Abstract
The epithelial-mesenchymal transition (EMT) is an essential step in invasion and metastasis of human cancers. Identification of EMT status would help us to properly understand the mechanism of cancer metastasis and progression. In the present study, tissue microarray and immunohistochemical staining of two important markers, E-cadherin and Vimentin, were used to characterize the EMT status in human esophageal cancer. We selected the appropriate cut-off values of expression levels of E-cadherin and Vimentin, and found 63 out of 105 cases of esophageal cancers underwent EMT. And we also found that in the subgroup with (T3 + T4), the ratio of patients undergoing EMT was significantly higher than that in the subgroup with (T1 + T2) (P = 0.0097), and in the subgroup with metastasis, the ratio of patients undergoing EMT was significantly higher than that in the subgroup with no metastasis (P = 0.0253). The log-rank survival analysis showed that the overall survival rate of the patients undergoing EMT was significantly poorer than that of the patients with wide type status (P = 0.0278, HR = 2.470, 95% CI: 1.971~2.970). In the COX model analysis, we also found that the EMT status of the esophageal cancer patients could be used as an independent risk factor for the prediction of prognosis of this malignancy (P = 0.026, HR = 2.306, 95% CI: 1.103~4.824). Thus, our present study successfully established a method by using tissue microarray and the markers, E-cadherin and Vimentin, to conveniently and properly identify the EMT status in human esophageal cancer, and revealed that the EMT status significantly associated with invasion, metastasis and prognosis in this malignancy.
Keywords: Epithelial-to-mesenchymal transition, esophageal cancer, tissue microarray, immunohistochemistry
Introduction
The epithelial-mesenchymal transition (EMT) roles importantly in embryonic development, furthermore, this physio-pathological process is also needed in wound healing, tissue regeneration, organ fibrosis, and cancer progression [1-4]. EMT process could confer more mesenchymal fibroblast-like and more motile on the polarized epithelial cells, thus potentially enable and promote the epithelial cancer cells to detach from the primary tumor location, traverse the basement membrane, and finally leads to invasion and metastasis of cancer cells [5]. It has been demonstrated that a series of distinct molecular processes are involved in the initiation, progression and completion of the EMT, such as the activation of some specific transcription factors and the change of surface markers of some specific proteins [6].
The esophageal cancer is one of the most common types of human cancer, it ranks sixth among all cancers in mortality and it is one of the deadliest cancers worldwide because of its extremely aggressive characteristics and poor postoperative prognosis [7,8]. According to the histological classification, the squamous cell carcinoma and the adenocarcinoma are the two main types of human esophageal cancer, and the squamous cell carcinoma represents 90% of all esophageal cancer cases worldwide [9,10]. Despite the development of therapeutic options such as surgery, chemotherapy, and radiotherapy, it’s still very important for us to identify novel biomarkers, and to benefit the early detection and the prognostic evaluation of esophageal cancer patients.
E-cadherin is highly expressed in epithelial cells, and its expression is decreased during EMT in embryonic development, tissue fibrosis, and cancer, and loss of E-cadherin function could promote EMT [1]. E-cadherin has been shown to function as a tumor suppressor independent of its function in an adhesion complex by sequestering β-catenin away from the nucleus [11]. Vimentin is an important surface marker of the cell type from mesenchymal origin, and it has been found that this protein could also be up-regulated in some migratory epithelial cells, which contributes to the migratory and the invasive process of metastatic epithelial cancer cells [12]. In the present study, we tried to use two surface bio-markers, E-cadherin and Vimentin to classify the EMT status of esophageal cancer patients in a tissue microarray. And we found that EMT status in human esophageal was significantly associated with tumor invasion, metastasis and patients’ postoperative prognoses. Therefore, the EMT issue and its status as well, could be recognized as an essential marker to predict metastasis and prognosis in human esophageal cancer.
