Abstract
Pulmonary mucoepidermoid carcinoma (PMEC) is a rare malignant neoplasm, and little is known about the prognostic factors. The aim of the present study was to identify the relationship between tumor’s histological features and clinical behaviors and to analyze the survival of patients with PMEC. A total of 34 patients with PMEC from May 2001 to April 2013 were included in the investigation. The clinical data, radiological manifestation, pathological findings, treatment strategy, and prognoses of all patients were analyzed retrospectively. The patients were classified into low-grade group (n = 25) and high-grade group (n = 9), based on histological grades. High-grade PMEC was more common in patient with elevated serum carcinoembryonic antigen (CEA) (P = 0.033), advanced tumor-node-metastasis (TNM) stage (P = 0.004) and lymph node metastasis (P < 0.001). The 5-year PFS and OS of all patients were 75.7% and 83.6%, respectively. Age, pathological grade, lymph node metastasis and TNM stage were correlated with the survival of PMEC patients. Lymph node metastasis was an independent predictor of OS (HR, 0.080; P = 0.029) and PFS (HR, 0.090; P = 0. 004). A higher tumor histological grade indicated a more aggressive behavior. Patients who had undergone complete resection for PMEC without any lymph node metastasis were expected to be cured.
Keywords: Pulmonary mucoepidermoid carcinoma (PMEC), histopathology, treatment, prognosis
Introduction
Pulmonary mucoepidermoid carcinoma (PMEC) is a rare tumor. It represents only 0.1-0.2% of all primary pulmonary malignancies and usually considered to originate from the bronchial gland of minor salivary gland-type lining the tracheobronchial tree [1-4]. PMEC is now defined as a tumor characterized by a combination of mucus-secreting, squamous, and intermediate cell types and is classified into low grade and high grade according to histology appearance, cellular atypia, mitotic activity, local invasion, and necrosis [4,5].
Unlike the common types of lung cancer (adenocarcinoma and squamous carcinoma),PMEC is a low malignant tumor with favorable prognosis and high survival rate after surgical resection [1,2]. In previous studies, PMEC was mostly reported in small series or described in case reports. Thus, the relationship between the tumor histology, clinical behavior and prognosis is uncertain. Consequently, there is no consensus on an optimal treatment strategy. The purpose of this study, therefore, was to review PMEC with emphasis on clinical behavior, radiological features, pathologic features, treatment, and prognostic factors.
Methods
Patients
Between May 2001 and April 2013, thirty-four patients underwent surgical treatment for primary PMEC at the Sun Yat-Sen University Cancer Center. Clinicopathologic data collected from retrospective analysis included age, gender, symptoms, radiographic, bronchoscopic and histopathological features of the tumors, tumor location, tumor size, stage, treatment strategy and outcome.
The chest computed tomography (CT) scan and the bronchoscopy were performed in all patients. All patients underwent pulmonary resection and systematic mediastinal lymph node dissection. The tumors were classified into low and high histological grades according to the criteria described by Colby [4].Tumor specimens were reviewed by two experienced pathologists (Zhang and Xiao) for histopathological confirmation; the histological grading and cellular composition were also reviewed. The pathologic staging was based on the tumor-node-metastasis (TNM) staging system of the American Joint Committee on Cancer (7th edition). Follow-ups were conducted via medical records or telephone interview. This retrospective study was approved by the Institutional Review Board of Sun Yat-Sen University Cancer Center and by the Ethics Committees of Sun Yat-Sen University Cancer Center.
Statistical analysis
Categorical variables were presented as counts and percentages, and comparisons were conducted using Fisher’s exact tests. Continuous variables were presented as median and range, and comparisons were conducted using Mann-Whitney u test. The Kaplan-Meier method was used to analyze survival curves, and the log-rank test was used to compare statistical differences in the univariate analysis of survival. The variables statistically significant in the univariate analysis were used for multivariate analysis. Prognostic factors related to survivals were determined by forward stepwise Cox proportional hazard regression model. A two-sided P values < 0.05 were considered statistically significant. All analyses were performed with SPSS software (SPSS Standard version 16.0, SPSS, Chicago, IL, USA).
