Abstract
Primary central nervous system (CNS) germ cell tumors (GCTs) are a rare heterogeneous group of lesions, which the clinicopathological features have a marked degree of heterogeneity comparing with that of gonadal GCTs. Accurately diagnosing CNS GCTs might be extremely difficult and requires immunohistochemical verification. This study was to investigate the biological feature of CNS GCTs and diagnostic value of immunohistochemical markers OCT3/4, C-kit, PLAP, and CD30 in CNS GCTs. A retrospective study was performed on 34 patients with CNS germ cell tumors between 1990 and 2014. 34 CNS GCTs account for 9.2% of all primary CNS neoplasms. The sellar region (35.3%) and pineal gland (17.6%) were the most common sites of intracranial GCTs. Hydrocephalus (82.4%) and diplopia (46.9%) were the two most common clinical presentations. The most common histological subtypes were germinoma (67.6%). PLAP, c-kit, OCT3/4 were highly expressed in gernimomas. CD30 and CK AE1/3 stainings were positive in embryonal carcinoma. Yolk sac tumor component showed positive staining for AFP and CK AE1/3. β-HCG staining was positive in choriocarcinoma and STGC. Patients with mature teratomas and germinomas had a better prognosis (a 5-year survival rate) than those with embryonal carcinoma and choriocarcinoma (a 5-year survival rates were 0). Our finding suggest that the incidences of primary CNS GCTs are higher in South China than in the West, but mixed GCTs are uncommon in our study. The judicious use of a panel of selected markers is helpful in diagnosing and predicting the prognosis for CNS GCTs.
Keywords: Central nervous system, germ cell tumors, C-kit, PLAP, OCT3/4, CD30
Introduction
Primary central nervous system (CNS) germ cell tumors (GCTs) are a rare heterogeneous group of lesions in the CNS. CNS GCTs occur predominantly in children and adolescents with approximately 90% of the cases before the age of 20 years. The peak incidence of CNS-GCT is between 10 and 20 years of age. CNS GCTs are more common in male. Histologically, these tumors are very similar to those in gonadal and other extragonadal sites. CNS GCTs most frequently locate in the midline sites of body (pineal, suprasella region, thalamus, basal ganglia, et al). Pathological classifications of CNS GCTs by the WHO contain germinoma, teratoma, yolk sac tumor, embryonal carcinoma, choriocarcinoma and mixed germ cell tumor. CNS GCTs can be divided into two broad groups: germinomas and non-germinoamtous germ cell tumors (NGGCT) [1-4].
Compared with that of gonadal GCTs, the clinical and histological characteristics of CNS GCTs have a marked degree of heterogeneity. The incidence of CNS GCTs varies significantly according to geography. In western countries, CNS GCTs account for 0.3%~0.5% of all primary CNS tumors, compared with an incidence rate of 2% in Asian countries (even up to 15% in Japan, South Korea and Taiwan) [4-6]. It has been hypothesized that the incidence of CNS GCTs is also high in mainland of China [5]. However, to our knowledge, limited data are available on the clinicopathological features of CNS GCTs in mainland of China. The specimens from CNS GCT are often very limited in size as special lesion sites. Accurately diagnosing CNS GCTs might be extremely difficult and requires immunohistochemical verification. This study was to investigate the biological feature of CNS GCTs, and diagnostic value of immunohistochemical markers OCT3/4, C-kit, PLAP, and CD30 in CNS GCTs. In addition, we assessed treatment outcomes and prognostic factors.
Materials and methods
Patient data
Between 1990 and 2014, 25 male and 9 female Chinese patients (male: female ratio 2.8:1), with median age of 16.9 years (ranging from 7 to 36 years) diagnosed with primary CNS GCTs from Ren Ji Hospital, School of Medicine, Shanghai Jiaotong University. Diagnoses are based on the combination of clinical symptoms, tumor imaging characteristics, serum tumor markers, as well as cytological and histological confirmation. None of the patients had a previous history of gonadal GCTs or non-CNS extragonadal GCTs. The study was approved by the ethics committee of Ren Ji Hospital, School of Medicine, Shanghai Jiaotong University.
Follow-up study
Thirty-four patients were followed up by telephone or mail and only 29 patients had completed follow-up results. Median follow-up period was 6.5 years, ranging from 4 months to 17 years.
