Abstract
Solid pseudopapillary neoplasms (SPNs) of the pancreas are very rare neoplasms. The present study is to summarize our experience of the diagnosis, surgical treatment and prognosis of SPNs. The clinical data of 19 cases that underwent surgery for pathologically confirmed SPNs, admitted in our hospital from Mar. 2007 to Mar. 2013, were analyzed retrospectively. The clinicopathologic feature, surgical treatment and prognosis were described in detail. The 19 patients were 17 females and 2 males, with a median age of 29 years. All patients had curative resections, including eight distal pancreatectomies with splenectomy, four spleen-preserving distal pancreatectomies, two pancreaticoduodenectomies, two pylorus-preserving pancreaticoduodenectomies, two duodenum-preserving pancreatic head resections and one central pancreatectomy. The tumors were 6.3 cm in diameter on average, and were mostly located in the body or tail of the pancreas (63.2%). Pathologically, the tumors contained a mixture of solid, cystic, and pseudopapillary patterns in various proportions. None of the patients had lymph nodes metastases and local invasion. All patients were alive and disease-free at a median follow-up of 38.4 months. SPNs are rare neoplasms, typically affecting young women without notable symptoms, with a low malignant potential but excellent prognosis. Radical surgical resection with clear margins is the treatment of choice.
Keywords: Solid pseudopapillary tumor, pancreatic neoplasm, clinicopathologic feature, treatment, prognosis
Introduction
The solid pseudopapillary neoplasm (SPN) of the pancreas was first described by Frantz in 1959 [1]. It is a rare neoplasm of low malignant potential, accounting for 0.17-2.7% of all pancreatic tumors [2]. The tumors have been given several different names such as a papillary epithelial neoplasm, solid and cystic tumor, solid and papillary tumor, papillary cystic tumor, and solid and papillary epithelial neoplasm because of its typical histological features including cystic, solid, and pseudopapillary structures [3]. The World Health Organization (WHO) defined these tumors as solid-pseudopapillary tumors (SPTs) in 1996 [4] and reclassified them as solid-pseudopapillary neoplasms (SPNs), a low-grade malignant neoplasm of the exocrine pancreas in 2010 [5]. SPNs primarily affect young women and are usually treated with surgical resection [6]. Recently, the number of cases reported in the literature has been steadily rising [7-10]; however, the pathogenesis and guidelines for SPNs treatment remain challenging and still enigmatic. Herein, we report the clinical, histopathological, immunohistochemical, therapeutic characteristics and outcomes of 19 cases of SPNs.
Materials and methods
The clinical data of 19 cases that underwent surgery for pathologically confirmed SPNs, admitted in the Department of General Surgery, Affiliated Hospital of Xuzhou Medical College from Mar. 2007 to Mar. 2013, were analyzed retrospectively. The clinical presentation, radiological details, clinicopathologic feature, type of surgical procedure, operative time, postoperative complications, and prognosis were collected and described in detail. Pancreatic fistulas were defined according to the concept of International Study Group on Pancreatic Fistula [11]. Independent pathological assessments were made of tumor location, size, the resection margin status, pattern of growth, lymphovascular space invasion, perineural invasion, cellular atypia. Fourteen patients were assessed by immunohistochemical staining to confirm the diagnosis. Pathologically, SPNs were defined as malignant if they demonstrated extrapancreatic invasion, distant metastases, pancreatic parenchymal invasion, or perineural or vascular invasion [12]. Long-term survival data and follow-up information were collected using the patients’ medical records and telephone interviews. The study protocol was approved by the Institutional Review Board.
Results
Patient characteristics
The 19 patients included 17 females and 2 males (female: male=8.5:1), with a median age of 29 years (range 15-70 years). The clinical presentation was unspecific including abdominal discomfort (6, 31.6%), abdominal dull pain (4, 21.1%), abdominal distension (2, 10.5%), and vomiting (2, 10.5%). Five patients (26.3%) were asymptomatic and their SPNs were found incidentally during routine physical examinations. The patients had median symptom duration of 2.6 months (range 3 days to 12 months). The tumors were 6.3 cm in diameter on average (range 3.5 cm to 13 cm), and were located in the body or tail in twelve patients, the head in six patients, and the neck in one patients. The tumor markers (CA19-9, CEA, CA125 and AFP) detected in 15 of the 19 patients were normal. None of the patients has jaundice and history of pancreatic neoplasm or pancreatitis. The clinical features of the 19 patients were listed in Table 1.
Table 1.
