Abstract
Objectives: To investigate the association of shear wave velocity (SWV) and its ratio (SWV ratio) using virtual touch tissue quantification (VTQ) imaging with clinicopathologic prognostic factors in women with invasive ductal breast cancer. Methods: 138 consecutive women with invasive ductal breast cancer, who were diagnosed by pathological examination, were recruited between September 2011 and October 2013. Clinicopathologic findings were investigated in each participant, including age, invasive size, lymph node status, histological grade, estrogen receptor (ER) expression, progesterone receptor (PR) expression and human epidermal growth factor receptor 2 (C-erbB-2) expression. SWV and its ratio (breast mass/adjacent breast tissue) were measured by the VTQ imaging, and univariate analysis and multivariate regression analyses were applied to investigate their relationship with all clinicopathologic abnormalities. Results: In univariate analyses, large mass size (P < 0.001), lymph node involvement (P < 0.001), High histological grade (P = 0.001) and C-erbB-2 expression (P = 0.029) were significantly associated with SWV, whereas large invasive size (P < 0.001), lymph node involvement (P = 0.001) and high histological grade (P = 0.007) were significantly related to SWV ratio. Multiple linear regression indicated that invasive size was the strongest pathologic determinant of SWV and its ratio (P < 0001). Conclusion: SWV and its ratio by the VTQ imagining were significantly associated with clinicopathologic abnormalities, and may therefore provide prognostic information in patients with invasive ductal breast cancer.
Keywords: Invasive ductal breast cancers, shear wave velocity, shear wave velocity ratio
Introduction
Acoustic radiation force impulse (ARFI) imaging is an emerging and promising ultrasound-based imaging modality for evaluation of tissue stiffness, which consists of virtual touch tissue imaging (VTI) and virtual touch tissue quantification (VTQ). When ARFI imaging is performed, the tissue in a fixed target region of interest (ROI) is mechanically excited using short-duration (less than 1 ms) acoustic pulses to generate small (1-10 mm) localized tissue displacements without the need for an external compression [1-3]. By real-time ultrasound monitoring, the result of ARFI could show immediately, which reflected the application of Young’s modulus, and provide information about tissue properties [1-3]. Impulsive acoustic radiation force (ARFI) can propagate displacement in parenchymal tissue, which is the imaging foundation of VTI and VTQ. By measuring the time of peak displacement at each lateral location, shear wave velocity (SWV) within the tissue can be calculated and presented in m/s [1]. The VTI directly reflects tissue stiffness using black and white images, whereas the VTQ presented as an objective quantitative evaluation of tissue stiffness. Increased tissue stiffness is represented by greater SWV value as it crosses through the ROI. VTI and VTQ are therefore the qualitative and quantitative diagnostic tools to non-invasively and feasibly evaluate patients’ tissue stiffness [4-7].
Accounting for 23% total cancer cases and 14% total cancer deaths, breast cancer is the most common cancer and the leading cause of cancer death among females [8], especially those in economically developing countries. The prognostic factors related to breast cancer are age, tumor size, nodal status, histological tumor grade, steroid hormone (estrogen and progesterone) receptor status, and C-erbB-2 expression. The indicators mentioned above were widely used in clinical practice, which were proven to have great prognostic value in breast cancer [9,10]. On the other hand, invasive ductal breast cancer is a group of masses which has the characteristic of histological heterogeneity, with its elasticity varying with different masses. Therefore we aimed to investigate the relationship between SWV and its ratio with clinicopathologic prognostic factors in women with invasive ductal breast cancer.
Subjects and methods
Subjects
This study was conducted in accordance with the ethical guidelines of the Helsinki Declaration and approved by the Ethics Committee of the Tenth People’s Hospital of Tongji University. From September 2011 to October 2013, 260 consecutive patients with suspected malignant breast masses were detected by VTQ, with 138 patients with invasive ductal breast cancer, who were confirmed by histopathology after surgery, were enrolled. Flowchart of the patient selection was shown in Figure 1. The inclusion criteria for the patients were as follows: (1) The diameter of lesion was larger than 7 mm, because the size of the sample ROI for VTQ is 6 mm x 5 mm; (2) No treatment was performed on the lesions; (3) Pathological result of the masses was invasive ductal cancers. According to the local legislation, all patients who were older than 18 years old gave their verbal informed consent prior to entering the study. The committee approved the consent procedures because the noninvasive technique used in this study was incorporated in a commercially available ultrasound (US) machine and its safety has been well documented [11]. The consent process was documented in a separate file after the verbal informed consent was obtained.