Materials and methods
Patients and tissue microarray
Formalin-fixed, paraffin-embedded esophageal cancer tissue samples were collected from 105 patients who underwent surgical resection between February 2005 and May 2006 in our hospital (82 men and 23 women; median age at diagnosis was 59 years). In addition, 5 normal tissues from the non-malignant portion of esophagus were resected from surgery and used as controls. No patients received pre-operative chemotherapy or radiotherapy. All tumor tissues were confirmed as the esophageal squamous cell carcinoma by using hematoxylin and eosin (H&E) staining after surgical resection. The clinical parameters of the patients are shown in Table 1. Among all the patients, the survival data of 56 patients were available. Then, the paraffin blocks of 105 cases of esophageal cancer tissues were used in the construction of tissue microarray. In brief, the H&E-stained standard slides were reviewed from each section of esophageal cancer tissues, and a representative tumor region and the corresponding formalin-fixed paraffin-embedded tissue block were selected for use in the tissue microarray. The viable invasive carcinoma tissue (epithelial cells) and surrounding tumor stroma from central parts within the tumors were carefully selected and marked on the H&E slides, and then were sampled for the tissue microarray block which was assembled using a tissue-arraying instrument (Beecher Instruments, Silver Springs, MD, USA).
Table 1.
Correlation between patients’ clinical parameters and EMT transition
| Clinical parameters | Cases | EMT status | P-value | ||
|---|---|---|---|---|---|
|
| |||||
| Wide type | Undergoing EMT | χ2 | |||
| Gender | |||||
| Male | 82 | 31 (37.8%) | 51 (62.2%) | 0.7516 | 0.3860 |
| Female | 23 | 11 (47.8%) | 12 (52.2%) | ||
| Age (years) | |||||
| < 60 | 54 | 19 (35.2%) | 35 (64.8%) | 1.074 | 0.3001 |
| ≥ 60 | 51 | 23 (45.1%) | 28 (54.9%) | ||
| Tumor size (cm) | |||||
| < 3.5 | 37 | 14 (37.8%) | 23 (62.2%) | 0.1113 | 0.7387 |
| ≥ 3.5 | 68 | 28 (41.2%) | 40 (58.8%) | ||
| Tumor invasion depth (T) | |||||
| T1 + T2 | 37 | 21 (56.8%) | 16 (43.2%) | 6.684 | 0.0097 |
| T3 + T4 | 68 | 21 (30.9%) | 47 (69.1%) | ||
| Nodal metastasis (N) | |||||
| Yes | 35 | 12 (34.3%) | 23 (65.7%) | 0.7143 | 0.3980 |
| No | 70 | 30 (42.9%) | 40 (57.1%) | ||
| Distant metastasis (M) | |||||
| Yes | 7 | 0 (0.0%) | 7 (100.0%) | 5.000 | 0.0253 |
| No | 78 | 42 (53.9%) | 56 (46.1%) | ||
| TNM stage | |||||
| I | 6 | 4 (66.7%) | 2 (33.3%) | 2.955 | 0.0856 |
| II | 60 | 24 (40.0%) | 36 (60.0%) | ||
| III | 32 | 14 (43.8%) | 18 (56.2%) | ||
| IV | 7 | 0 (0.0%) | 7 (100.0%) | ||
Values in bold signify P < 0.05.
Antibodies
Mouse anti-human E-cadherin monoclonal antibody and mouse anti-human Vimentin monoclonal antibody (both are ready to use) were was purchased from Maxim Biotechnology Limited Corporation (Fuzhou, China). The horseradish peroxidase (HRP)-labeled goat anti-mouse/rabbit secondary antibody used in immunohistochemical staining was purchased from Dako (Glostrup, Denmark).
Immunohistochemistry
The paraffin-embedded esophageal cancer tissue microarray block was cut into serial 3-µm-thick sections, and then the sections dewaxed in xylene, rehydrated and graded ethanol solutions. Antigen was retrieved by heating the tissue sections at 100°C for 30 min under citrate solution (10 mmol/L, PH 6.0). Sections were cooled and immersed in presence of 0.3% hydrogen peroxide for 15 min to block endogenous peroxidase activity, and then rinsed in PBS for 5 min, blocked with 3% BSA at room temperature for 20 min, and incubated with primary polyclonal antibodies, mouse anti-human E-cadherin monoclonal antibody and mouse anti-human Vimentin monoclonal antibody, respectively, at 4°C overnight. A negative control was performed by omitting the primary antibodies. The sections were then incubated with HRP-labeled goat anti mouse/rabbit secondary antibody. Diaminobenzene was used as the chromogen and hematoxylin as the nuclear counterstain. The sections were dehydrated, cleared and mounted.