Clinicopathologic features of 19 patients with SPNs
| Parameter | Patient number (n=19) | % |
|---|---|---|
| Age (years, mean) | 29 (15-70) | |
| Sex | ||
| Female | 17 | 89.5% |
| Male | 2 | 10.5% |
| Symptoms | ||
| Abdominal dull pain | 4 | 21.1% |
| Abdominal discomfort | 6 | 31.6% |
| Abdominal distension | 2 | 10.5% |
| Vomiting | 2 | 10.5% |
| Asymptomatic | 5 | 26.3% |
| Size (cm, mean) | 6.3 (3.5-13) | |
| Location | ||
| Body or tail | 12 | 63.2% |
| Head | 6 | 31.6% |
| Neck | 1 | 5.2% |
| Tumor feature | ||
| Solid and cystic | 16 | 84.2% |
| Solid | 3 | 15.8% |
| Surgical treatment | ||
| Distal pancreatectomy with splenectomy | 8 | 42.1% |
| Spleen-preserving distal pancreatectomy | 4 | 21.1% |
| Duodenum-preserving pancreatic head resection | 2 | 10.5% |
| Pylorus-preserving pancreaticoduodenectomy | 2 | 10.5% |
| Pancreaticoduodenectomy (Whipple) | 2 | 10.5% |
| Central pancreatectomy | 1 | 5.6% |
| Follow-up (months, mean) | 38.4 (3-72) | |
| Outcome | ||
| Alive | 19 | 100% |
| Dead | 0 | 0% |
Preoperative examination and diagnosis
All 19 patients underwent radiological investigations before operation, including computed tomography (CT) in twelve patients, ultrasonography (US) in eight patients, magnetic resonance imaging (MRI) in four patients, and US-guided fine needle aspiration cytology (FNAC) in two patients. Figure 1 showed CT and MRI images of the SPNs, located in the head, neck and body or tail of pancreas. The characteristic imaging findings included a well-encapsulated, heterogeneous mass with solid and cystic lesions (16, 84.2%; Figure 1), only a solid component (3, 15.8%), calcification (6, 31.6%; Figure 1), hemorrhage or necrosis (5, 26.3%). None of the patients was found to have hepatic and lymph nodes metastases and portal vein invasion on imaging. MRI can help to identify intratumoral hemorrhage. A definitive preoperative diagnosis was difficult and a correct diagnosis was made in 5 patients, suspected diagnosis in 2 patients. Two patients underwent preoperative US-guided fine needle aspiration cytology, which correctly diagnosed as SPNs. Misdiagnoses included pancreatic adenocarcinomas in 3 patients, cystadenomas in 3 patients, islet cell tumors in 1 patient, pancreatic cysts in 3 patients and gastrointestinal stromal tumors in 2 patients.
Figure 1.
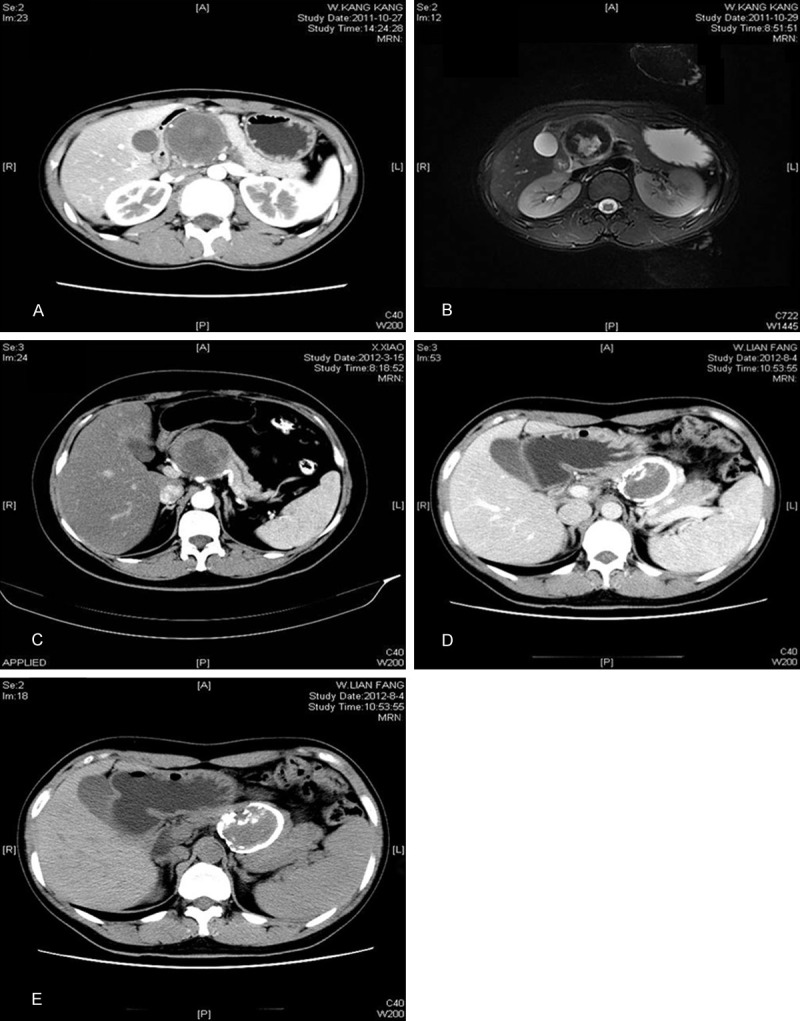
CT and MRI scans. Contrast-enhanced CT (A) and MRI (B) show a mixture-density mass located in the head of the pancreas. Contrast-enhanced CT (C) shows a solid and cystic mass located in the neck of the pancreas. CT (D and E) shows a solid mass with an annula calcification located in the body tail of the pancreas.
