscopy in Renji Hospital between May 1st, 2010 and September 30th, 2010. Samples were obtained by endoscopic biopsy. According to the New Sydney System, the gastric biopsy specimens were divided into 5 groups, including normal group (n = 10), chronic superficial gastritis group (n = 35), atrophy/intestinal metaplasia group (n = 35), the dysplasia group (n = 34) and the gastric carcinoma group (n = 34), based on endoscopic and histological results. H. pylori infection was detected by rapid urease test and Geimsa staining. A total of 80 samples were H. pylori positive. 28 were superficial gastritis, 26 were atrophy/intestinal metaplasia, 16 were dysplasia and 10 were gastric carcinoma.
Immunohistochemistry
For immunohistological analyses, tissue specimens were fixed in 10% formalin buffered at pH 7.0 for 24 hours and paraffin embedded. After the deparaffinization and gradient hydration, the tissue slides were submitted to antigen retrieval. The slides were incubated with polyclonal anti-TLR4 antibody (1:500 dilution, Usbiological, Massachusetts, USA) and polyclonal anti-TLR9 antibody (1:1000 dilution, Usbiological, Massachusetts, USA) at 4°C overnight. As a secondary reagent, the bound antibody was detected by applying HRP-conjugated anti-TLR4 secondary antibody (1:100, Maixin Biotech-nology, Fujian Province, China) and anti-TLR9 secondary antibody. (1:50, Usbiological, Massachusetts, USA). Then, the slides were washed and incubated in 3, 3-diaminobenzidine (DAB, Maixin Biotechnology, Fujian, China). Following counterstaining with hematoxylin, the slides were washed, dehydrated and mounted with neutral balsam.
Immunohistochemical evaluation
The slide incubated with PBS instead of primary antibody was taken as the negative control. Excluding the edge, we randomly selected a certain part of the sample and divided it into epithelial area and interstitial area. Five high powered (HP) fields were observed in these two areas. The number of positive cells (n) was counted in both 100 epithelial cells and interstitial cells. A score (p) of 0 to 3 was considered according to a subjective evaluation of the intensity of stained cells [no staining - negative (p = 0); weak positive staining - light yellow (p = 1); moderate positive staining-brown yellow (p = 2); intense positive staining-brownish black (p = 3)]. The mean number (N) of positive cells in each sample was calculated by the following formula: N = (n1 × p1 + n2 × p2 + n3 × p3 + n4 × p4 + n5 × p5)/5. All the samples were evaluated and quantified by two independents pathologists.
Statistical analysis
Data analysis was performed using the SPSS for windows (version 11.0). Categorical variables were presented as mean ± standard deviation. Independent samples test was used for the means of two groups. One-way ANOVA was used to compare the means of more than two groups. Chi-square test was used for categorical variables. Statistical significance was set at P < 0.05.
Results
Distribution of TLR4+ and TLR9+ cells
The expression of TLR4 and TLR9 were not detectable in normal gastric epithelium. TLR4 was detected in gastric epithelium with superficial gastritis, atrophy/intestinal metaplasia, dysplasia or carcinoma. TLR4 could also be found in monocytes/macrophages, which increased significantly compared with in gastric epithelium (41.01 vs. 21.94, P < 0.01). However, TLR9 was rarely detected in gastric epithelium of superficial gastritis, atrophy/intestinal metaplasia or dysplasia, while it could be found in some epithelium of gastric carcinoma. TLR9 was mainly accentuated in monocytes/macrophages.
TLR4 and TLR9 correlated with H. pylori infection
In superficial gastritis group, TLR4 positive cells in epithelium and in monocytes/macrophages with H. pylori infection were much more than those without H. pylori infection. In atrophy/intestinal metaplasia, dysplasia and carcinoma groups, similar results were also found (Figure 1). Thus, these results might indicate that TLR4 plays some role in mediating multiple types or stages of H. pylori-associated gastric lesions.
Figure 1.