Evaluation of immunohistochemical staining
All slides were examined independently by two senior pathologists who were not informed of patients’ clinical parameters, and the immunostaining intensity of E-cadherin was assessed according to the H-score method described by our previous reports [13,14]: H-score = (% tumor cells unstained x0) + (% tumor cells stained weak x1) + (% tumor cells stained moderate x2) + (% tumor cells stained strong x3). The H-scores ranged from 0 (100% negative tumor cells) to 300 (100% strong staining tumor cells). For the evaluation of Vimentin staining, the rate of Vimentin positive cancer cells in all cancer cells was calculated and the positive rate was recorded. Results from the two pathologists were averaged and used in the statistical analysis.
Statistical analyses
Statistical analysis was performed using the GraphPad Prism 5.0 software package (GraphPad Software, Inc., San Diego, USA). Paired Student’s t-test, the Wilcoxon signed rank test or the survival analysis were used where appropriate. The Cox model was analyzed by the SPSS13.0 software package. A P-value of < 0.05 was deemed significant.
Results
Immunohistochemical staining of EMT makers, E-cadherin and Vimentin, in human esophageal cancer tissue microarray
In the present study, we defined the cancer patients with H-score of E-cadherin > 60 plus the rate of positive vimentin staining cells as the group of undergoing EMT, and other patients were defined as the group of wide type. As shown in Figure 1, in the esophageal cancer tissue from wide type group, we could found high level of E-cadherin expression and low level vimentin expression, while in the esophageal cancer tissue from the group of undergoing EMT, we could found low level E-cadherin expression and high level expression of vimentin. In contrast to esophageal cancer tissues, we also could found that high level of E-cadherin and negative vimentin expression in normal esophageal tissues. Thus our results from tissue microarray and immunohistochemistry demonstrated that normal expression level of E-cadherin as well as vimentin could be found in normal esophageal tissues, however, loss of E-cadherin expression and increased vimentin expression could be found in some cases of esophageal cancer tissues, suggesting those cases were undergoing EMT process.
Figure 1.
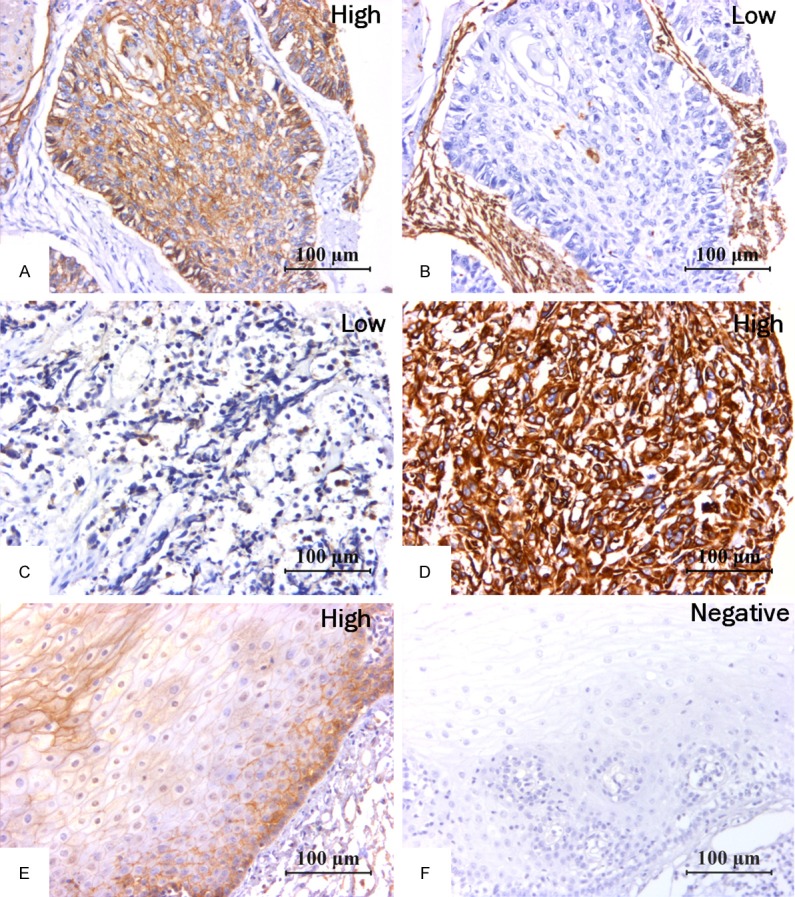
Immunohistochemical staining of EMT biomarkers in human esophageal tissues. In cancer tissues, we could found high E-cadherin expression (A) and low Vimentin (B) expression in esophageal cancer tissues from wide type group, and low E-cadherin expression (C) and high Vimentin expression (D) in esophageal cancer tissues from undergoing EMT group. In contrast to esophageal cancer tissues, we can found that positive E-cadherin expression (E) and negative Vimentin expression (F) were present in adjacent normal tissues from the esophagus.