Immunohistochemistry and staining evaluation
Surgical specimens were fixed in 10% formalin and embedded in paraffin. 4 μm sections were cut and stained with hematoxylin and eosin. Additional 4 μm sections were deparaffinized with xylene and rehydrated in a graded series of ethanol. The deparaffinised sections were then incubated with 3% H2O2 to inhibit the endogenous peroxidase, followed by microwave-treated or trypsin digestion for antigen retrieval before incubation with different primary antibodies, using a two-step polymer method (EnVisionTM). The sections were incubated in a humid chamber at 4°C overnight after adding primary antibodies and the Table 1 showed the details of primary antibodies used in our study. Subsequently, second antibodies were added after PBS rinse. The sections were incubated at room temperature for 30 minutes, and then colored with DAB for 15 minutes, and finally light counterstained with hematoxylin. As positive control staining we used placental tissue, testicular neoplasm and Gastrointestinal stromal tumor, respectively. Negative controls were performed using blocking serum in place of primary antibody. Immunohistochemical expression was graded using a semi-quantitative scoring system based on the proportion of positive cells over total cells (percent positivity) ranging from 0 to 100% where 0% was negative expression, <50% was weak expression, ≥50% was strong expression [7,8]. This study protocol was approved by the Human Ethics Committee of Renji Hospital, School of Medicine, Shanghai Jiao Tong University.
Table 1.
Summary of immunohistochemical antibodies
| Antibdy | Type | pre-treated | dilution | location | details |
|---|---|---|---|---|---|
| PLAP | mouse anti-human | heated | WS | cytoplasm | DaKo (GM719102) |
| OCT3/4 | goat anti-IgG | heated | 1:50 | nucleus | Santa Cruz (c-20, sc-8629) |
| c-kit | rabbit anti-human | heated | WS | membrane | DaKo (A450202) |
| β-HCG | rabbit anti-human | heated | WS | cytoplasm | DaKo (GA023102) |
| AFP | rabbit anti-human | None | WS | cytoplasm | DaKo (GA000804) |
| CK AE1/3 | mouse anti-human | typsin digestion | 1:50 | cytoplasm | DaKo (GM082110) |
| CD30 | mouse anti-human | heated | WS | membrane | DaKo (GM075102) |
WS: working solution; goat anti-mouse, rabbit anti-goat, goat anti-rabbit.
Statistics analysis
Statistical analyses were performed using SPSS for Windows (version 17.0). The chi-square test or Fisher exact tests were used for categorical and ordinal variables. P values of <0.05 were considered statistically significant, and all reported P values are two-sided.
Results
Clinical characteristics of the patients
Thirty-four CNS GCTs accounted for 9.2% of all primary CNS neoplasms and 37.7% of extragonadal GCTs, respectively. The duration of symptoms before diagnosis ranged from 5 days to 5 years, with a mean time of 10 months. 7 patients (20.6%) were younger than 10 years, 9 patients (26.5%) were older than 20 years, and 18 patients (52.9%) were between 10 and 20 years. Affected sites included intracranial (88.2%) and intraspinal canal (11.8%). The sellar region and pineal gland were the most common sites for intracranial GCTs, accounting for 35.3% and 17.6% respectively. The hypothalamus, third ventricle and suprasella were also common tumor locations. Other locations included: basal ganglia and medulla spinalis (Table 2). There is an overall male predominance in CNS GCTs except suprasella (n = 2, both female). Clinical presentations of CNS GCTs were dependent on the location and size of the tumor in the CNS. Symptoms varied at diagnosis including endocrine abnormalities, headache, visual disturbances and signs of increased intracranial pressure. 10 patients displayed endocrine abnormalities, including precocious puberty (n = 6), growth retardation (n = 2, one with Cushing syndrome), low libido (n = 1) and amenorrhoea (n = 1).
Table 2.