Figure 1.
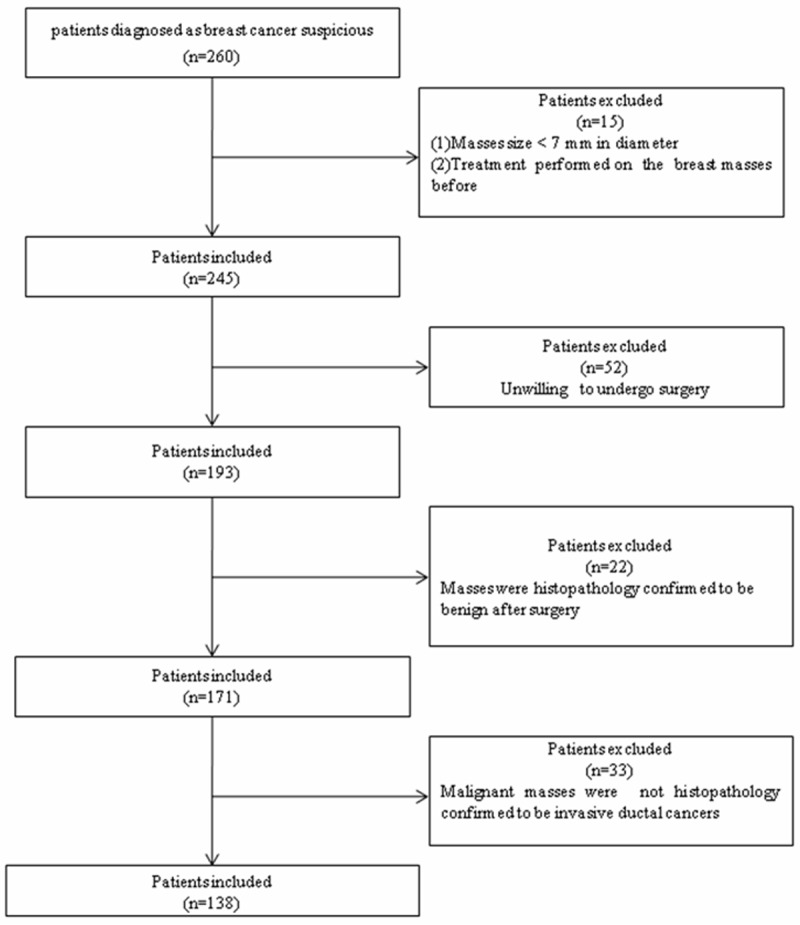
The flowchart of the selection of the patients with breast invasive ductal cancer.
Methods
The ACUSON S2000 US system (Siemens Medical Solutions, Mountain View, CA, USA) equipped with a linear array transducer 9L4 (6-18 MHz), conventional B-mode ultrasound and VTQ elasticity imaging technology was applied in the present study. In order to avoid inter-observer variability, a single sonographer with more than 5 years experience in breast examination performed all the measurements in this study. Patients with respiratory disease or large respiratory amplitude were asked to hold breath. After the image was stabilized and lesion was clearly showed, operator clicked “Update” button, the SWV was automatically measured and recorded by the inbuilt software, as well as the lesion depths (cm) from skin. The calcified regions of masses were avoided in the measurement. When ARFI is initiated, the probe emitted a acoustic pulse that propagates at a given velocity that is proportional to the tissues shear modulus, and that velocity is then estimated. SWV values were expressed as m/s, and measurement depths (cm) were recorded. In cases that were beyond the VTQ limitations of 0-9 m/s, the SWV values were shown as “x.xx m/s”. The value of “x.xx m/s” was confirmed to be 9 m/s after excluding the possible image instability influencing factors such as patient’s respiration or motion and operator’s inappropriate gesture. Then the SWV of adjacent normal breast parenchyma was also measured and recorded using the same procedure. The SWV of each lesion and adjacent normal breast parenchyma were measured seven times in the same position, direction and depth and with the same probe. In order to minimize, the highest and lowest values were removed and the rest five measurements were calculated for further analyses [11-13].