Clinical association and prognostic value of EMT in human esophageal cancer
As shown in Table 1, we found that in the subgroup with (T3 + T4), the ratio of patients undergoing EMT was significantly higher than that in the subgroup with (T1 + T2) (P = 0.0097). Moreover, we also found that in the subgroup with metastasis, the ratio of patients undergoing EMT was significantly higher than that in the subgroup with no metastasis (P = 0.0253). The log-rank survival analysis also showed that the overall survival rate of the patients undergoing EMT was significantly poorer than that of the patients with wide type status (Figure 2, P = 0.0278, HR = 2.470, 95% CI: 1.971~2.970). In the COX model analysis, we also found that the EMT status of the esophageal cancer patients could be used as an independent risk factor for the prediction of prognosis of this malignancy (P = 0.026, HR = 2.306, 95% CI: 1.103~4.824, Table 2).
Figure 2.
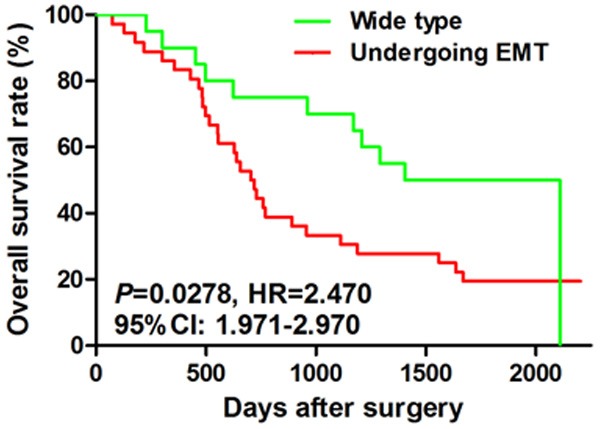
Prognostic value of EMT in human esophageal cancer. The log-rank survival analysis also showed that the overall survival rate of the patients undergoing EMT was significantly poorer than that of the patients with wide type status (Figure 2, P = 0.0278, HR = 2.470, 95% CI: 1.971~2.970).
Table 2.
Prognostic factors in Cox’s proportional hazards model
| Clinical parameters | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | ||
| Gender | |||||||
| Male/Female | 1.111 | 0.437-1.415 | 0.768 | 0.856 | 0.407-1.803 | 0.683 | |
| Age (years) | |||||||
| ≥ 60/< 60 | 0.744 | 0.395-1.399 | 0.358 | 0.873 | 0.454-1.680 | 0.685 | |
| Tumor size (cm) | |||||||
| ≥ 3.5/< 3.5 | 1.125 | 0.569-2.156 | 0.723 | 1.098 | 0.546-2.209 | 0.793 | |
| TNM Stage | |||||||
| SIII+IV/SI+II | 2.922 | 1.417-6.025 | 0.004 | 2.599 | 1.336-5.055 | 0.005 | |
| EMT status | |||||||
| EMT/Wide type | 2.470 | 1.971-2.970 | 0.028 | 2.306 | 1.103-4.824 | 0.026 | |
Values in bold signify P < 0.05.
Discussion
It has been suggested that the frequent recurrence and metastasis of human esophageal cancer leading to the failure of treatments, was due to the invasive and metastatic nature of this malignancy [15], which needs us to reveal the molecular and cellular mechanism of this physio-pathological process. As we know, the EMT is an initial step toward cancer invasion and metastasis, and is also an identified phenotype of cancer invasion and metastasis in human cancers [16,17]. In addition, the cells underwent epithelial-mesenchymal transition also showed some phenotypes of stem cells. Mani et al. [18] reported that the induction of an EMT in immortalized human mammary epithelial cells contributed to the acquisition of mesenchymal traits and in the expression of stem-cell markers. Thus it’s necessary for us to investigate the clinical applications and the detailed mechanism of EMT in human cancers. Our present study combined the two important protein markers, E-cadherin and vimentin, to identify the EMT in human esophageal cancer, and our results showed that, the EMT status in human esophageal cancer, was significantly associated with tumor invasion, metastasis and patients’ postoperative prognoses.