Distribution of locations in different histological subtype of 34 CNS GCTs
| Tumor Location (male: Female) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Histology | No. | age | S | P | H | TV | SS | BG | MS | Total ratio |
| G | 23 | 15.8 | 5:3 | 4:1 | 3:1 | 3:0 | 0:1 | 1:0 | 1:0 | 17:6 |
| MT | 6 | 23.2 | 1:0 | 1:0 | - | - | 0:1 | - | 3:0 | 5:1 |
| IT | 1 | 7 | - | - | - | 1:0 | - | - | - | 1:0 |
| MGCT | 2 | 16.5 | 1:1 | - | - | - | - | - | - | 1:1 |
| EC | 1 | 12 | 0:1 | - | - | - | - | - | - | 0:1 |
| CC | 1 | 20 | - | - | - | 1:0 | - | - | - | 1:0 |
| Total | 34 | 16.9 | 7:5 | 5:1 | 3:1 | 5:0 | 0:2 | 1:0 | 4:0 | 25:9 |
| Of pts | - | - | 12/34 | 6/34 | 4/34 | 5/34 | 2/34 | 1/34 | 4/34 | |
| Of pts (%) | - | - | 35.3% | 17.6% | 11.8% | 14.7% | 5.9% | 2.9% | 11.8% | |
G: germinoma; MT: mature teratoma; IT: immature teratoma; EC: embryonal carcinoma; CC: choriocarcinoma; MGCTs: mixed germ cell tumors; STGCs: syncytiotrophoblastic giant cells. S: sellar; SS: suprasella; P: pineal; H: hypothalamus; TV: third ventricle; BG: basal ganglia; MS: medulla spinalis.
Elevations of serum beta-human chorionicgonadotropin (β-HCG) have been detected in one germinoma patient with syncytiotrophoblastic (1,3900 mIU/ml, normal < 25 mIU/ml) and one choriocarcinoma patient (2,5980 mIU/ml, normal < 25 mIU/ml), respectively. Both the serum β-HCG levels dropped dramatically after the operation. Elevation of serum alpha fetoprotein (AFP) level was found in one mixed GCT patient (G+YST+EC). Additionally, 7 patients with pure germinoma had normal AFP, β-HCG, TT3, TT4, TSH, ACTH, LH, FSH and PRL levels.
Histological diagnosis
The sizes of tumor varied from 2 cm × 1.8 cm × 1.5 cm to 5 cm × 4 cm × 4 cm. 34 patients had undergone surgical resection. 23 patients were histologically diagnosed for germinoma (67.6%), one for germinoma with STGCs, 7 for teratoma (20.6%, 1 immature teratoma and 6 mature teratoma), 2 for mixed malignant GCTs (5.9%, 1 G+YST+EC, 1 G+EC), one for embryonal carcinoma (2.9%) and choriocarcinoma (2.9%) respectively.
Macroscopic examination: The macroscopic appearance of the germinoma was pale grey solid nodule with less hemorrhage, necrosis and cystic change. 17 of 34 CNS GCTs cases had well defined tumor borders with well circumscribed, and the other 17 had poorly defined tumor borders with infiltrative growth pattern. Two mixed GCTs, one embryonal carcinoma, and one choriocarcinoma also had poorly defined borders with hemorrhage, necrosis.
Microscopic examination: The histological features of 23 germinoma were similar to seminoma of testis and dysgerminoma of ovarian. The tumor cell of germinoma in CNS was round or polygonal with abundant pale to clear cytoplasm and moderate sized polygonal nuclei and prominent nucleoli. The architecture was a sheet or nest pattern associated lymphocytic infiltrates along fibrovascular septae (Figure 1A). One of the 23 germinoma cases revealed a germinoma containing syncytiotrophoblastic giant cells (STGCs). 6 mature teratomas had tissue originating from three fully differentiated tissue elements of ectoderm, mesoderm, and endoderm. One immature teratoma contained incompletely tissue from embryonic mesenchyme-like stroma. 2 mixed GCT contained germinoma, yolk sac tumor and embryonal carcinoma component (G+YST+EC), and germinoma and embryonal carcinoma (G+EC), respectively. One embryonal carcinoma was composed of large cells with indistinct cell borders, prominent nucleoli, mitoses forming irregular gland-like architecture. One choriocarcinoma was composed of cytotrophoblasts and syncytiotrophoblasts with severe hemorrhage, necrosis and scattered syncytiotrophoblastic giant cells.
Figure 1.