Surgical data
All 19 patients underwent surgical exploration. The surgical procedures included distal pancreatectomy with splenectomy (8, 42.1%), spleen-preserving distal pancreatectomy (4, 21.1%), pancreaticoduodenectomy (Whipple, 2, 10.5%), pylorus-preserving pancreaticoduodenectomy (2, 10.5%), duodenum-preserving pancreatic head resection (2, 10.5%) and central pancreatectomy (1, 5.6%). The mean operative time was 3.3 hours (range 2-5 hours). The intraoperative blood loss was estimated about 250 mL (range 100-1200 mL). Blood transfusion requirements were mean 2.8 units (range 2-4 units) in five patients during surgery.
All the patients underwent R0 resections and there were no surgical mortalities. Postsurgical complications occurred in five patients. One patient had wound infection four days after surgery. Another patient had been found to have a pseudocyst (3 cm in diameter) 3 months after surgery. Three patients had pancreatic fistula. These patients were conservatively managed with a successful outcome. The median postsurgical stays were 10.5 days (range 7 to 19 days).
Pathological features
All the tumors were solitary and isolated mass with cystic and solid components. Grossly, the tumors were well encapsulated and well demarcated from the pancreas. The cut surface showed white-gray and large spongy areas of hemorrhage alternating with both solid and cystic degeneration. Microscopically, the tumors contained a mixture of solid, cystic, and pseudopapillary patterns in various proportions. There were fibrous capsules around the tumors and the lesions were sometimes accompanied by calcification like egg shell. The tumors contained small and uniform tumor cells with round nuclei with both solid and cystic growth patterns (Figure 2). The typical features included a pseudopapillary pattern with fibrovascular stalks, formed by several layers of tumor cells surrounding delicate vascular cores (Figure 2A). Solid areas were composed of sheets and cords of discohesive tumor cells with extensive microcystic space formation and apparent hyaline degeneration in the stroma (Figure 2B). Blood lakes were present at the periphery of the neoplasm. Tumor cell nucleus was relatively uniform and round-ovoid, with few mitotic figures. Neither invasive neoplastic components nor metastasis to lymph nodes was identified in all tumors. Figure 2 shows the histopathologic image results.
Figure 2.
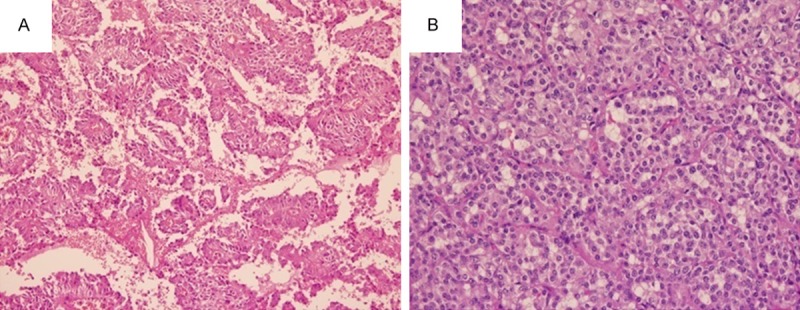
Histopathology of SPN. (H&E × 100). A. the tumor showed papillary structures with intervening cyst-like spaces; B. solid areas showed monotonous cell population with myxohyline stroma, arranged around delicate fibrovascular septa.