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences, version 18.0 (SPSS Inc., Chicago, IL, USA). The clinical characteristics and SWV measurements of patients with invasive ductal breast cancer were expressed as means ± standard deviations (SD). Relationships between SWV, SWV ratio and clinicopathologic variables were investigated by using univariate analysis. Multiple linear regressions were applied to perform multivariate analysis to evaluate which clinicopathologic variables were most influential factor on SWV and its ratio. P < 0.05 was considered statistically significant.
Results
According to surgical results, clinicopathologic characteristics included age, mass size, lymph node status, histological grade, estrogen and progesterone receptor status, and C-erbB-2 expression at pathologic examination were compared with SWV and SWV ratio. The 3.30 m/s for SWV and 2.16 for SWV ratio (breast mass/adjacent breast tissue) were the best cut-off value in differentiating benign and malignant breast masses in our previous preliminary study [14], while 7.03 m/s for SWV and 3.95 for SWV ratio were mean value in all masses of this study, respectively.
138 women with invasive ductal breast cancer, at a mean age of 58.1 ± 11.5 years (median, 57 year) were recruited in the present study. Mean size of breast mass was 19.97 ± 10.28 mm (median, 18.00 mm). Mean SWV were 7.03 ± 2.39 m/s (median, 7.72 m/s) for breast mass and 1.94 ± 0.64 m/s (median, 1.83 m/s) for adjacent breast tissue, respectively, whereas mean SWV ratio was 3.95 ± 1.90 (median, 3.77) (Table 1).
Table 1.
Clinical and VTQ characteristics in the 143 invasive breast ductal cancers
| Characteristic | Mean value | Median value |
|---|---|---|
| Age, year | 58.1 ± 11.50 | 57 |
| Tumor size, mm | 19.97 ± 10.28 | 18.00 |
| Mass SWV, m/s | 7.03 ± 2.39 | 7.72 |
| Adjacent breast tissue SWV, m/s | 1.94 ± 0.64 | 1.83 |
| SWV ratio | 3.95 ± 1.90 | 3.77 |
Higher histological grade was associated with higher SWV (P = 0.001) and SWV ratio values (P = 0.007) (Tables 2, 3; Figures 2, 3 and 4). In univariate analysis, large invasive size (P < 0.001), lymph node involvement (P < 0.001) and C-erbB-2 expression (P = 0.029) were statistically associated with high SWV. Large invasive size (P < 0.001) and lymph node involvement (P = 0.001) all showed significantly association with high SWV ratio (Table 3). There were no correlation between SWV or SWV ratio and certain clinicopathologic prognostic factors in invasive ductal cancers, including age, ER expression, and PR expression (P > 0.05), and the same result was found between SWV ratio and C-erbB-2 expression (Tables 2, 3). Multiple linear regression indicated that invasive size was the strongest pathologic determinant of SWV and its ratio (P < 0.001), while histological grade (P = 0.018) for SWV and lymph nodal metastasis (P = 0.031) for SWV ratio also had specific influence, respectively (Tables 4, 5).
Table 2.