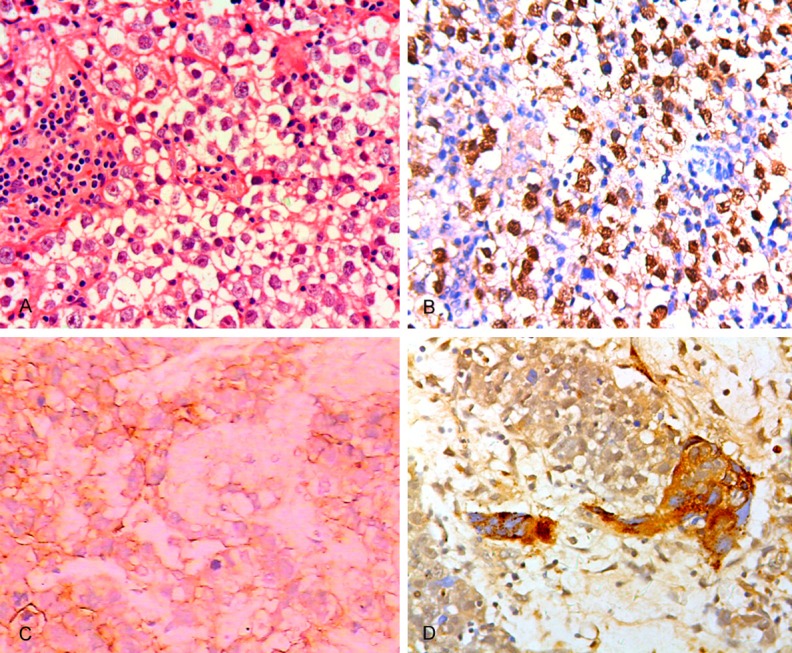
A: Histological features of CNS germinoma: A tumor cells with abundant clear cytoplasm, sheet growth pattern, lymphocytic infiltrates along fibrovascular septae. (HE×200). B: OCT3/4 immunolabeling of tumor cells in CNS germinoma (×200). C: CNS Mixed GCT showing embryonal carcinoma component with CD30 immonolabelling (×200). D: Immunohischemical features of germinoma with syncytiotrophoblastic giant cells. Immunostaining for β-HCG in STGCs (×200).
Immunohistochemical results (Table 3)
Table 3.
Expression of immunohischemical markers in 34 CNS GCTs
| Histology | PLAP | C-kit | OCT3/4 | HCG | AFP | CD30 | CK AE1/3 |
|---|---|---|---|---|---|---|---|
| G | + | + | + | - | - | - | - |
| STGC | - | - | - | + | - | + | + |
| G+EC | + | + | + | - | - | + | + |
| G+YST+EC | + | + | + | - | + | + | + |
| T(MT+IT) | - | - | - | - | - | - | + |
| EC | - | - | + | - | - | + | + |
| CC | + | - | - | + | - | - | + |
G: germinoma; MT: mature teratoma; IT: immature teratoma; MGCTs: mixed germ cell tumors; EC: embryonal carcinoma; YST: yolk sac tumor; CC: choriocarcinoma; STGCs: syncytiotrophoblastic giant cells.
Placental alkaline phosphatase (PLAP) was mainly located in the cell membrane and cytoplasm. It was detected in 19 of 23 germinomas (82.6%), 2 mixed GCTs, one embryonal carcinoma and one choriocarcinoma. Strong positive staining was found in 14/23 germinomas, weak positive in 5/23, negative in 4/23. None of 7 patients with teratoma (mature and immature) had a positive expression of PLAP. YST component of mixed GCT showed negative expression of PLAP. An uncertain immunohischemical evaluation was performed in 2 cases with weak positive expression because of insufficient tumor samples and obscuration by crushing artifact. C-kit was detected in all germinomas (23/23) and 2 mixed GCTs. Strong positive staining was found in 18/23 germinomas, and weak positive in 5/23. None of 7 patients with teratoma (mature and immature), one patient with embryonal carcinoma, and one patient with choriocarcinoma had a positive expression. C-kit was positive in YST component in mixed GCT, but negative in embryonal component. STGC was also negative.
OCT3/4 was detected in all germinomas (23/23), germinoma component and embryonal carcinoma component in 2 mixed GCTs. Strong positive staining was detected in 19/23 germinomas, and weak positive in 4/23. None of 7 patients with teratoma (mature and immature), YST component, and STGCs had a positive expression (Figure 1B).