Immunohistochemical studies were performed in 14 cases. Results were typically positive for vimentin (Vim, 11/11, 100%), progesterone receptor (PR, 14/14, 100%), synaptophysin (Syn, 12/12, 100%), CD56 (11/11, 100%), neuronspecific enolase (NSE, 9/10, 90%), CD10 3/3 (100%). In addition, chromaffin granule protein A (CgA) was detected in 9 patients and the positive rate was 2/9 (22.2%). Cytokeratin (CK) was detected in 2/3 (66.7%), CK8 in 4/6 (66.7%), CK18 in 5/6 (83.3%), estrogen receptor (ER) in 0/2 (0%), S100 in 0/2 (0%). Ki-67 was detected in 5 patients without pancreatic parenchyma invasion and the index was < 1%. The immunohistochemical features of the tumors were characterized in 14 patients (Table 2).
Table 2.
Immunohistochemical features of SPNs (n=14)
| Antigen | Total number | Positive (%) | Negative (%) |
|---|---|---|---|
| Neuron-specific enolase | 10 | 9 (90%) | 1 (10%) |
| Vimentin | 11 | 11 (100%) | 0 (0%) |
| Progesterone receptor | 14 | 14 (100%) | 0 (0%) |
| β-catenin | 12 | 12 (100%) | 0 (0%) |
| Synaptophysin | 12 | 12 (100%) | 0 (0%) |
| CD56 | 11 | 11 (100%) | 0 (0%) |
| Cytokeratin | 3 | 2 (66.7%) | 1 (33.3%) |
| CK8 | 6 | 4 (66.7%) | 2 (33.3%) |
| CK18 | 6 | 5 (83.3%) | 1 (16.7%) |
| Estrogen receptor | 2 | 0 (0%) | 2 (100%) |
| Chromaffin granule protein A | 9 | 7 (77.8%) | 2 (22.2%) |
| CD10 | 3 | 3 (100%) | 0 (0%) |
| S100 | 2 | 0 (0%) | 2 (100%) |
| Ki-67 | 5 | < 1% |
Follow-up
Eighteen of 19 patients had been followed up, including clinical examination, routine laboratory tests, abdominal US, and CT or MRI every 3 months. The follow-up time was for a mean duration of 38.4 months (range 3 to 72 months) and all patients were alive with no evidence of disease recurrence or metastasis. Adjuvant chemotherapy or radiotherapy was not given to any of the patients.
Discussion
SPNs represent exceedingly rare neoplasms, accounting for 1-2% of exocrine pancreas neoplasms and 3% of all cystic pancreas neoplasms [13,14]. SPNs can be observed in patients of all ages, but usually affect young women at a mean age of 27.2 years with a female: male ratio of 8.37:1 [15]. In our series, the proportion of women was 89.5% (17/19), and the female to male ratio was 8.5:1. The current study suggested that SPNs occurred in patients between 15 and 70 years of age, and the median age at diagnosis was 29 years. The clinical presentation of SPNs was nonspecific. Most patients have unclear clinical features including abdominal discomfort, mild abdominal pain and nearly one third of the patients were asymptomatic as presented in our cases. Other uncommon clinical symptoms are poor appetite and nausea, loss of weight, vomiting and (very rarely) jaundice and hematemesis [16,17]. Due to its slow growth and asymptomatic presentation, the tumors reached large proportions at the time of identification. The mean size was 9.5 cm; however, tumors measuring 30 cm in size had been described [14,18]. In our series, the largest tumor was 13 cm, the smallest was 3.5 cm, and the mean tumor size was 6.3 cm. The most common localization of SPNs was the tail and body of the pancreas (64%) [14]. Unusual presentations include multicentric tumors in the pancreas [19]. The current study found that all the tumors were solitary and isolated mass, and 63.2% tumors (12/19) were located in the pancreatic body or tail.
The origin of SPNs is unclear, some of the studies have suggested that they arise from pancreatic pluripotent stem cells [3,20] or from cell lines of the female genital bud [21]. Beta-catenin mutations, alterations of the Wnt pathway and disorganisation of E-cadherin have been implicated in the occurrence of SPNs [22,23]. The common expression of progesterone receptor and the strong predilection for females suggest that they might be hormone-dependent tumors [24]. However, estrogen receptors have not been demonstrated. Another hypothesis is an extra pancreatic origin from genital ridge anlage related cells [25,26]. Park M et al [27] provided a molecular mechanism for solid-pseudopapillary neoplasm tumorigenesis. They proposed that SPNs were characterized by activation of Wnt/b-catenin, Hedgehog, androgen receptor signaling pathways and epithelial-mesenchymal transition based on the signature of differentially expressed mRNAs in SPNs and the downregulated miR-200 family and miR-192/215 were related to the upregulation of genes belong to epithelial-mesenchymal transition in SPNs.