Results
Clinical features
The main clinical features of the 34 patients are summarized in Table 1. This study included 19 male and 15 female patients, with a median age of 41 years (range 16-78 years). The most common presenting symptoms were cough (n = 26, 76.5%) and hemoptysis (n = 14, 41.2%), and were followed closely by sputum (n = 6, 17.6%), chest pain (n = 4, 11.8%), wheezing (n = 3, 8.8%), fever (n = 2, 5.9%) and weight loss (n = 3, 8.8%). Seven patients (20.6%) were asymptomatic, and the tumors were detected during routine health examination. Based on histological grades the patients were classified as high-grade group (n = 9, 26.5%) (Figure 1A, 1B) and low-grade group (n = 25, 73.5%) (Figure 1C, 1D), and its relationship with clinical features is summarized in Table 1. There were statistically significant differences in the age (P = 0.031), CEA (P = 0.033), TNM stage (P = 0.004) and lymph node involvement (p < 0.001) between patients with high grade and those with low grade. The median age of patients with the high grade (65 years) was older than that of patients with low grade (40 years), although it was not statistically significant (P = 0.058).
Table 1.
Characteristics of Patients with Pulmonary Mucoepidermoid Carcinoma (n = 34)
| No. of Patients (%) | ||||
|---|---|---|---|---|
|
|
||||
| Parameters | Total (n = 34) | High Grade (n = 9) | Low Grade (n = 25) | p-value |
| Median Age, Range | 41 (16-78) | 65 (24-78) | 40 (16-76) | 0.058 |
| < 60 years | 28 (82.4) | 5 (55.6) | 23 (92.0) | 0.031 |
| ≥ 60 years | 6 (17.6) | 4 (44.4) | 2 (8.0) | |
| Gender | 0.462 | |||
| Female | 15 (44.1) | 5 (55.6) | 10 (40.0) | |
| Male | 19 (55.9) | 4 (44.4) | 15 (60.0) | |
| Smoking | 11 (32.4) | 4 (44.4) | 7 (28.0) | 0.692 |
| CEA | 0.033 | |||
| ≤ 5 ng/ml | 23 (67.6) | 3 (33.3) | 20 (80.0) | |
| > 5 ng/ml | 11 (32.4) | 6 (66.7) | 5 (20.0) | |
| TNM Stage | 0.004 | |||
| I-IIA | 25 (73.5) | 3 (33.3) | 22 (88.0) | |
| IIB-IV | 9 (26.5) | 6 (66.7) | 3 (12.0) | |
| Resection Margin | 0.465 | |||
| Positive | 2 (5.9) | 1 (11.1) | 1 (4.0) | |
| Negative | 32 (94.1) | 8 (88.9) | 24 (96.0) | |
| LN Metasasis | 0.000 | |||
| Yes | 7 (20.6) | 6 (66.7) | 1 (4.0) | |
| No | 27 (79.4) | 3 (33.3) | 24 (96.0) | |
Figure 1.
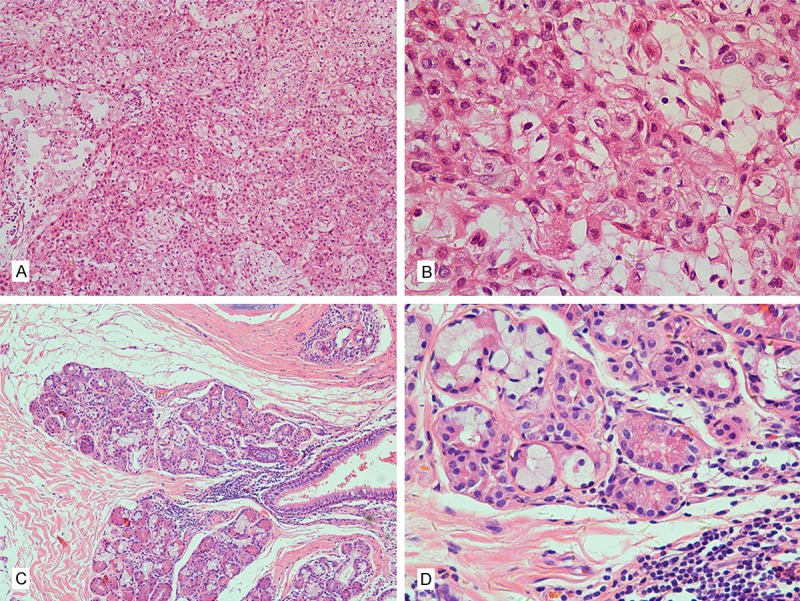
Representative images of hematoxylin and eosin-stained pathologic specimens in pulmonary mucoepidermoid carcinoma (PMEC). A: High grade, 100×; B: High grade, 400×; C: Low grade, 100×; D: Low grade, 400×.