In addition, CD30 and CK AE1/3 staining were positive in embryonal carcinoma component of mixed GCT and embryonal carcinoma (Figure 1C). AFP and CK AE1/3 staining were positive in YST. β-HCG and CK AE1/3 staining were both positive in STGC. β-HCG staining was positive in choriocarcinoma (Figure 1D).
Treatment outcomes
Total excision and subtotal excision were performed in 15 and 34 patients, respectively. All patients except 6 patients with mature teratomas received subsequent adjuvant radiotherapy and chemotherapy after surgical resection. 2 patients with germinomas recurrence 4/8 months after primitive surgical excision received tumor excision again, and neither local recurrence nor distal metastasis was observed according to a followed up of 3 and 6 years, respectively. One patient with mature teratoma showed local relapse 4 years after surgical treatment. One death case of germinoma was reported two weeks after operation for serious intranoperative bleeding. One patient with mixed GCT (G+YST) had recurrence and died 4 years after operation. One patient with embryonal carcinoma had recurrence 4 month after tumor resection and died 10 months for repeated surgical resection. One death case of choriocarcinoma was reported 6 months after operation. One mixed GCT (G+EC) recurrence 2 months after primitive surgical excision received tumor excision again, and neither local recurrence nor distal metastasis was observed according to a followed up of 5 years. Neither local recurrence nor distal metastasis was observed in the rest 23 patients with germinoma, mature teratomas and immature teratomas, according to follow-up records.
The overall 5-year survival rate of the patients with CNS GCTs was 86.2%. Teratomas and germinomas had good prognosis with a 5-year survival rates of 100% and 94.4%, respectively. The overall 3 and 5-year survival rates of the patients with malignant NG-GCTs were 60% and 40%. The prognosis of the patients with germinoma was significantly better than that of the patients with malignant NGGCT (P < 0.05). The overall 5-year survival rates of the patients with or without recurrence were 66.7% and 95.7%, but there was no significantly difference.
Discussions
Germ cell tumor most frequently occurs in the gonads (ovary and testis), and rarely occurs in the extragonadal sites of the midline of the body including pineal gland, suprasellar region, mediastinum and sacrococcygeal region. Primary CNS GCTs are rare neoplasms with the incidence rate higher in the Far East than in the West [4,6]. Our data confirmed the incidence of CNS GCT is high in China. In this study, CNS GCTs account for 9.2% of all primary CNS tumors, with a high rate in the prepubertal children (73.5%), and especially in the second ten years of the life (52.9%). There is an overall male predominance in CNS GCTs except 2 cases of suprasella. 2 patients in suprasella region tumors were female, suggesting a female predominance in this site. This result was in accordance with Lee et al who reported that the suprasella region was the only anatomical site demonstrating a female predominance in South Korea [3].
Clinical features of CNS GCTs are similar to that of other tumors, such as gliomas, pituitary adenoma, or pineoblastomas, whose symptoms were largely caused by tumor effect on surrounding structures. Clinical presentations of CNS GCTs frequently include signs of increased intracranial pressure, visual changes, and endocrine abnormalities depending on the tumor location, size of lesion, and patient’s age. In our study, the sellar region (35.3%) and pineal gland (17.6%) were the most common sites of intracranial GCTs. Patients with tumors located in pineal region usually present with progressive hydrocephalus and signs of increased intracranial pressure, which result from compression and obstructive of the aqueduct of midbrain. Patients with GCTs in sellar and suprasellar most commonly present with visual symptoms including failure of upper gaze and obtundation related to compression of the optic chiasm, or present with hypothalamic/pituitary axis dysfunction such as delayed sexual development, hypopituitarism, precocious puberty, and diabetes insipidus [3,6,9]. In this study, 6 patients with precocious puberty were all male, and the histological types were pure germinona, G+STGCs, and choriocarcinoma, respectively, with the locations of sella regions, pineal region, hypothalamic and medulla spinal. Though the cause of precocious puberty in patients with CNS GCTs has not been completely clarified, we found some pure germinomas can produce β-HCG at a high level, which may be responsible for precocious puberty. This speculation was also supported by previous studies that germinoma with β-HCG secreting syncytiotrophoblasts and choriocarcinomas seems to be more likely to present with precocious puberty than any other type of GCTs [10-12].