Relationships between mean SWV and clinicopathologic features of 143 invasive ductal cancers
| Clinicopathologic features | < 3.30 m/s | 3.30-7.03 m/s | ≥ 7.03 m/s | Mean SWV m/s | Median SWV m/s |
|---|---|---|---|---|---|
| Agea | |||||
| < 50 (n = 28) | 4 | 5 | 19 | 6.96 ± 2.33 | 7.69 |
| ≥ 50 (n = 115) | 16 | 23 | 76 | 7.04 ± 2.41 | 7.72 |
| Tumor sizeb | |||||
| < 10 mm (n = 11) | 7 | 4 | 0 | 3.07 ± 0.73 | 2.99 |
| 10-20 mm (n = 53) | 10 | 15 | 28 | 6.30 ± 2.55 | 7.40 |
| ≥ 20 mm (n = 79) | 3 | 9 | 67 | 8.07 ± 1.51 | 9.00 |
| Lymph nodal metastasisc | |||||
| Negative (n = 89) | 17 | 25 | 47 | 6.46 ± 2.48 | 7.31 |
| Positive (n = 54) | 3 | 3 | 48 | 7.96 ± 1.89 | 9.00 |
| Histological graded | |||||
| Grade 1 (n = 12) | 5 | 3 | 4 | 5.38 ± 2.76 | 6.29 |
| Grade 2 (n = 86) | 12 | 19 | 55 | 6.76 ± 2.39 | 7.61 |
| Grade 3 (n = 45) | 3 | 6 | 36 | 7.98 ± 1.90 | 9.00 |
| ER expressiona | |||||
| Negative (n = 59) | 6 | 14 | 39 | 7.21 ± 2.21 | 7.73 |
| Positive (n = 84) | 14 | 14 | 56 | 6.90 ± 2.51 | 7.71 |
| PR expressiona | |||||
| Negative (n = 67) | 8 | 15 | 44 | 7.18 ± 2.36 | 9.00 |
| Positive (n = 76) | 12 | 13 | 51 | 6.90 ± 2.42 | 7.65 |
| C-erbB-2 expressione | |||||
| Negative (n = 39) | 11 | 6 | 22 | 5.58 ± 0.52 | 5.74 |
| Positive (n = 104) | 9 | 22 | 73 | 7.30 ± 2.22 | 7.94 |
ER: estrogen receptor, PR: C-erbB-2: progesterone receptor human epidermal growth factor receptor 2, SWV: shear wave velocity.
P > 0.05;
P < 0.001;
P < 0.001;
P = 0.001;
P = 0.029.
Table 3.
Relationships between mean SWV ratio and clinicopathologic features of 143 invasive ductal cancers
| Clinicopathologic features | < 2.16 | 2.16-3.95 | ≥ 3.95 | Median SWV ratio |
|---|---|---|---|---|
| Agea | ||||
| < 50 (n = 28) | 5 | 13 | 10 | 3.17 |
| ≥ 50 (n = 115) | 20 | 55 | 40 | 3.88 |
| Tumor sizeb | ||||
| < 10 mm (n = 11) | 9 | 2 | 0 | 1.63 |
| 10-20 mm (n = 53) | 14 | 22 | 17 | 3.13 |
| ≥ 20 mm (n = 79) | 2 | 29 | 48 | 4.29 |
| Lymph nodal metastasisc | ||||
| Negative (n = 89) | 20 | 37 | 32 | 3.43 |
| Positive (n = 54) | 5 | 10 | 39 | 4.34 |
| Histological graded | ||||
| Grade 1 (n = 12) | 4 | 3 | 5 | 3.50 |
| Grade 2 (n = 86) | 18 | 35 | 33 | 3.34 |
| Grade 3 (n = 45) | 3 | 15 | 27 | 4.25 |
| ER expressiona | ||||
| Negative (n = 59) | 7 | 25 | 27 | 3.78 |
| Positive (n = 84) | 18 | 28 | 38 | 3.72 |
| PR expressiona | ||||
| Negative (n = 67) | 9 | 28 | 30 | 3.82 |
| Positive (n = 76) | 16 | 25 | 35 | 3.70 |
| C-erbB-2 expressiona | ||||
| Negative (n = 39) | 1 | 10 | 28 | 3.78 |
| Positive (n = 104) | 14 | 41 | 49 | 3.76 |
ER: estrogen receptor, PR: C-erbB-2: progesterone receptor human epidermal growth factor receptor 2, SWV ratio (breast mass/adjacent breast tissue): shear wave velocity ratio.
P > 0.05;
P < 0.001;
P = 0.001;
P = 0.007.
Figure 2.