Radiographic findings
The findings were obtained by CT scan before treatment in 34 patients (Table 2). The CT images were assessed by an experienced radiologist (Xiao) who was unaware of the histopathological diagnoses. The tumor diameter ranged from 6 to 60 mm. The tumors were located in the trachea (n = 2), main bronchus (n = 5), right upper lobe (n = 4), right middle lobe (n = 6), right lower lobe (n = 5), left upper lobe (n = 7), or left lower lobe (n = 5). The contour of the tumors were round (n = 12), oval (n = 9) or lobulated (n = 13). Twenty-two tumor masses showed a well-defined margin. Four cases showedspiculation and pleural indentation sign. Punctate calcifications were found in 4 cases. On enhanced CT scan, the tumors appeared mild (23.5%), or moderate to marked (76.5%) homogeneous or heterogeneous contrast enhancement. Ten cases may have led to the obstructive pneumonia or atelectasis. Seven cases showed metastasis to hilar and mediastinal lymph nodes; one of them also showed pleural metastases. There was no statistically significant difference observed between patients with high grade and with low grade for the CT features assessed (Table 2).
Table 2.
Computed Tomography Finding in Patients with Pulmonary Mucoepidermoid Carcinoma
| No. of Patients (%) | ||||
|---|---|---|---|---|
|
|
||||
| Parameters | Total (n = 34) | High Grade (n = 9) | Low Grade (n = 25) | p-value |
| Tumor Size, Range | 3 (0.6-6) | 3.5 (1.5-5) | 2.5 (0.6-6) | |
| Tumor Sharp | 0.254 | |||
| Round or Oval | 21 | 4 | 17 | |
| Lobulated | 13 | 5 | 8 | |
| Tumor Margin | 0.687 | |||
| Well Defined | 22 | 5 | 17 | |
| Poorly Defined | 12 | 4 | 8 | |
| Degrees of Contrast Enhancement | 1.000 | |||
| Mild | 8 | 2 | 6 | |
| Moderate to Marked | 26 | 7 | 19 | |
| Punctate Calcification | 4 | 2 | 2 | 0.281 |
Bronchoscopic findings
All 34 patients underwent bronchoscopy. The tumors were easily visualized in 27 cases except 7 peripheral cases. Most of these tumors appeared as either a round, smooth polypoid nodule or a protruding cauliflower mass with a sessile base, and wholly or partially obstructed the bronchus. Bronchoscopic brushing or biopsy, was performed in 27 cases, however, a preoperative diagnosis of mucoepidermoid carcinoma was made in only 14 (51.9%) of them. In other cases revealed a non-typed malignant tumor or non-diagnostic inflammatory cells.
Treatment
The treatment of all patients was summarized in Table 3. Thirty-two patients were operated by a standard posterolateral thoracotomy and 2 by video assisted thoracoscopic surgery (VATS). One patient received postoperative radiotherapy due to positive resection margin. Eight patients with more advanced tumor staging were treated with a combination of systemic chemotherapy (n = 7) or radiotherapy (n = 1).
Table 3.
Treatment in Patients with Pulmonary Mucoepidermoid Carcinoma
| Surgical | No. of Patients |
|---|---|
| Tracheal Resection | 3 |
| Sleeve Lobectomy | 2 |
| Wedge Lobectomy | 4 |
| Lobectomy | 15 |
| Bilobectomy | 7 |
| Pneumonectomy | 3 |
| Postoperative Treatment | 9 |
| Radiotherapy | 2 |
| Chemotherapy | 7 |
Clinical follow-up
The median follow-up for all patients was 63 months (range 22-118 months), during which the tumor recurrence or metastasis was identified in 7 patients. Among these patients, 3 survived and 4 died. They lived 13, 25, 33 and 41 months after treatment, respectively. The 5-year OS and PFS of all patients were 84.6% and 81.6%, respectively. Univariate analysis showed that age > 60, stage III/IV, high grade, and lymph node metastasis were associated with unfavorable OS (Figure 2) or PFS (Figure 3). In the forward conditional Cox regression model, lymph node metastasis was found to be a strong unfavorable predictor of OS (HR, 0.080; 95% CI, 0.008-0.775; P = 0.029) as well as PFS (HR, 0.090; 95% CI, 0.017-0.468; P = 0.004) for patients with PMEC.