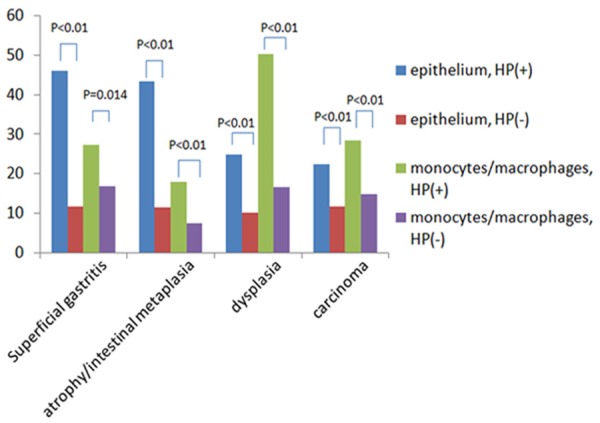
Expression of TLR4 in epithelium and monocytes/macrophages in different lesions.
TLR9 positive cells in monocytes/macrophages with H. pylori infection were much more than those without H. pylori infection in superficial gastritis (27.39 vs. 12.71, P < 0.01), atrophy/intestinal metaplasia (22.26 vs. 9.13, P < 0.01), dysplasia (15.89 vs. 8.56, P < 0.01), and carcinoma groups (35.81 vs. 12.36, P < 0.01). There results also suggested that TLR9 might be involved in H. pylori-associated gastric lesions.
The changed expression of TLR4 in the progression of gastric carcinogenesis
When gastric epithelium was accompanied with H. pylori infection, TLR4 was significant higher in superficial gastritis and atrophy/intestinal metaplasia groups compared with dysplasia and carcinoma groups (Figure 2). On the contrary, there was no significant difference of TLR4 expression among superficial gastritis, atrophy/intestinal metaplasia, dysplasia or carcinoma group without H. pylori infection (15.34 vs. 11.43 vs. 11.09 vs. 11.78, P < 0.05).
Figure 2.
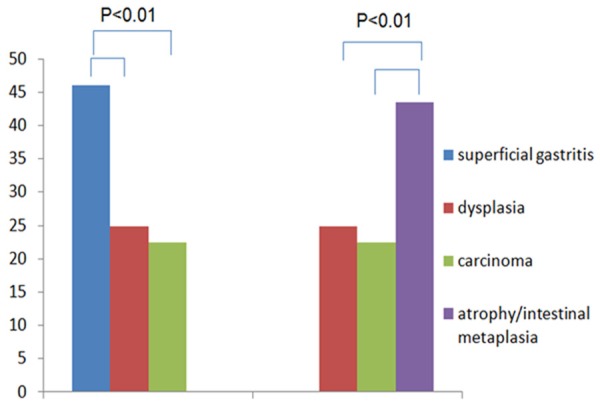
Expression of TLR4 in different gastric lesions with HP infection.
When gastric epithelium was infected by H. pylori, TLR9 was significant higher in carcinoma group compared with superficial gastritis (35.81 vs. 27.39, P = 0.48), atrophy/intestinal metaplasia (35.81 vs. 22.26, P = 0.03) and dysplasia (35.81 vs. 15.89, P < 0.01). When gastric epithelium was not infected by H. pylori, there was no group whose expression of TLR9 was significant higher than one of the other three groups (15.34 vs. 9.1 vs. 8.56 vs. 12.36, P > 0.05).
TLR4 and TLR9 determine the degree of chronic inflammation
The degree of chronic inflammation was classified into four levels (Grades 0, 1, 2 and 3) according to the New Sydney System. In the four groups of our study, the degree of chronic inflammation with TLR4 and TLR9 positive samples was significantly higher compared with TLR4 and TLR9 negative samples. It is indicated that the expression of TLR4 (r = 0.77, P < 0.01) and TLR9 (r = 0.65, P < 0.01) were significantly correlated with the severity of chronic inflammation.