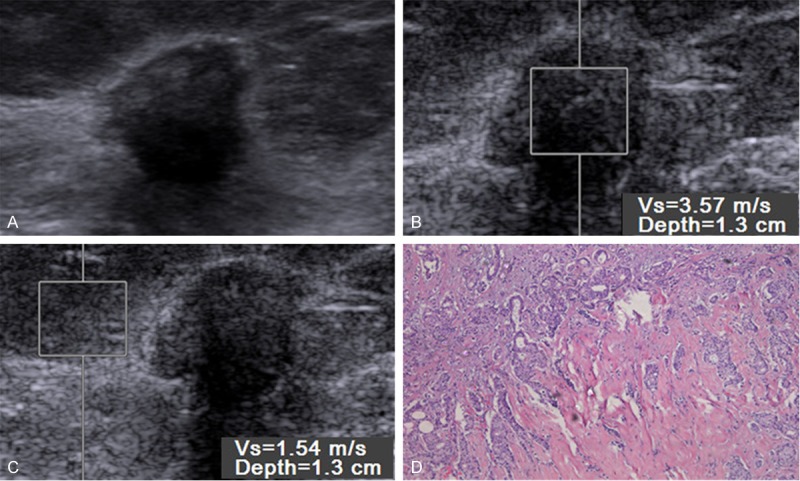
Gray-scale images (A), virtual touch tissue quantification (VTQ) imaging (B, C) and histopathology (D) in 80-year-old woman with a 9 mm, grade 1, negative-lymph nodal metastasis and human epidermal growth factor receptor 2 (C-erbB-2) expression, positive-estrogen receptor (ER) and progesterone receptor (PR) expression. VTQ of the mass (B) shows the SWV is 3.57 m/s and the SWV of the adjacent breast tissue (D) is 1.54 m/s.
Figure 3.
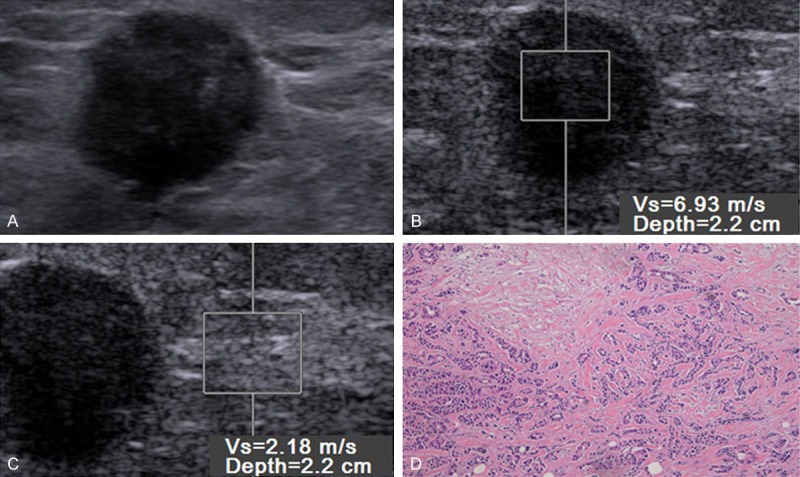
Gray-scale images (A), virtual touch tissue quantification (VTQ) imaging (B, C) and histopathology (D) in 55-year-old woman with a 15 mm, grade 2, negative-lymph nodal metastasis, positive-estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (C-erbB-2) expression. VTQ of the mass (B) shows the SWV is 6.93 m/s and the SWV of the adjacent breast tissue (D) is 2.18 m/s.
Figure 4.
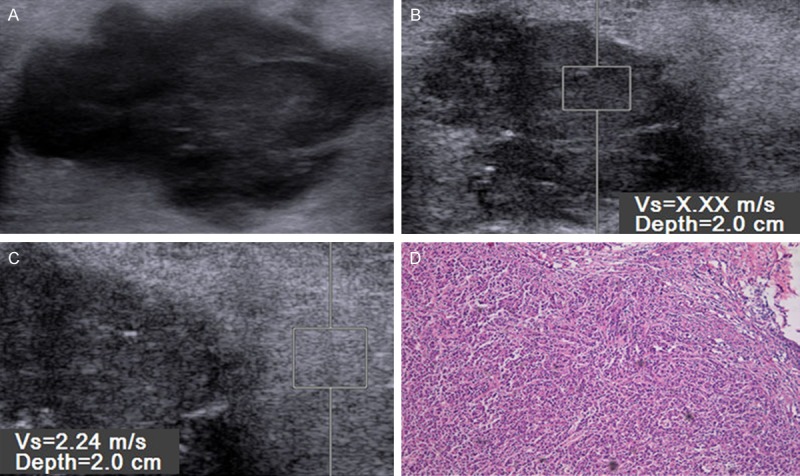
Gray-scale images (A), virtual touch tissue quantification (VTQ) imaging (B, C) and histopathology (D) in 62-year-old woman with a 35 mm, grade 3, positive-lymph nodal metastasis, estrogen receptor (ER) and progesterone receptor (PR) expression, and human epidermal growth factor receptor 2 (C-erbB-2) expression. VTQ of the mass (B) shows the SWV is x.xx m/s and the SWV of the adjacent breast tissue (D) is 2.24 m/s.