Macroscopically, these tumors manifest both cystic and solid components with hemorrhagic areas, fibrous capsules and sometimes calcification. On cross-section, the tumor is yellow-brown in color with hemorrhagic areas. Histologically, SPNs are commonly well-circumscribed and -encapsulated with irregular degenerative cystic cavities and hemorrhages. The tumors contain a mixture of solid, cystic, and pseudopapillary patterns in various proportions. The solid portions of the tumors are composed of uniform and polygonal epithelioid cells with well-vascularized stroma and a discohesive arrangement [21]. The key histological hallmarks are solid and pseudopapillary proliferation of homomorphous cells without increased mitoses or cytological atypia [25,26]. Immunohistochemically, SPNs include positive staining for β-catenin, vimentin, progesterone receptor, CD56, neuron-specific enolase, CD10, cyclin D1 and more recently, negative membranous E-cadherin [28,29]. In our cases, results were typically positive for vimentin (11/11, 100%), progesterone receptor (14/14, 100%), β-catenin (12/12, 100%), synaptophysin (12/12,100%), CD56 (11/11, 100%), CD10 (3/3, 100%), neuronspecific enolase (9/10, 90%), chromaffin granule protein A (7/9, 77.8%). The Ki-67 index has been suggested as a potential indicator of malignant potential and poor outcome of SPNs [30]. The low Ki-67 index (≤ 5%) indicates a slow growth of the tumors. Our 5 patients displayed a low Ki-67 index of < 1%. Vassos N et al suggested that abnormal nuclear and cytoplasmic β-catenin expression and presence of progesterone receptor were fairly common features of SPNs [31].
The preoperative diagnosis of SPNs is difficult. In our cases, only 7 patients were diagnosed as or suspected of SPNs, and the misdiagnosis rate was 63.2%. Imaging techniques are of great importance. Intratumoral hemorrhage and septa are characteristic features of SPNs. Ultrasound and CT/MRI-scans typically reveal a sharply well-circumscribed, heterogeneous mass with varying solid and cystic components, generally demarcated by a peripheral capsule and occasional calcification. CT is the most frequently used method for diagnosing SPNs, and shows the presence of a heterogeneously enhanced solid and cystic mass [32]. MRI is superior to CT in distinguishing certain tissue characteristics, such as haemorrhage, cystic degeneration or the presence of a capsule and may suggest correct diagnosis [33]. Therefore, a radiologic diagnosis would be helpful when surgery is planned. The preoperative histological diagnosis can be made definitely by an endoscopic ultrasound scan (EUS) with fine-needle aspiration (FNA) biopsy [34,35] or percutaneous core needle biopsy with ultrasound or CT-guidance [36]. The US-guided FNAC was used in 2 of our 19 patients without any complications and preoperative diagnosis could be confirmed. The differential diagnosis of SPNs is wide and includes in particular solid and cystic lesions such as serous microcystic adenoma, cystadenocarcinoma, mucinous cystic neoplasms, cystic neuroendocrine tumors, cystic acinar cell carcinoma, teratoma, pancreatoblastoma as well as a variety of congenital and acquired dysontogenetic, post-inflammatory and infectious cysts [37]. According to our experience, the typical constellation of a pancreas-associated solid and cystic mass in a young woman should always alert to the possibility of SPNs. FNAC should be performed when preoperative diagnosis need to be confirmed.
SPNs usually behave in an indolent fashion, but certainly have low malignant potential. Malignant behaviors were observed in about 10-15% of the cases; some of them had been treated with aggressive resection [31]. Currently, complete aggressive surgical resection is the treatment of choice for SPNs, even with local invasion or metastasis [38]. The surgical approach depends on the location, size, and nature of the neoplasms, as well as the time of surgery [39]. In our study, distal pancreatectomies with/without splenectomy (12, 63.2%) were the most common surgical procedures because the tumors were frequently located in the body and tail of the pancreas. Tumor enucleation and incomplete excision should be avoided due to the risk of tumor dissemination, development of a pancreatic fistula [40] and the higher recurrence rate [31]. Surgeons should always aim for complete en-bloc resection including adjacent structures preferably with microscopically clear margins. Intraoperative frozen section may be helpful to ascertain the adequate of the resection margins [10]. Extensive lymphatic dissection or resection of adjacent structures is not warranted, due to the rare incidence of lymph node metastasis (< 2%) [41,42]. In our cases, all the patients underwent R0 resections and all histologically examined lymph nodes were negative. Unlike other pancreatic tumors, the stage of the disease does not play any role for the treatment of SPNs [41]. If veins were infiltrated, vascular en-bloc resection and reconstruction with vein grafts had been proposed and the results were encouraging [41]. Resection of distant metastases should be performed at the time of primary resection or even for recurrences.