CNS GCTs are composed of several different tumor types showing varying degrees of malignancy. However, researchers found various histological types of GCTs may share a common origin of primordial germ cells (PGCs) at different development stages. For example, yolk sac tumor and choriocarcinoma were thought to originate from PGCs at extraembryonic differentiation, while germinoma were thought to originate from undifferentiated PGCs, and teratoma from PCGs at embryonal differentiation [2,13,14]. In this study, 67.6% of GCTs were germinomas, followed by mature and immature teratomas (20.6%), mixed GCTs (5.9%), embryonal carcinoma (2.9%), and choriocarcinoma (2.9%). The results were rather similar to a report based on 389 published cases by Jennings et al, in which 65% of GCTs were germinomas, 18% were teratomas, 5% were embryonal carcinomas, 7% were endodermal sinus tumors, and 5% were choriocarcinomas [4]. However, the present study reveals a markedly lower proportion of mixed GCTs (2.9%) compared with 27.4% and 13.8% that reported in the studies performed in Korea and Taiwan, respectively [3,5]. In our study, germinomas with STGCs constituted 4.3% of geminoma, which is similar with a reported proportion of 5.2% by Matsutani et al [6]. It should be paid more attention that scattered STGCs in the germinoma cannot be misdiagnosed as choriocarcinoma [11,12].
Therapeutic strategies and prognosis of CNS GCTs are highly dependent on histological subtypes. Total or partial surgical resection is the cornerstone of treatment and also beneficial for histological diagnosis. CNS GCTs except mature teratomas require subsequent therapy including adjuvant radiotherapy and chemotherapy after surgical resection. Complete surgical resection of mature teratomas usually predicts a favorable outcome of patients [15,16]. In mixed GCTs, the prognosis and treatment depend on the most malignant component. In this study, germinomas are highly sensitive to radiation therapy, with a five-year survival rate of 94.4%. Mixed GCTs, embryonal cell carcinomas, and choriocarcinomas are less radiosensitive than pure germinomas and their prognosis are relatively poor with five-year survival rates of 50%, 0% and 0%, respectively. Some studies reported that following the treatment, there was no difference in five years PFS or EFS between patients with a residual lesion and those without it [16-18]. Matsutani reported that 5-years, 10-year, 15-year and 20-year survival rates for pure germinomas were 95.4%, 92.7%, 87.9% and 80.6%, respectively. The 1-year survival rates for embryonal carcinomas, yolk sac tumors and choriocarcinomas was 80%, 33.3% and 0, respectively. The 5-year survival rates for mixed GCTs and immature and malignant teratomas were 57.1% and 70.7%, respectively [6]. A previous study reported that germinomas with STGCs usually has a poor prognosis largely for high incidence of recurrence [12]. In our study, one case of germinoma with STGCs has a good prognosis without occurrence and metastasis 6 years after operation.
Compared with other extragonadal sites GCTs, CNS GCTs are usually diagnosed based on histological assessments, neuroimaging characteristics, and tumor markers. However, the neuroimaging characteristics of all types of CNS GCTs are similar, and the tumor markers are usually nonspecific. The diagnosis of CNS GCTs usually requires a tumor biopsy; however it is sometimes difficult to perform complete resection of the tumor due to its special sites in CNS. Accurately diagnosing CNS GCTs can be very difficult, since tumor specimens from these lesions are often so limited in size that it is difficult to obtain them. Immunohistochemical staining is often necessary for the diagnosis of CNS GCTs. This may be the reason why more patients were diagnosed during 2001~2014 than during 1990~2000 (8 vs. 26 cases) in our series.