Table 4.
Multivariate Linear regression analysis of pathologic factors and mean SWV
| Characteristic | Beta value | P value |
|---|---|---|
| Mass Size | 0.504 | < 0.001 |
| Histological grade | 0.170 | 0.025 |
| Lymph nodal metastasis | 0.124 | 0.086 |
Beta: Standardized coefficients.
Table 5.
Multivariate Linear regression analysis of pathologic factors and mean SWV ratio
| Characteristic | Beta value | P value |
|---|---|---|
| Mass Size | 0.399 | < 0.001 |
| Histological grade | 0.139 | 0.088 |
| Lymph nodal metastasis | 0.166 | 0.034 |
Beta: Standardized coefficients.
Discussion
Incidence of breast cancer is increasing in most countries, reaching a 1.5% average annual rise since 1995 [15]. Breast cancer caused great harm to female health, and invasive ductal cancer is the most common pathological type of the disease. Prognosis of invasive ductal cancers is determined by several factors, such as age, invasive size, lymph node status, vascular invasion status, histological grade, hormonal receptors (ER, PR) and C-erbB-2 expression. Tumor molecular biology is the decisive factor of biological behavior and histopathological appearance, and then has a great influence on elastic features. Correlations between radiologic findings and pathologic prognostic factors of invasive ductal breast cancer have shown that there is a poor correlation between mammographic and ultrasound size of invasive lobular carcinoma with the histological size [16]. Breast cancer has higher cure rate when detected early and treated appropriately. Our research shows that SWV and its ratio are related with clinicopathologic factors, which would help to initiate an early intervention to this malignant disease. Secondly, in recent years, number of young breast cancer patients (< 35 year) are growing, therefore, more attention should be paid about this situation. Age is an independent prognostic factor for the overall survival and recurrence rate [17], but no correlation was found between age and SWV or its ratio in women with invasive ductal breast cancer.
Mass size, as the most important prognostic indicator, was confirmed by tumor autopsy specimens [18-21]. Within each tumor-size group (< 10 mm, 10-20 mm, and ≥ 20 mm), SWV and SWV ratio are higher if the diameter is greater or mass stiffness is harder. It is well known that axillary lymph node status and histological grade have been found to be independent prognosis factor, and will be beneficial for the adjuvant therapeutic planning [19-22]. Survival rates are close to 80% in patients with axillary lymph nodal metastasis negative, for 21% only four or more lymph nodal metastasis. In this study, the SWV and its ratio in lymph nodal metastasis (+) are higher than those in lymph nodal metastasis (-). Higher pathological grade was associated with higher SWV and its ratio.
Although there are several molecular markers with prognostic utility in breast cancer, hormonal receptors, including ER and PR, and C-erbB-2 play a significant role in tumor prognosis in patients with breast cancer. As biomarkers, hormonal receptors (ER and PR) are currently used to determine whether a patient needs adjuvant endocrine therapy [23]. Patients with estrogen receptor (ER and PR) negative cancer have a poor prognosis compared with those with ER-positive cancer [24], accounting for approximately 30% of the primary breast cancer [23]. SWV and its ratio are not associated with ER and PR expression. Phosphorylation of signal transduction was activated by C-erbB-2 protein, and overexpression of the C-erbB-2 protein, reaching approximately 30% of breast cancers, holds an important status in breast cancer pathogenesis, which is commonly considered as a predictor of a poor prognosis in early breast cancer. In addition, it has shown that positive-C-erbB-2 expression might be related to poor prognosis, and possible drug resistance in numerous clinical and pre-clinical results [25-28]. In this study, low SWV in breast cancer mass was associated with reduced C-erbB-2 expression (P = 0.029), while SWV ratio was irrelevant to C-erbB-2 expression.
In multiple linear regression, mass size was the most significantly influential factor on SWV and its ratio as measured with VTQ, followed by histological grade lymph for SWV and nodal metastasis for SWV ratio, which indicates that mass size may be extremely useful as a predictor of prognosis in patients with invasive ductal cancer.
