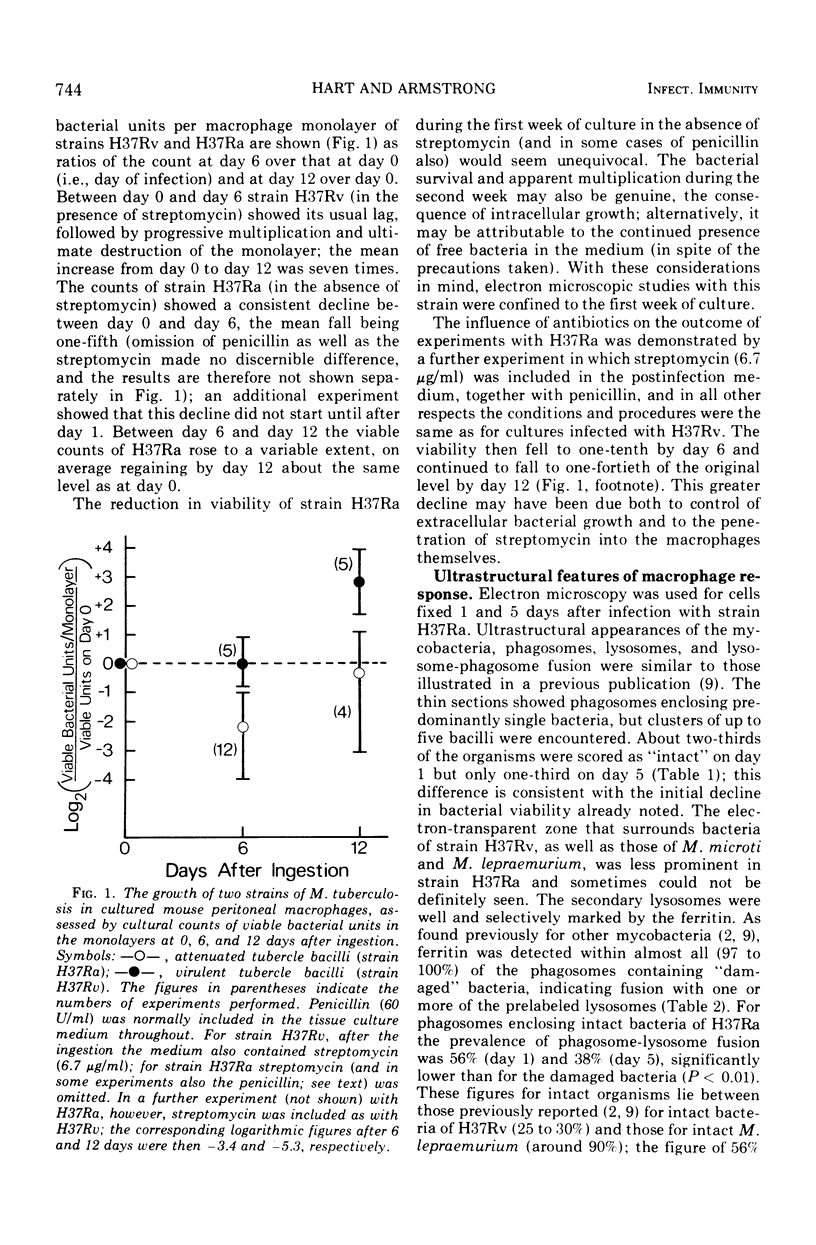
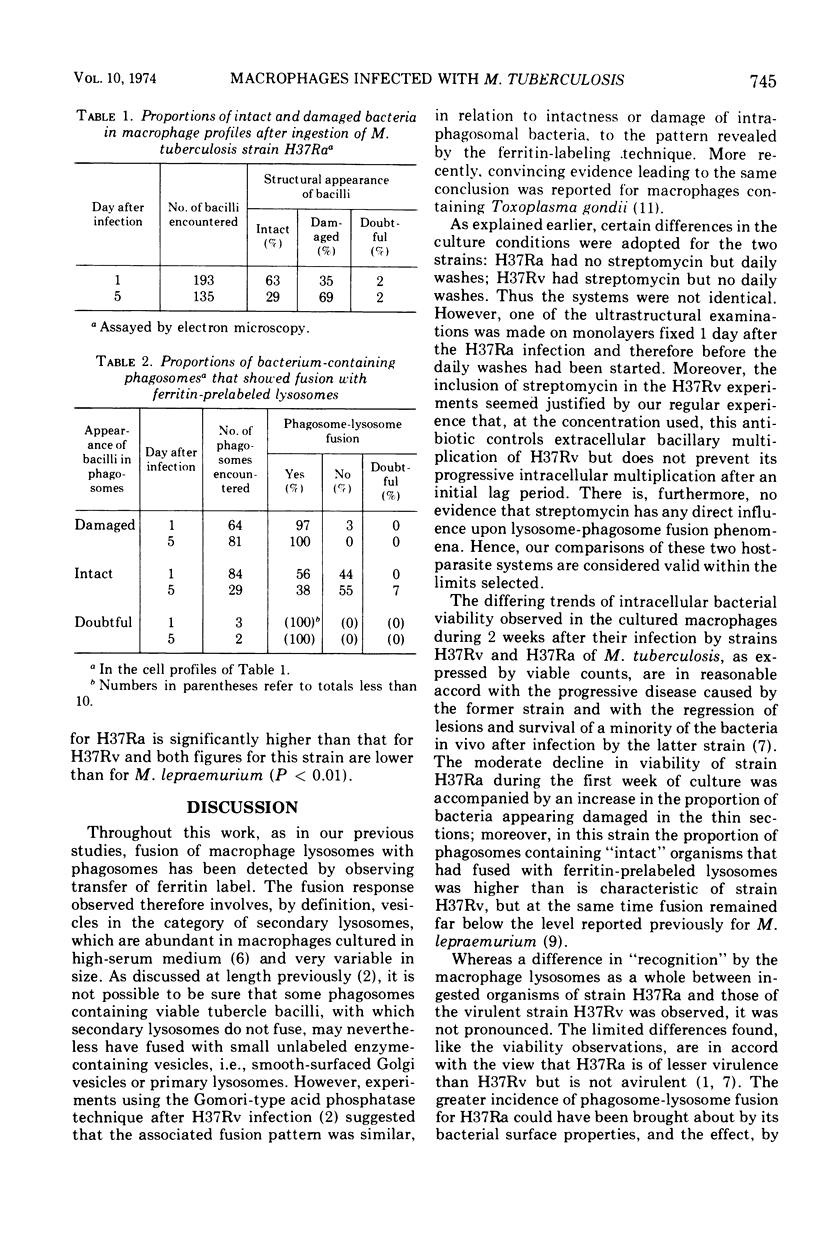
Abstract
Strains H37Ra and H37Rv, attenuated and virulent variants, respectively, of the original human strain H37 of Mycobacterium tuberculosis, were used to infect cultures of mouse peritoneal macrophages. Bacterial viability of each strain was assessed over a 2-week period, and the cellular response to H37Ra during the first week was observed using electron microscopy. Prelabeling of secondary lysosomes with ferritin was used to facilitate the estimation of fusion of the lysosomes with phagosomes containing the bacteria. Streptomycin was excluded from the medium of cell cultures infected with H37Ra. The intracellular viability of strain H37Rv (in the presence of streptomycin) showed a lag during the first week after infection and then rose progressively to a mean figure seven times the starting level. In contrast, the viability of strain H37Ra declined, on the average, to one-fifth of the starting level during the first week; moreover, this decline occurred in the absence of antibiotics. In the second week a variable rise in the viable count took place, usually regaining the starting level. Electron microscopy of macrophages infected with H37Ra revealed a higher proportion of “damaged” bacteria 5 days after infection than at 1 day, in keeping with the decline in viability. Phagosomes containing these “damaged” (and presumed dead) organisms showed virtually universal fusion with prelabeled lysosomes. Phagosomes containing “intact” bacteria of this strain showed a prevalence of fusion varying from 38 to 56%, somewhat higher than the level previously reported for “intact” organisms of H37Rv. Nevertheless, the lysosome-phagosome fusion response to “intact” H37Ra was still far less extensive than that observed previously towards “intact” M. lepraemurium (around 90%). In conclusion, a difference between the macrophage lysosome-phagosome fusion response towards viable organisms of strain H37Ra and to the virulent strain H37Rv was observed, but was not pronounced, and the present findings are in keeping with the increasingly held view that H37Ra should be regarded as a low-virulence or attenuated strain rather than truly avirulent.