Discussion
Members of TLR family were identified in the mucosa of the entire gastrointestinal tract. They widely diffused in the mature and immature epithelial cells of the stomach, small intestine and colon [10,16-19]. TLR family was also detected in monocytes/macrophages and dendritic cells of the gastrointestinal mucosa [20-22]. H. pylori does not invade the gastric mucosa, which causes inflammation through interaction with gastric epithelia and secretion of proinflammatory cytokines or chemokines [2]. TLRs may play a role in gastrointestinal epithelial innate immune response.
In our present study, TLR4 could be detected in gastric epithelia and monocytes/macrophages of superficial gastritis, atrophy/intestinal metaplasia, dysplasia or carcinoma. Patients with H. pylori infection showed higher TLR4 level. Thus, it suggested that in H. pylori-induced gastritis, the increased level of TLR4 may play some roles in mediating the process of inflammation. Furthermore, TLR4 increased in superficial gastritis and atrophy/intestinal metaplasia compared with dysplasia and carcinoma. TLR4 may mainly participate in the inflammatory pathways in superficial gastritis and atrophy/intestinal metaplasia. The interaction of TLR4 with H. pylori has been demonstrated to confer LPS responsiveness, which leaded to activation of NF-κB and secretion of proinflammatory cytokines, such as IL-8 and TNF [10,23].
In this study, we also found that TLR9 increased in patients with gastric carcinoma. Besides, TLR9 may increase along with the infection of H. pylori. These findings indicated that TLR9 was involved in H. pylori-associated gastric carcinoma. Some researches have shown that H. pylori could activate NF-κB through TLR2/TLR9 pa-thway, which resulted in the expression of COX-2 gene [24]. COX-2 might be involved in the early events of gastric carcinoma occurrence. COX-2 overexpression enhances lymphatic invasion and metastasis in patients with gastric carcinoma [25]. In addition, the activation of NF-κB induced by TLR9 promotes the secretion of proinflammatory cytokines, such as IL-8 and TNF [26]. IL-8 promotes tumor growth, invasion and metastasis by angiogenic potential and directly via the corresponding chemokine receptors CXCR1 and CXCR2 [27].
In conclusion, H. pylori infection was associated with increased expression of TLR4 and TLR9 in gastric mucosa. In superficial gastritis and atrophy/intestinal metaplasia, the inflammation was predominately mediated by TLR4. While in gastric cancer, the inflammation was mainly mediated by TLR9. The expression of TLR4 and TLR9 were significantly correlated with the severity of chronic inflammation. These finding may be helpful for elucidation the mechanisms of inflammation and carcinogenesis caused by H. pylori.
Acknowledgements
This work was supported by grants from the National Science Foundation of China to Zhi Hua Ran (No. 81170362 and No. 81370508).
Disclosure of conflict of interest
None.
References
- 1.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 2.Segal ED, Lange C, Covacci A, Tompkins LS, Falkow S. Induction of host signal transduction pathways by Helicobacter pylori. Proc Natl Acad Sci U S A. 1997;94:7595–7599. doi: 10.1073/pnas.94.14.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 5.Underhill DM, Ozinsky A. Toll-like receptors: key mediators of microbe detection. Curr Opin Immunol. 2002;14:103–110. doi: 10.1016/s0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- 6.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 7.Oldenburg M, Kruger A, Ferstl R, Kaufmann A, Nees G, Sigmund A, Bathke B, Lauterbach H, Suter M, Dreher S, Koedel U, Akira S, Kawai T, Buer J, Wagner H, Bauer S, Hochrein H, Kirschning CJ. TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification. Science. 2012;337:1111–1115. doi: 10.1126/science.1220363. [DOI] [PubMed] [Google Scholar]