In this study, SWV ratio had ruled out the influence of adjacent breast tissue stiffness, since there are differences in stiffness. This study had some limitations. First, although the results of tumor and adjacent breast tissue SWV values were prospectively recorded, the selection bias may have existed under this retrospective design. Second, our study was only conducted in patients with invasive ductal cancer, but not in all patients with various breast cancer. Third, vascular invasion factor was taken into consideration in this study, which is a crucial prognosis indicator, since only one patient with invasive ductal cancer has vascular invasion investigation. Fourth, this study did not include long-term follow-up. A large series study with a prospective design and long-term follow-up is warranted. Lastly, the one person only assigning all the measurements which is another bias. In conclusion, on the basis of univariate analyses, large mass size, lymph node involvement, and high histological grade were significantly associated with high SWV and SWV at VTQ elastography. Positive-C-erbB-2 expression showed its associations with increased SWV ratio by VTQ elastography. In multivariate analyses, large invasive size and lymph node involvement were significantly related to high SWV, whereas large invasive size and high histological grade show significant associations with increased SWV ratio. Virtual touch tissue quantification may be useful in predicting prognosis of invasive ductal breast cancer.
Disclosure of conflict of interest
None.
References
- 1.Palmeri ML, Wang MH, Dahl JJ, Frinkley KD, Nightingale KR. Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med Biol. 2008;34:546–558. doi: 10.1016/j.ultrasmedbio.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nightingale K, Soo MS, Nightingale R, Trahey G. Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med Biol. 2002;28:227–235. doi: 10.1016/s0301-5629(01)00499-9. [DOI] [PubMed] [Google Scholar]
- 3.Nightingale K, McAleavey SA, Trahey G. Shear-wave generation using acoustic radiation force: in vivo and ex vivo results. Ultrasound Med Biol. 2003;29:1715–1723. doi: 10.1016/j.ultrasmedbio.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Fahey BJ, Nelson RC, Bradway DP, Hsu SJ, Dumont DM, Trahey GE. In vivo visualization of abdominal malignancies with acoustic radiation force elastography. Phys Med Biol. 2008;53:279–293. doi: 10.1088/0031-9155/53/1/020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nightingale K, Palmeri M, Trahey G. Analysis of contrast in images generated with transient acoustic radiation force. Ultrasound Med Biol. 2006;32:61–72. doi: 10.1016/j.ultrasmedbio.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Gallotti A, D’Onofrio M, Mucelli RP. Acoustic Radiation Force Impulse (ARFI) technique in ultrasound with Virtual Touch tissue quantification of the upper abdomen. Radiol Med. 2010;115:889–897. doi: 10.1007/s11547-010-0504-5. [DOI] [PubMed] [Google Scholar]
- 7.Goertz RS, Amann K, Heide R, Bernatik T, Neurath MF, Strobel D. An abdominal and thyroid status with Acoustic Radiation Force Impulse Elastometry--a feasibility study: Acoustic Radiation Force Impulse Elastometry of human organs. Eur J Radiol. 2011;80:e226–230. doi: 10.1016/j.ejrad.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt M, Voelker HU, Kapp M, Krockenberger M, Dietl J, Kammerer U. Glycolytic phenotype in breast cancer: activation of Akt, up-regulation of GLUT1, TKTL1 and down-regulation of M2PK. J Cancer Res Clin Oncol. 2010;136:219–225. doi: 10.1007/s00432-009-0652-y. [DOI] [PubMed] [Google Scholar]
- 10.Singletary SE, Connolly JL. Breast cancer staging: working with the sixth edition of the AJCC Cancer Staging Manual. CA Cancer J Clin. 2006;56:37–47. doi: 10.3322/canjclin.56.1.37. [DOI] [PubMed] [Google Scholar]