Figure 2.
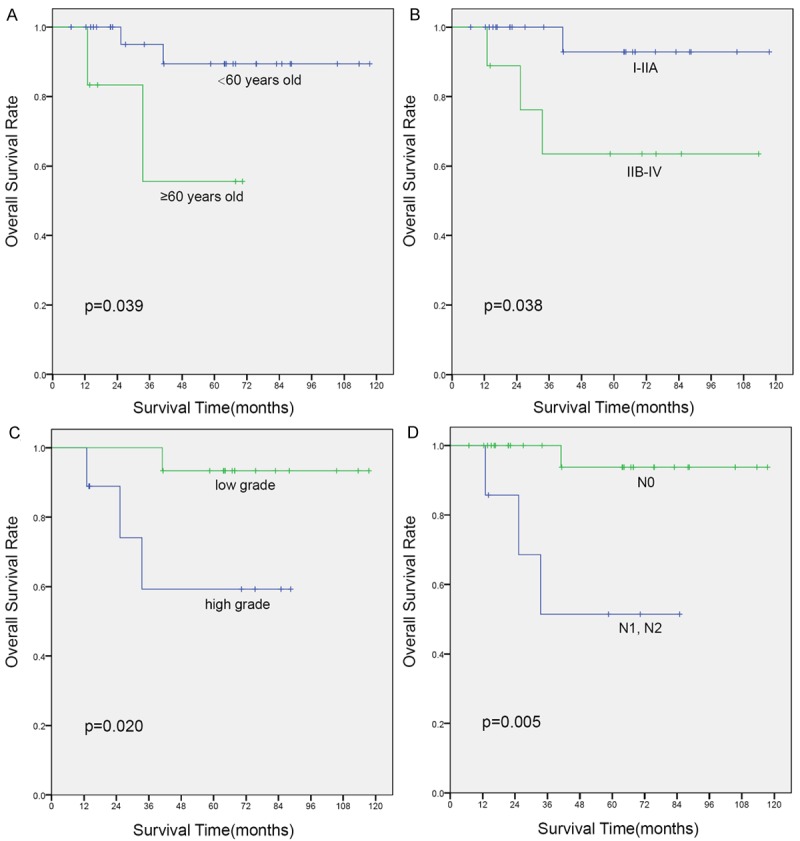
Kaplan-Meier curves of overall survival (OS) for variates: (A) age, (B) TNM stage, (C) pathological grade, and (D) lymph node metastasis.
Figure 3.
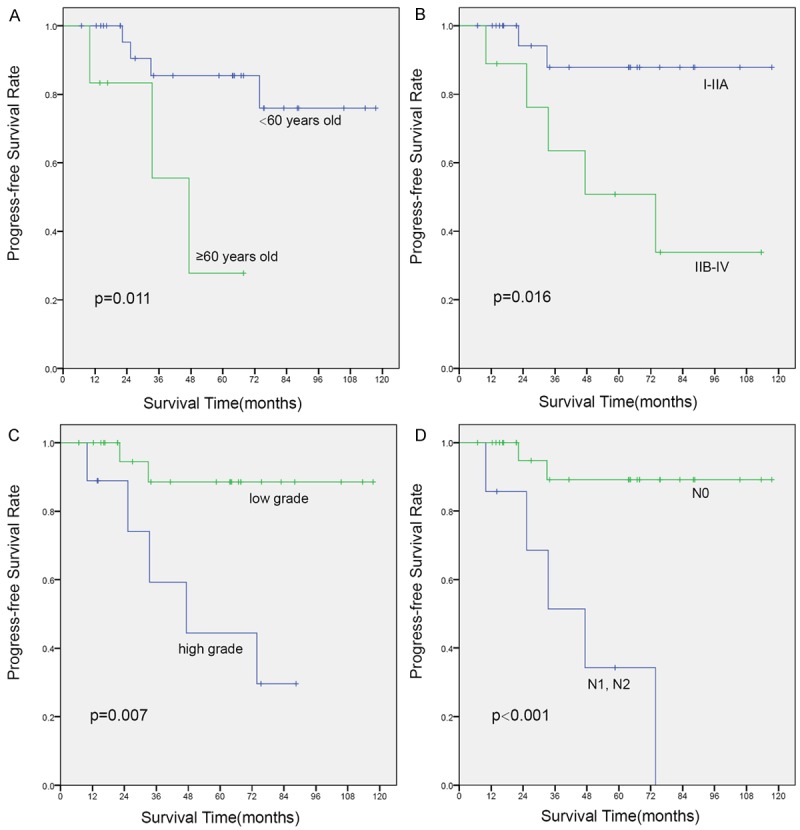
Kaplan-Meier curves of progress-free survival (PFS) for variates: (A) age, (B) TNM stage, (C) pathological grade, and (D) lymph node metastasis.