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsaadi A. I., Smith D. W. The fate of virulent and attenuated Mycobacteria in guinea pigs infected by the respiratory route. Am Rev Respir Dis. 1973 Jun;107(6):1041–1046. doi: 10.1164/arrd.1973.107.6.1041. [DOI] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOCH H., SEGAL W. Viability and multiplication of vaccines in immunization against tuberculosis. Am Rev Tuberc. 1955 Feb;71(2):228–248. doi: 10.1164/artpd.1955.71.2.228. [DOI] [PubMed] [Google Scholar]
- Bonventre P. F., Imhoff J. G. Uptake of h-dihydrostreptomycin by macrophages in culture. Infect Immun. 1970 Jul;2(1):89–95. doi: 10.1128/iai.2.1.89-95.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE IN VITRO DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. II. THE INFLUENCE OF SERUM ON GRANULE FORMATION, HYDROLASE PRODUCTION, AND PINOCYTOSIS. J Exp Med. 1965 May 1;121:835–848. doi: 10.1084/jem.121.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. T. Suppressive activity of streptomycin on the growth of Mycobacterium lepraemurium in macrophage cultures. Appl Microbiol. 1969 May;17(5):750–754. doi: 10.1128/am.17.5.750-754.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. The relative immunogenicity of virulent and attenuated strains of tubercle bacilli. Am Rev Respir Dis. 1973 Jun;107(6):1030–1040. doi: 10.1164/arrd.1973.107.6.1030. [DOI] [PubMed] [Google Scholar]
- Ekzemplyarov O. N. Penetration of tetracycline and streptomycin into macrophages cultured in vitro. Fed Proc Transl Suppl. 1966 Mar-Apr;25(2):312–314. [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. S. The fate of Mycobacterium tuberculosis within macrophages of guinea pigs. Am Rev Respir Dis. 1971 May;103(5):607–611. doi: 10.1164/arrd.1971.103.5.607. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANAI K., KATSUYAMA S., YANAGISAWA K. An interference phenomenom between virulent and avirulent tubercle bacilli concerning their multiplication and survival in guinea pigs, with special reference to the mechanism of virulence-enhancement of mycobacterial strains by animal passage. Jpn J Med Sci Biol. 1955 Jun;8(3):207–218. doi: 10.7883/yoken1952.8.207. [DOI] [PubMed] [Google Scholar]