E-cadherin, an important regulator of the differentiated epithelial phenotype, could suppress the tumor invasion and metastasis, and maintain the stable cell-to-cell adhesion, and the polarity of the epithelial cells [1]. The transcription factor Snail, a member of the Snail super-family of zinc-finger transcription, regulates E-cadherin expression in various physio-pathological processes [19]. Loss of E-cadherin expression has been demonstrated to correlate with different clinical parameters, such as invasion, metastasis, tumor stage, patients’ postoperative prognoses in a series of human cancers, for example, lung, breast, uterine cervix, gastric carcinomas, and ovary [12,20-24]. E-cadherin expression level in human ovarian cancer also suggested the response to first line platinum-based chemotherapy and platinum sensitivity [24]. Recently, many studies also focused on the epigenetic changes of E-cadherin, and correlated it with cancer progression and clinical significance, Wu et al. demonstrated that methylation in the promoters of E-cadherin was more common in than that in normal skin tissue, and was correlated with differentiation, lymph node metastasis, and clinical stage, implicating that aberrant methylation in promoters of E-cadherin may promote occurrence and progression of skin squamous cell carcinoma [25].
Vimentin, an important mesenchymal marker, usually expressed by fibroblasts, endothelial cells, and some cells from the hematopoietic lineages, also could be found in some epithelial cancer cells when they are involved in EMT [26,27]. Vimentin is firstly recognized as a controversial marker of EMT, because in adult epithelial cells, it could be transiently expressed in response to various insults [1]. But it could be used as an EMT marker in many cancers to identify invasiveness and metastasis, namely type 3 EMT, due its up-regulation in some epithelial cancer cells, and positively correlates with invasion and metastasis [1]. Zhao et al. [28] showed that positive staining of vimentin could be found in 30 of all 121 cases of bladder cancer, and its expression level significantly associated with tumor grade, recurrence and patient’s prognosis. It has been suggested that, the combination of the two markers, E-cadherin and vimentin, could be reasonably to identify the EMT in many human cancers. For example, by using E-cadherin and vimentin, Yamada et al. identified EMT status in human pancreatic cancer, and found that EMT status is an important prognostic factor for pancreatic cancer and associated with portal vein invasion and lymph node metastasis [29]. Thus, our present study successfully established a method by using tissue microarray and the markers, E-cadherin and vimentin, to conveniently and properly identify the EMT status in human esophageal cancer, and revealed that the EMT status significantly associated with invasion, metastasis and prognosis in this malignancy.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No. 81301960, 81171653), Natural Science Foundation of Jiangsu Province (BK2011246, BK2011247), the project of Six Batch of Major Talent Summit (BRA2010037), Society Development Plans, Department of Science and Technology Changzhou (CJ20112020, CZ20110024, CS20102020), and the Innovative Talents Training Project of Changzhou Health Bureau.
Disclosure of conflict of interest
None.