Discussion
Mucoepidermoid carcinoma (MEC) is the most common salivary gland tumor and usually located in the parotid gland [6]. As a malignant tumor of bronchial gland origin, PMEC was first described by Smetana in 1952 [7]. PMEC is now defined as a malignant epithelial tumor, composed of mucus-secreting, squamous, and intermediate cells arranged in solid, glandular or cystic patterns [8]. The tumors are classified as high grade or low grade based on their histological appearance (Figure 1). High-grade PMEC was characterized by necrosis, nuclear pleomorphism, frequent mitoses, and a solid or nested pattern of growth for the intermediate or squamous cells. Low-grade PMEC usually demonstrated bland cytological features, few mitoses, frequent cystic components and mucous cells. High-grade PMEC is often difficult to differentiate from adenosquamous carcinoma. Keratinization, a feature of adenosquamous carcinoma, is absent in high-grade PMEC. In our study, most pulmonary mucoepidermoid carcinomas (over 70%) were categorized as low grade similar to the most previous study [9-11]. However, in a few series, most PMEC were of intermediate grade because of different grading systems [12,13].
The clinical behavior of MEC in the head and neck was believed to be associated with pathological grade of the tumor [6]. Similar findings have been shown for PMEC. Yousem and Hochholzer reported that low-grade PMEC was more commonly found in women or in younger patients compared with high-grade tumor [1]. In the present study, the median age of patients with high-grade tumors was significantly greater than that of patients with low-grade tumors (65 years vs. 40 years), despite the fact that it was not statistically significant (p = 0.058). In high-grade tumors, the incidence of lymph node metastasis was reported to be more frequent (45% to 75%) [9]. In accordance with previous studies, our data also showed that the incidence of lymph node metastasis was more frequent (66.7%) in high-grade tumors.
CT examination provided significant information in the evaluation of pulmonary tumors and played an important role in the diagnosis of PMEC. The CT appearance of the tumors in our series, such as a well defined round or oval shape with a smooth margin, were similar to those reported by Kim et al [14]. The study conducted by Fisher et al. showed punctate calcification in 6 cases (50%). In our series, punctuate calcification within the tumor was seen in 4 patients (11.7%), which was lower than that in the study by Fisher et al [15]. According to the report of 16 patients reviewed by Li et al., the majority of these tumors (n = 12, 75%) showed moderate to marked enhancement and a minority (n = 4, 25%) showed mild enhancement [10]. Our results supported these findings. The tumor with spiculation and the pleural indentation sign has been seen in 4 cases, which were also reported in the study by Li et al [10].
Radical surgery is the primary choice of treatment for PMEC [16,17] in which tumor location determines the surgical approach. PMEC of the lung could be surgically treated by lobectomy, sleeve resection, local resection, segmental resection, or even endoscopic removal [18]. In our cases, lobectomy was the most frequently performed procedure and pneumonectomy was the last choice. Sleeve lobectomy should be considered when the primary bronchus was invaded, and the rate was relatively low. The conventional surgical approach for the resection of the bronchus or pulmonary resection with clear resection margin is the posterolateral thoracotomy. Advances in minimally invasive surgery that included VATS have led to a less traumatic approach for lung cancer treatment [19].