- LARSON C. L., WICHT W. C. INFECTION OF MICE WITH MYCOBACTERIUM TUBERCULOSIS, STRAIN H37RA. Am Rev Respir Dis. 1964 Nov;90:742–748. doi: 10.1164/arrd.1964.90.5.742. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B., SMITH N. The bactericidal action of isoniazid, streptomycin and terramycin on extracellular and intracellular tubercle bacilli. Am Rev Tuberc. 1953 Mar;67(3):322–340. doi: 10.1164/art.1953.67.3.322. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B., SMITH N., WELLS A. Q. The growth of intracellular tubercle bacilli in relation to their virulence. Am Rev Tuberc. 1954 Apr;69(4):479–494. doi: 10.1164/art.1954.69.4.479. [DOI] [PubMed] [Google Scholar]
- PIERCE C. H., DUBOS R. J., SCHAEFER W. B. Multiplication and survival of tubercle bacilli in the organs of mice. J Exp Med. 1953 Feb 1;97(2):189–206. doi: 10.1084/jem.97.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Multiplication of Mycobacterium tuberculosis Within Normal and "Immune" Mouse Macrophages Cultivated With and Without Streptomycin. Infect Immun. 1970 Jan;1(1):30–40. doi: 10.1128/iai.1.1.30-40.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVER J. L., YOUMANS G. P. The enumeration of nonpathogenic viable tubercle bacilli from the organs of mice. Am Rev Tuberc. 1957 Feb;75(2):280–294. doi: 10.1164/artpd.1957.75.2.280. [DOI] [PubMed] [Google Scholar]
- SUTER E. The multiplication of tubercle bacilli within normal phagocytes in tissue culture. J Exp Med. 1952 Aug;96(2):137–150. doi: 10.1084/jem.96.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder M. S., Edberg J. C. Interaction of virulent and avirulent Listeria monocytogenes with cultured mouse peritoneal macrophages. Infect Immun. 1973 Mar;7(3):409–415. doi: 10.1128/iai.7.3.409-415.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