There is no consensus of treatment plan for the unresectable SPNs. Other therapeutic options such chemotherapy and radiotherapy had been applied in some cases [43], Fried et al observed substantial shrinkage of an unresectable tumor after 6 weeks of radiotherapy [43]. A favorable response to radiotherapy in locally advanced unresectable disease had also been reported [44]. The role of neoadjuvant chemotherapy was described in a case of advanced disease with local invasion and unresectable SPNs with good response [45,46]. S. Krug et al reported that the selective internal radiotherapy (SIRT) was a suitable approach for inducing long-term remissions of the strongly vascularized liver metastases, and the patient was in a good condition without any evidence for hepatic recurrence four years after SIRT [47]. In our cases, none of the tumors had local invasion or metastasis. Adjuvant chemotherapy or radiotherapy was not given to any of the patients.
The prognosis of SPNs patients even with local recurrence and metastasis or invasion is generally excellent. The overall 5-year survival rate of SPNs is about 95% [2]. Although the malignant potential of SPNs is low, approximately 10-15% of patients develop metastatic disease, most often involving the liver or peritoneum [20]. Therefore, all SPNs patients need long-term follow-up. In our cases, all patients were alive with no evidence of disease recurrence or metastasis for a mean duration of 38.4 months (range 3 to 72 months).
In conclusion, SPNs are rare neoplasms of unclear histogenesis that typically affects young females without notable symptoms, with a malignant potential, but an excellent prognosis. Appearance on imaging is fairly characteristic and may suggest diagnosis, but in unclear cases preoperative diagnosis should be accomplished by percutaneous CT/US-guided core needle biopsy. Adequate surgical resection is the only effective treatment option and is warranted even in the presence of local invasion or metastases as patients demonstrate excellent long-term survival. The role of adjuvant therapy remains to be studied.
Disclosure of conflict of interest
None.
References
- 1.Frantz VK. Atlas of Tumor Pathology, Section 7, Fascicles 27 and 28. Washington, DC, USA: Armed Forces Institute of Pathology; 1959. Papillary tumors of the pancreas: Benign or malignant? Tumors of the pancreas; pp. 32–3. [Google Scholar]
- 2.Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965–72. doi: 10.1016/j.jamcollsurg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Mortenson MM, Katz MH, Tamm EP, Bhutani MS, Wang H, Evans DB, Fleming JB. Current diagnosis and management of unusual pancreatic tumors. Am J Surg. 2008;196:100–13. doi: 10.1016/j.amjsurg.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Kloppel G, Solcia E, Longnecker DS, Capella C, Sobin LH. World Health Organization. International histological classification of tumors. 2nd edition. Berlin, Heidelberg, New York: Springer; 1996. Histological typing of tumors of the exocrine pancreas; pp. 120–128. [Google Scholar]
- 5.Klöppel G, Hruban RH, Klimstra DS, Maitra A, Morohoshi T, Notohara K, Shimizu M, Terris B. Solid-pseudopapillary tumor of pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. World Health Organization Classification of Tumours of the digestive system. Lyon: IARC; 2010. pp. 327–330. [Google Scholar]
- 6.Kasem A, Ali Z, Ellul J. Papillary cystic and solid tumour of the pancreas: report of a case and literature review. World J Surg Oncol. 2005;3:62. doi: 10.1186/1477-7819-3-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Liang TB, Wang WL, Shen Y, Ren GP, Zheng SS. Diagnosis and treatment of solid- pseudopapillary tumor of the pancreas. Hepatobiliary Pancreat Dis Int. 2006;5:454–8. [PubMed] [Google Scholar]