- 8.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 9.Schmausser B, Andrulis M, Endrich S, Lee SK, Josenhans C, Muller-Hermelink HK, Eck M. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin Exp Immunol. 2004;136:521–526. doi: 10.1111/j.1365-2249.2004.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishihara S, Rumi MA, Kadowaki Y, Ortega-Cava CF, Yuki T, Yoshino N, Miyaoka Y, Kazumori H, Ishimura N, Amano Y, Kinoshita Y. Essential role of MD-2 in TLR4-dependent signaling during Helicobacter pylori associated gastritis. J Immunol. 2004;173:1406–1416. doi: 10.4049/jimmunol.173.2.1406. [DOI] [PubMed] [Google Scholar]
- 11.Kawahara T, Kuwano Y, Teshima-Kondo S, Kawai T, Nikawa T, Kishi K, Rokutan K. Toll-like receptor 4 regulates gastric pit cell responses to Helicobacter pylori infection. J Med Invest. 2001;48:190–197. [PubMed] [Google Scholar]
- 12.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 13.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 14.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 15.Pimentel-Nunes P, Soares JB, Roncon-Albuquerque R Jr, Dinis-Ribeiro M, Leite-Moreira AF. Toll-like receptors as therapeutic targets in gastrointestinal diseases. Expert Opin Ther Targets. 2010;14:347–368. doi: 10.1517/14728221003642027. [DOI] [PubMed] [Google Scholar]
- 16.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 17.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fusunyan RD, Nanthakumar NN, Baldeon ME, Walker WA. Evidence for an innate immune response in the immature human intestine: toll-like receptors on fetal enterocytes. Pediatr Res. 2001;49:589–593. doi: 10.1203/00006450-200104000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 20.Smith PD, Smythies LE, Mosteller-Barnum M, Sibley DA, Russell MW, Merger M, Sellers MT, Orenstein JM, Shimada T, Graham MF, Ku- bagawa H. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J Immunol. 2001;167:2651–2656. doi: 10.4049/jimmunol.167.5.2651. [DOI] [PubMed] [Google Scholar]
- 21.Otte JM, Rosenberg IM, Podolsky DK. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology. 2003;124:1866–1878. doi: 10.1016/s0016-5085(03)00403-7. [DOI] [PubMed] [Google Scholar]
- 22.Maaser C, Heidemann J, von Eiff C, Lugering A, Spahn TW, Binion DG, Domschke W, Lugering N, Kucharzik T. Human intestinal microvascular endothelial cells express Toll-like receptor 5: a binding partner for bacterial flagellin. J Immunol. 2004;172:5056–5062. doi: 10.4049/jimmunol.172.8.5056. [DOI] [PubMed] [Google Scholar]
- 23.Brandt S, Kwok T, Hartig R, Konig W, Backert S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci U S A. 2005;102:9300–9305. doi: 10.1073/pnas.0409873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang YJ, Wu MS, Lin JT, Sheu BS, Muta T, Inoue H, Chen CC. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-kappaB activation. Mol Pharmacol. 2004;66:1465–1477. doi: 10.1124/mol.104.005199. [DOI] [PubMed] [Google Scholar]
- 25.Murata H, Kawano S, Tsuji S, Tsuji M, Sawaoka H, Kimura Y, Shiozaki H, Hori M. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol. 1999;94:451–455. doi: 10.1111/j.1572-0241.1999.876_e.x. [DOI] [PubMed] [Google Scholar]
- 26.Xue YW, Zhang QF, Zhu ZB, Wang Q, Fu SB. Expression of cyclooxygenase-2 and clinico pathologic features in human gastric adenocarcinoma. World J Gastroenterol. 2003;9:250–253. doi: 10.3748/wjg.v9.i2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitadai Y, Takahashi Y, Haruma K, Naka K, Sumii K, Yokozaki H, Yasui W, Mukaida N, Ohmoto Y, Kajiyama G, Fidler IJ, Tahara E. Transfection of interleukin-8 increases angiogenesis and tumorigenesis of human gastric carcinoma cells in nude mice. Br J Cancer. 1999;81:647–653. doi: 10.1038/sj.bjc.6690742. [DOI] [PMC free article] [PubMed] [Google Scholar]