- 11.Zhang YF, Xu HX, He Y, Liu C, Guo LH, Liu LN, Xu JM. Virtual touch tissue quantification of acoustic radiation force impulse: a new ultrasound elastic imaging in the diagnosis of thyroid nodules. PLoS One. 2012;7:e49094. doi: 10.1371/journal.pone.0049094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu JM, Xu XH, Xu HX, Zhang YF, Zhang J, Guo LH, Liu LN, Liu C, Zheng SG. Conventional US, US Elasticity Imaging, and Acoustic Radiation Force Impulse Imaging for Prediction of Malignancy in Thyroid Nodules. Radiology. 2014;33:585–595. doi: 10.1148/radiol.14132438. [DOI] [PubMed] [Google Scholar]
- 13.Guo LH, Xu HX, Fu HJ, Peng A, Zhang YF, Liu LN. Acoustic radiation force impulse imaging for noninvasive evaluation of renal parenchyma elasticity: preliminary findings. PLoS One. 2013;8:e68925. doi: 10.1371/journal.pone.0068925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao MH, Wu J, Zou LL, Xu G, Xie J, Wu R, Xu HH. Diagnostic value of virtual touch tissue quantification for breast lesions with different size. Biomed Res Int. 2014;2014:142504. doi: 10.1155/2014/142504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 16.Mann RM. The effectiveness of MR imaging in the assessment of invasive lobular carcinoma of the breast. Magn Reson Imaging Clin N Am. 2010;18:259–276. doi: 10.1016/j.mric.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Han W, Kim SW, Park IA, Kang D, Kim SW, Youn YK, Oh SK, Choe KJ, Noh DY. Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer. 2004;17:82. doi: 10.1186/1471-2407-4-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming ST, Rastogi A, Dmitrienko A, Johnson KD. A comprehensive prognostic index to predict survival based on multiple comorbidities: a focus on breast cancer. Med Care. 1999;37:601–614. doi: 10.1097/00005650-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Cho N, Kim SJ, Cha JH, Cho KS, Ko ES, Moon WK. Correlation between high resolution dynamic MR features and prognostic factors in breast cancer. Korean J Radiol. 2008;9:10–18. doi: 10.3348/kjr.2008.9.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuncbilek N, Karakas HM, Okten OO. Dynamic magnetic resonance imaging in determining histopathological prognostic factors of invasive breast cancers. Eur J Radiol. 2005;53:199–205. doi: 10.1016/j.ejrad.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Szabó BK, Aspelin P, Kristoffersen WM, Tot T, Boné B. Invasive breast cancer: correlation of dynamic MR features with prognostic factors. Eur Radiol. 2003;13:2425–2435. doi: 10.1007/s00330-003-2000-y. [DOI] [PubMed] [Google Scholar]
- 22.Fisher ER, Sass R, Fisher B. Pathologic findings from the National Surgical Adjuvant Project for Breast Cancers (protocol no. 4). X. Discriminants for tenth year treatment failure. Cancer. 1984;53:712–723. doi: 10.1002/1097-0142(19840201)53:3+<712::aid-cncr2820531320>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 23.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J. Clin. Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 24.Yaghan R, Stanton PD, Robertson KW, Going JJ, Murray GD, McArdle CS. Oestrogen receptor status predicts local recurrence following breast conservation surgery for early breast cancer. Eur J Surg Oncol. 1998;24:424–426. doi: 10.1016/s0748-7983(98)92341-1. [DOI] [PubMed] [Google Scholar]
- 25.Lovekin C, Ellis IO, Locker A, Robertson JF, Bell J, Nicholson R, Gullick WJ, Elston CW, Blamey RW. c-erbB-2 oncoprotein expression in primary and advanced breast cancer. Br J Cancer. 1991;63:439–443. doi: 10.1038/bjc.1991.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dittadi R, Gion M. More about: prognostic importance of low c-erbB2 expression in breast tumors. J Natl Cancer Inst. 2000;92:1443–1444. doi: 10.1093/jnci/92.17.1443. [DOI] [PubMed] [Google Scholar]
- 27.Muss HB, Thor AD, Berry DA, Kute T, Liu ET, Koerner F, Cirrincione CT, Budman DR, Wood WC, Barcos M. C-erb-B2 expression and response to adjuvant therapy in women with node-positive early breast cancer. N Engl J Med. 1994;330:1260–1266. doi: 10.1056/NEJM199405053301802. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton A, Piccart M. The contribution of molecular markers to the prediction of response in the treatment of breast cancer: A review of the literature on HER2, p53 and BCL-2. Ann Oncol. 2000;11:647–663. doi: 10.1023/a:1008390429428. [DOI] [PubMed] [Google Scholar]