- 8.Chen SQ, Zou SQ, Dai QB, Li H. Clinical analysis of solid-pseudopapillary tumor of the pancreas: report of 15cases. Hepatobiliary Pancreat Dis Int. 2008;7:196–200. [PubMed] [Google Scholar]
- 9.Cai H, Zhou M, Hu Y, He H, Chen J, Tian W, Deng Y. Solid-pseudopapillary neoplasms of the pancreas: clinical and pathological features of 33 cases. Surg Today. 2013;43:148–54. doi: 10.1007/s00595-012-0260-3. [DOI] [PubMed] [Google Scholar]
- 10.Wang XG, Ni QF, Fei JG, Zhong ZX, Yu PF. Clinicopathologic features and surgical outcome of solid pseudopapillary tumor of the pancreas:analysis of 17 cases. World J Surg Oncol. 2013;11:38. doi: 10.1186/1477-7819-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Goh BK, Tan YM, Cheow PC, Chung AY, Chow PK, Wong WK, Ooi LL. Solid pseudopapillary neoplasms of the pancreas: an updated experience. J Surg Oncol. 2007;95:640–4. doi: 10.1002/jso.20735. [DOI] [PubMed] [Google Scholar]
- 13.Klimstra DS, Adsay NV. Tumors of the pancreas and ampulla of Vater. In: Odze RD, Goldblum JR, editors. Surgical pathology of the GI tract, liver, biliary tract and pancreas. 2nd edition. Philadelphia: WB Saunders; 2009. pp. 944–6. [Google Scholar]
- 14.Thompson LDR, Heffess CS. Pancreas. In: Mills SE, Carter D, Greenson JK, Reuter VE, Stoler MH, editors. Diagnosticsurgical pathology. 5th edition. Philadelphia: Lippincott Williams & Wilkins; 2010. pp. 1469–71. [Google Scholar]
- 15.Yu PF, Hu ZH, Wang XB, Guo JM, Cheng XD, Zhang YL, Xu Q. Solid pseudopapillary tumor of the pancreas: a review of 553 cases in Chinese literature. World J Gastroenterol. 2010;16:1209–14. doi: 10.3748/wjg.v16.i10.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy S, Cameron JL, Scudiere J, Hruban RH, Fishman EK, Ahuja N, Pawlik TM, Edil BH, Schulick RD, Wolfgang CL. Surgical management of solid-pseudopapillary neoplasms of the pancreas: a large single-institutional series. J Am Coll Surg. 2009;208:950–9. doi: 10.1016/j.jamcollsurg.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apostolidis S, Papavramidis TS, Zatagias A, Michalopoulos A, Papadopoulos VN, Paramythiotis D, Harlaftis N. Hematemesis, a very rare presentation of solid-pseudopapillary tumors of the pancreas: a case report. J Med Case Rep. 2008;2:271. doi: 10.1186/1752-1947-2-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Zhou GW, Zhou HJ, Peng CH, Li HW. Diagnosis and treatment of solid-pseudopapillary tumors of the pancreas. Hepatobiliary Pancreat Dis Int. 2005;4:456–9. [PubMed] [Google Scholar]
- 19.Li HX, Zhang Y, Du ZG, Tang F, Qi XQ, Yin B, Jiang YJ, Yang F, Subedi S. Multi-centric solid-pseudopapillary neoplasm of the pancreas. Med Oncol. 2013;30:330. doi: 10.1007/s12032-012-0330-9. [DOI] [PubMed] [Google Scholar]
- 20.Campbell F, Azadeh B. Cystic neoplasms of the exocrine pancreas. Histopathology. 2008;52:539–51. doi: 10.1111/j.1365-2559.2007.02856.x. [DOI] [PubMed] [Google Scholar]
- 21.Tang LH, Aydin H, Brennan MF, Klinstra DS. Clinically aggressive solid pseudopapillary tumor of pancreas: A report of cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol. 2005;29:512–9. doi: 10.1097/01.pas.0000155159.28530.88. [DOI] [PubMed] [Google Scholar]
- 22.El-Bahrawy MA, Rowan A, Horncastle D, Tomlinson I, Theis BA, Russell RC, Stamo G. E-cadherin/catenin complex status in solid-pseudopapillary tumor of the pancreas. Am J Surg Pathol. 2008;32:1–7. doi: 10.1097/PAS.0b013e31813e0676. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka Y, Kato K, Notohara K, Hojo H, Ijiri R, Miyake T, Nagahara N, Sasaki F, Kitagawa N, Nakatani Y, Kobayashi Y. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001;61:8401–4. [PubMed] [Google Scholar]
- 24.Morales A, Ruíz Molina JM, Estéves HO, Robles-Díaz G, Díaz-Sánchez V. Papillary-cystic neoplasm of the pancreas. A sex-steroid dependent tumor. Int J Pancreatol. 1998;24:219–25. doi: 10.1007/BF02788425. [DOI] [PubMed] [Google Scholar]