PLAP, a characteristic marker of primordial germ cells, is detected in 82.6% of germinomas. However, the PLAP marker has its own shortcomings of heavy staining background due to surface membrane and diffuse cytoplasmic staining. It was very difficult to interpret in small biopsies where tumor cells were obscured by crushing artifact and focal necrosis in 2 cases. C-kit and OCT3/4 are also characteristic sensitive markers of germinomas with membrane staining and nuclear staining, respectively, and the staining sensitivities are higher than PLAP (100% positive staining). CK AE1/3 staining is usually negative or focal weak positive in germinomas, but positive in embryonal carcinomas, yolk sac tumors, teratomas and choriocacinomas. STGCs show strong immunopositive reactions for CK AE1/3, human placental lactogen and β-HCG [7,8,10,13]. Embryonal carcinomas and embryonal carcinoma component show positive stainings for CK AE1/3, EMA, OCT3/4 and CD30. CD30 staining is negative in germinoma, yolk sac tumors and choriocacinomas [19,20]. PLAP, OCT3/4, C-kit and CD30 used in combination are useful for distinguishing germinoma from embryonal carcinoma. Yolk sac tumors are positive for AFP, CK AE1/3 and EMA. Immunoreactivity for AFP of yolk sac tumors is characteristic and valuable in distinguishing these tumors from embryonal carcinomas [20,21]. Choriocarcinomas show immunopositivity for β-HCG, HPL, and CK AE1/3 [21,22].
In summary, the incidences of primary CNS GCTs are higher in South China than that in West countries. The mixed GCTs are uncommon in our study and the incidences are very lower than that in other countries. Mature teratomas and germinomas have a better prognosis. The prognosis of the patients with germinoma was significantly better than that of the patients with malignant NGGCT. An accurate diagnosis of CNS GCTs is critical for patient management. Immunohistochemistry has played a major role in accurately diagnosing and distinguishing the different histological types. The judicious use of a panel of selected markers is helpful in diagnosing and predicting the prognosis for CNS GCTs.
Acknowledgements
This work was partly supported by the funding of Science and Technology Commission of Shanghai Municipality (NO. 134119a9502). 12DZ2260600.
Disclosure of conflict of interest
None.
References
- 1.Thakkar JP, Chew L, Villano JL. Primary CNS germ cell tumors: current epidemiology and update on treatment. Med Oncol. 2013;30:496–515. doi: 10.1007/s12032-013-0496-9. [DOI] [PubMed] [Google Scholar]
- 2.Packer RJ, Cohen BH, Cooney K. Intracranial germ cell tumors. Oncologist. 2000;5:312–320. [PubMed] [Google Scholar]
- 3.Lee D, Suh YL. Histologically confirmed intracranial germ cell tumors; an analysis of 62 patients in a single institute. Virchows Arch. 2010;457:347–357. doi: 10.1007/s00428-010-0951-3. [DOI] [PubMed] [Google Scholar]
- 4.Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg. 1985;63:155–167. doi: 10.3171/jns.1985.63.2.0155. [DOI] [PubMed] [Google Scholar]
- 5.Liang SY, Yang TF, Chen YW, Liang ML, Chen HH, Chang KP, Shan IK, Chen YS, Wong TT. Neuropsychological functions and quality of life in survived patients with intracranial germ cell tumors after treatment. Neuro Oncol. 2013;15:1543–1551. doi: 10.1093/neuonc/not127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O, Funata N, Seto T. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86:446–455. doi: 10.3171/jns.1997.86.3.0446. [DOI] [PubMed] [Google Scholar]
- 7.Cheng L, Sung MT, Cossu-Rocca P, Jones TD, MacLennan GT, De Jong J, Lopez-Beltran A, Montironi R, Looijenga LH. OCT4: biological functions and clinical applications as a marker of germ cell neoplasia. J Pathol. 2007;211:1–9. doi: 10.1002/path.2105. [DOI] [PubMed] [Google Scholar]
- 8.Takeshima H, Kuratsu J. A review of soluble c-kit (s-kit) as a novel tumor marker and possible molecular target for the treatment of CNS germinoma. Surg Neurol. 2003;60:321–4. doi: 10.1016/s0090-3019(03)00430-0. discussion 324-5. [DOI] [PubMed] [Google Scholar]