Prognostic factors appearing to predict poor survival included the histological grade, TNM stage, completeness of resection, or age in previous studies [1,9,16,20]. Yousem and Hochholzer, separating PMEC tumors into low grade and high grade, reported that high-grade tumors showed worse prognosis, and these worse outcomes seemed to be associated with nodal staging in high-grade PMEC [1]. In our study, univariate analysis showed that age, histological grade, lymph node metastasis and TNM stage were correlated with OS and PFS. However, multivariate regression analysis revealed that lymph node metastasis was the only independent prognostic factor. Most patients with PMEC have a favorable outcome after a complete resection [21,22]. No evidence proved the effect of adjuvant chemotherapy or radiotherapy. Some studies reported that chemotherapy or radiotherapy was inefficient [2,23]. Our data showed that 6/9 of high-grade PMEC patients had lymph node metastases at the time of primary resection and the survival was inferior. As a type of NSCLC, adjuvant therapy is recommended for PMEC patients who undergo an incomplete resection or have lymph node metastasis. In our experience, patients having early staged tumors without lymph node metastasis, including those with high-grade tumor can be expected to be cured following complete resection of tumors with systematic mediastinal lymph node dissection. Despite the fact that the prognosis of PMEC patients with lymph node metastasis was poor, no subsequent therapy was proven to be optimal. Still, further studies on the role of adjuvant chemo- or radiotherapy are necessary due to the lack of experiences.
In conclusion, PMEC is a rare malignant neoplasm with a wide spectrum of clinical manifestations and non-specific radiographic presentation. A higher tumor histological grade indicated a more aggressive behavior. Patients who had undergone complete resection for PMEC without any lymph node metastasis had excellent prognoses, even in patients with high-grade tumor. Age, pathological grade, TNM stage, and lymph node metastasis were important prognostic factors. Further prospective studies and larger, multi-centric series are needed.
Acknowledgements
We thank the patients and their families and all the investigators, including the physicians, nurses, and laboratory technicians in this study for their participation. We thank also Pathologist Jiewei Chen for his contribution in providing histopathological pictures. This project was supported by State Key Laboratory of Oncology in South China, Sun Yat-Sen University Cancer Center.
Disclosure of conflict of interest
None.
References
- 1.Yousem SA, Hochholzer L. Mucoepidermoid tumors of the lung. Cancer. 1987;60:1346–1352. doi: 10.1002/1097-0142(19870915)60:6<1346::aid-cncr2820600631>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Heitmiller RF, Mathisen DJ, Ferry JA, Mark EJ, Grillo HC. Mucoepidermoid lung tumors. Ann Thorac Surg. 1989;47:394–399. doi: 10.1016/0003-4975(89)90380-9. [DOI] [PubMed] [Google Scholar]
- 3.Miller DL, Allen MS. Rare pulmonary neoplasms. Mayo Clin Proc. 1993;68:492–498. doi: 10.1016/s0025-6196(12)60199-2. [DOI] [PubMed] [Google Scholar]
- 4.Colby T, Koss M, Travis W. Tumors of the lower respiratory tract, Armed Forces Institute of Pathology fascicle. 3rd edition. Washington, DC: Armed Forces Institute of Pathology; 1995. [Google Scholar]
- 5.Travis WD, Brambilla E, Kourad H, Muller-Hermelink HK, Harris CC. Pathology and genetics of tumors of the lung, pleura, thymus and heart. In: Kleihues P, Sobin LH, editors. WHO Classification of Tumors. 2nd edition. Lyon, France: IARC Press; 2004. [Google Scholar]