References
- 1.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–37. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalluri R, Neilson EG. Epithelial-mesen-chymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–84. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 4.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 5.Pohl M, Radacz Y, Pawlik N, Schoeneck A, Baldus SE, Munding J, Schmiegel W, Schwarte-Waldhoff I, Reinacher-Schick A. SMAD4 mediates mesenchymal-epithelial reversion in SW480 colon carcinoma cells. Anticancer Res. 2010;30:2603–13. [PubMed] [Google Scholar]
- 6.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falk GW. Risk factors for esophageal cancer development. Surg Oncol Clin N Am. 2009;18:469–85. doi: 10.1016/j.soc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Luo G, Tan Y, Wei J, Wu C, Zheng L, Zhang X, Xu N. Immunolocalisation of tissue factor in esophageal cancer is correlated with intratumoral angiogenesis and prognosis of the patient. Acta Histochem. 2010;112:233–9. doi: 10.1016/j.acthis.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 10.Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan M, Shan BE, Lu BF, Zhang XG. B7-H4 expression associates with cancer progression and predicts patient’s survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60:1047–55. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowling VH, Cole MD. E-cadherin repression contributes to c-Myc-induced epithelial cell transformation. Oncogene. 2007;26:3582–6. doi: 10.1038/sj.onc.1210132. [DOI] [PubMed] [Google Scholar]
- 12.Blechschmidt K, Sassen S, Schmalfeldt B, Schuster T, Hofler H, Becker KF. The E-cadherin repressor Snail is associated with lower overall survival of ovarian cancer patients. Br J Cancer. 2008;98:489–95. doi: 10.1038/sj.bjc.6604115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Di D, Luo G, Zheng L, Tan Y, Zhang X, Xu N. Immunochemical staining of MT2-MMP correlates positively to angiogenesis of human esophageal cancer. Anticancer Res. 2010;30:4363–8. [PubMed] [Google Scholar]
- 14.Chen L, Sun J, Wu H, Zhou S, Tan Y, Tan M, Shan B, Lu B, Zhang X. B7-H4 expression associates with cancer progression and predicts patient’s survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60:1047–55. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong B, Wang T, Lun X, Zhang J, Zheng S, Yang W, Li W, Xiang AP, Chen Z. Contribution of nestin positive esophageal squamous cancer cells on malignant proliferation, apoptosis, and poor prognosis. Cancer Cell Int. 2014;14:57. doi: 10.1186/1475-2867-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta. 2009;1796:293–308. doi: 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–66. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 20.Prasad CP, Rath G, Mathur S, Bhatnagar D, Parshad R, Ralhan R. Expression analysis of E-cadherin, Slug and GSK3beta in invasive ductal carcinoma of breast. BMC Cancer. 2009;9:325. doi: 10.1186/1471-2407-9-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohta H, Aoyagi K, Fukaya M, Danjoh I, Ohta A, Isohata N, Saeki N, Taniguchi H, Sakamoto H, Shimoda T, Tani T, Yoshida T, Sasaki H. Cross talk between hedgehog and epithelial-mesenchymal transition pathways in gastric pit cells and in diffuse-type gastric cancers. Br J Cancer. 2009;100:389–98. doi: 10.1038/sj.bjc.6604846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomaskovic-Crook E, Thompson EW, Thiery JP. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res. 2009;11:213. doi: 10.1186/bcr2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miura N, Yano T, Shoji F, Kawano D, Takenaka T, Ito K, Morodomi Y, Yoshino I, Maehara Y. Clinicopathological significance of Sip1-associated epithelial mesenchymal transition in non-small cell lung cancer progression. Anticancer Res. 2009;29:4099–106. [PubMed] [Google Scholar]
- 24.Mise BP, Telesmanic VD, Tomic S, Sundov D, Capkun V, Vrdoljak E. Correlation Between E-cadherin Immunoexpression and Efficacy of First Line Platinum-Based Chemotherapy in Advanced High Grade Serous Ovarian Cancer. Pathol Oncol Res. 2014 doi: 10.1007/s12253-014-9827-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Wu J, Zhang JR, Qin J. Clinical significance of methylation of E-cadherin and p14ARF gene promoters in skin squamous cell carcinoma tissues. Int J Clin Exp Med. 2014;7:1808–12. [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson EW, Torri J, Sabol M, Sommers CL, Byers S, Valverius EM, Martin GR, Lippman ME, Stampfer MR, Dickson RB. Oncogene-induced basement membrane invasiveness in human mammary epithelial cells. Clin Exp Metastasis. 1994;12:181–94. doi: 10.1007/BF01753886. [DOI] [PubMed] [Google Scholar]
- 27.Franke WW, Schmid E, Osborn M, Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978;75:5034–8. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J, Dong D, Sun L, Zhang G. Prognostic significance of the epithelial-tomesenchymal transition markers e-cadherin, vimentin and twist in bladder cancer. Int Braz J Urol. 2014;40:179–89. doi: 10.1590/S1677-5538.IBJU.2014.02.07. [DOI] [PubMed] [Google Scholar]
- 29.Yamada S, Fuchs BC, Fujii T, Shimoyama Y, Sugimoto H, Nomoto S, Takeda S, Tanabe KK, Kodera Y, Nakao A. Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer. Surgery. 2013;154:946–54. doi: 10.1016/j.surg.2013.05.004. [DOI] [PubMed] [Google Scholar]