- 9.Kaur H, Singh D, Peereboom DM. Primary central nervous system germ cell tumors. Curr Treat Options Oncol. 2003;4:491–498. doi: 10.1007/s11864-003-0049-0. [DOI] [PubMed] [Google Scholar]
- 10.Allen J, Chacko J, Donahue B, Dhall G, Kretschmar C, Jakacki R, Holmes E, Pollack I. Diagnostic sensitivity of serum and lumbar CSF bHCG in newly diagnosed CNS germinoma. Pediatr Blood Cancer. 2012;59:1180–1182. doi: 10.1002/pbc.24097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogino H, Shibamoto Y, Akanaka T, Suzuki K, Ishihara S, Yamada T, Sugie C, Nomoto Y, Mimura M. CNS germinoma with elevated serum human chorionic gonadotropin level: clinical characteristics and treatment outcome. Int J Radiat Oncol Biol Phys. 2005;62:803–808. doi: 10.1016/j.ijrobp.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Shibamoto Y, Takahashi M, Sasai K. Prognosis of intracranial germinoma with syncytiophoblastic giant cells treated by radiation therapy. Int J Radiat Oncol Biol Phys. 1997;37:505–510. doi: 10.1016/s0360-3016(96)00611-6. [DOI] [PubMed] [Google Scholar]
- 13.Hoei-Hansen CE, Sehested A, Juhler M, Lau YF, Skakkebaek NE, Laursen H, Rajpert-de Meyts E. New evidence for the origin of intracranial germ cell tumours from primordial germ cells: expression of pluripotency and cell differentiation markers. J Pathol. 2006;209:25–33. doi: 10.1002/path.1948. [DOI] [PubMed] [Google Scholar]
- 14.Sano K. Pathogenesis of intracranial germ cell tumors reconsidered. J Neurosurg. 1999;90:258–264. doi: 10.3171/jns.1999.90.2.0258. [DOI] [PubMed] [Google Scholar]
- 15.Brandes AA, Pasetto LM, Monfardini S. The treatment of cranial germ cell tumours. Cancer Treat Rev. 2000;26:233–242. doi: 10.1053/ctrv.2000.0169. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa K, Toita T, Nakamura K, Uno T, Onishi H, Itami J, Shikama N, Saeki N, Yoshii Y, Murayama S. Treatment and prognosis of patients with intracranial nongerminomatous malignant germ cell tumors: a multiinstitutional retrospective analysis of 41 patients. Cancer. 2003;98:369–376. doi: 10.1002/cncr.11495. [DOI] [PubMed] [Google Scholar]
- 17.Kawabata Y, Takahashi JA, Arakawa Y, Shirahata M, Hashimoto N. Long term outcomes in patients with intracranial germinomas: a single institution experience of irradiation with or without chemotherapy. J Neurooncol. 2008;88:161–167. doi: 10.1007/s11060-008-9542-4. [DOI] [PubMed] [Google Scholar]
- 18.Kanamori M, Kumabe T, Saito R, Yamashita Y, Sonada Y, Ariga H, Takai Y, Tominaga T. Optimal treatment strategy for intracranial germ cell tumors: a single institution analysis. J Neurosurg Pediatr. 2009;4:506–514. doi: 10.3171/2009.7.PEDS08288. [DOI] [PubMed] [Google Scholar]
- 19.Ulbright TM. Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Mod Pathol. 2005;18(Suppl 2):S61–79. doi: 10.1038/modpathol.3800310. [DOI] [PubMed] [Google Scholar]
- 20.Kim A, Ji L, Balmaceda C, Diez B, Kellie SJ, Dunkel IJ, Gardner SL, Sposto R, Finlay JL. The prognostic value of tumor markers in newly diagnosed patients with primary central nervous system germ cell tumors. Pediatr Blood Cancer. 2008;51:768–773. doi: 10.1002/pbc.21741. [DOI] [PubMed] [Google Scholar]
- 21.Sugiyama K, Arita K, Tominaga A, Hanaya R, Taniguchi E, Okamura T, Itoh Y, Yamasaki F, Kurisu K. Morphologic features of human chorionic gonadotropin- or alpha-fetoprotein-producing germ cell tumors of the central nervous system: histological heterogeneity and surgical meaning. Brain Tumor Pathol. 2001;18:115–122. doi: 10.1007/BF02479424. [DOI] [PubMed] [Google Scholar]
- 22.Wildi-Runge S, Crevier L, Carret AS, Robitaille Y, Deal C. Pitutary choriocarcinoma in an adolescent male: tumor-derived CG and GH delay diagnosis. Growth Horm IGF Res. 2011;21:181–184. doi: 10.1016/j.ghir.2011.04.001. [DOI] [PubMed] [Google Scholar]