- 6.Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, Bodian C, Urken ML, Gnepp DR, Huvos A, Lumerman H, Mills SE. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25:835–845. doi: 10.1097/00000478-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Smetana HF, Iverson L, Swan LL. Bronchogenic carcinoma; an analysis of 100 autopsy cases. Mil Surg. 1952;111:335–351. [PubMed] [Google Scholar]
- 8.Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18:1059–1068. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]
- 9.Xi JJ, Jiang W, Lu SH, Zhang CY, Fan H, Wang Q. Primary pulmonary mucoepidermoid carcinoma: an analysis of 21 cases. World J Surg Oncol. 2012;10:232. doi: 10.1186/1477-7819-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Zhang W, Wu X, Sun C, Chen M, Zeng Q. Mucoepidermoid carcinoma of the lung: common findings and unusual appearances on CT. Clin Imaging. 2012;36:8–13. doi: 10.1016/j.clinimag.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Zhu F, Liu Z, Hou Y, He D, Ge X, Bai C, Jiang L, Li S. Primary salivary gland-type lung cancer: clinicopathological analysis of 88 cases from China. J Thorac Oncol. 2013;8:1578–1584. doi: 10.1097/JTO.0b013e3182a7d272. [DOI] [PubMed] [Google Scholar]
- 12.Roden AC, Garcia JJ, Wehrs RN, Colby TV, Khoor A, Leslie KO, Chen L. Histopathologic, immunophenotypic and cytogenetic features of pulmonary mucoepidermoid carcinoma. Mod Pathol. 2014 doi: 10.1038/modpathol.2014.72. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Lee GD, Kang do K, Kim HR, Jang SJ, Kim YH, Kim DK, Park SI. Surgical outcomes of pulmonary mucoepidermoid carcinoma: a review of 23 cases. Thorac Cardiovasc Surg. 2014;62:140–146. doi: 10.1055/s-0033-1342943. [DOI] [PubMed] [Google Scholar]
- 14.Kim TS, Lee KS, Han J, Im JG, Seo JB, Kim JS, Kim HY, Han SW. Mucoepidermoid carcinoma of the tracheobronchial tree: radiographic and CT findings in 12 patients. Radiology. 1999;212:643–648. doi: 10.1148/radiology.212.3.r99se09643. [DOI] [PubMed] [Google Scholar]
- 15.Fisher DA, Mond DJ, Fuchs A, Khan A. Mucoepidermoid tumor of the lung: CT appearance. Comput Med Imaging Graph. 1995;19:339–342. doi: 10.1016/0895-6111(95)00019-4. [DOI] [PubMed] [Google Scholar]
- 16.Vadasz P, Egervary M. Mucoepidermoid bronchial tumors: a review of 34 operated cases. Eur J Cardiothorac Surg. 2000;17:566–569. doi: 10.1016/s1010-7940(00)00386-9. [DOI] [PubMed] [Google Scholar]
- 17.Chin CH, Huang CC, Lin MC, Chao TY, Liu SF. Prognostic factors of tracheobronchial mucoepidermoid carcinoma-15 years experience. Respirology. 2008;13:275–280. doi: 10.1111/j.1440-1843.2007.01207.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Adams AL. Mucoepidermoid carcinoma of the bronchus: a review. Arch Pathol Lab Med. 2007;131:1400–1404. doi: 10.5858/2007-131-1400-MCOTBA. [DOI] [PubMed] [Google Scholar]
- 19.Santambrogio L, Cioffi U, De Simone M, Rosso L, Ferrero S, Giunta A. Video-assisted sleeve lobectomy for mucoepidermoid carcinoma of the left lower lobar bronchus: a case report. Chest. 2002;121:635–636. doi: 10.1378/chest.121.2.635. [DOI] [PubMed] [Google Scholar]
- 20.Ha SY, Han J, Lee JJ, Kim YE, Choi YL, Kim HK. Mucoepidermoid carcinoma of tracheobronchial tree: clinicopathological study of 31 cases. Korean Journal of Pathology. 2011;45:175–181. [Google Scholar]
- 21.Kang DY, Yoon YS, Kim HK, Choi YS, Kim K, Shim YM, Kim J. Primary salivary gland-type lung cancer: surgical outcomes. Lung Cancer. 2011;72:250–254. doi: 10.1016/j.lungcan.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Molina JR, Aubry MC, Lewis JE, Wampfler JA, Williams BA, Midthun DE, Yang P, Cassivi SD. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer. 2007;110:2253–2259. doi: 10.1002/cncr.23048. [DOI] [PubMed] [Google Scholar]
- 23.Roggenbuck C, Hau T, de Wall N, Buss H. [Simultaneous occurrence of tracheobronchopathia osteochondropastica and mucoepidermoid carcinoma] . Chirurg. 1995;66:231–234. [PubMed] [Google Scholar]