- 25.Matos JM, Grützmann R, Agaram NP, Saeger HD, Kumar HR, Lillemoe KD, Schmidt CM. Solid pseudopapillary neoplasms of the pancreas: a multi-institutional study of 21 patients. J Surg Res. 2009;157:e137–42. doi: 10.1016/j.jss.2009.03.091. [DOI] [PubMed] [Google Scholar]
- 26.Geers C, Moulin P, Gigot JF, Weynand B, Deprez P, Rahier J, Sempoux C. Solid and pseudopapillary tumor of the pancreas-review and new insights into pathogenesis. Am J Surg Pathol. 2006;30:1243–9. doi: 10.1097/01.pas.0000213311.28682.b2. [DOI] [PubMed] [Google Scholar]
- 27.Park M, Kim M, Hwang D, Park M, Kim WK, Kim SK, Shin J, Park ES, Kang CM, Paik YK, Kim H. Characterization of gene expression and activated signaling pathways in solid pseudopapillary neoplasm of pancreas. Mod Pathol. 2014;27:580–93. doi: 10.1038/modpathol.2013.154. [DOI] [PubMed] [Google Scholar]
- 28.Yeh TS, Jan YY, Chiu CT, Ho YB, Chen TC, Lee KF, Chan KM, Hsu JC, Hwang TL, Chen MF. Characterisation of oestrogen receptor, progesterone receptor, trefoil factor 1, and epidermal growth factor and its receptor in pancreatic cystic neoplasms and pancreatic ductal adenocarcinoma. Gut. 2002;51:712–6. doi: 10.1136/gut.51.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez P, Yorke R, Ayala AG, Ro JY. Solid-pseudopapillary neoplasm of pancreas with long delayed liver metastasis. Ann Diagn Pathol. 2012;16:380–4. doi: 10.1016/j.anndiagpath.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Serra S, Chetty R. Revision 2: an immunohistochemical approach and evaluation of solid pseudopapillary tumour of the pancreas. J Clin Pathol. 2008;61:1153–9. doi: 10.1136/jcp.2008.057828. [DOI] [PubMed] [Google Scholar]
- 31.Vassos N, Agaimy A, Klein P, Hohenberger W, Croner RS. Solid-pseudopapillary neoplasm (SPN) of the pancreas: case series and literature review on an enigmatic entity. Int J Clin Exp Pathol. 2013;6:1051–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Low G, Panu A, Millo N, Leen E. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics. 2011;31:993–1015. doi: 10.1148/rg.314105731. [DOI] [PubMed] [Google Scholar]
- 33.Cantisani V, Mortele KJ, Levy A, Glickman JN, Ricci P, Passariello R, Ros PR, Silverman SG. MR imaging features of solid pseudopapillary tumor of the pancreas in adult and pediatric patients. AJR Am J Roentgenol. 2003;181:395–401. doi: 10.2214/ajr.181.2.1810395. [DOI] [PubMed] [Google Scholar]
- 34.Jani N, Dewitt J, Eloubeidi M, Varadarajulu S, Appalaneni V, Hoffman B, Brugge W, Lee K, Khalid A, McGrath K. Endoscopic ultrasoundguided fine-needle aspiration for the diagnosis of solid-pseudopapillary tumors of the pancreas: a multicenter experience. Endoscopy. 2008;40:200–3. doi: 10.1055/s-2007-995364. [DOI] [PubMed] [Google Scholar]
- 35.Voss M, Hammel P, Molas G, Palazzo L, Dancour A, O’Toole D, Terris B, Degott C, Bernades P, Ruszniewski P. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244–9. doi: 10.1136/gut.46.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zamboni GA, D’Onofrio M, Principe F, Pozzi Mucelli R. Focal pancreatic lesions: accuracy and complications of US-guided fine-needle aspiration cytology. Abdom Imaging. 2010;35:362–6. doi: 10.1007/s00261-009-9527-6. [DOI] [PubMed] [Google Scholar]
- 37.Warshaw AL, Compton CC, Lewandrowski K, Cardenosa G, Mueller PR. Cystic tumors of the pancreas. New clinical, radiologic, and pathologic observations in 67 patients. Ann Surg. 1990;212:432–43. doi: 10.1097/00000658-199010000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JS, Han HJ, Choi SB, Jung CW, Song TJ, Choi SY. Surgical outcomes of solid pseudopapillary neoplasm of the pancreas: a single institution’s experience for the last ten years. Am Surg. 2012;78:216–9. [PubMed] [Google Scholar]
- 39.Yang F, Jin C, Long J, Yu XJ, Xu J, Di Y, Li J, Fu de L, Ni QX. Solid pseudopapillary tumor of the pancreas: a case series of 26 consecutive patients. Am J Surg. 2009;198:210–5. doi: 10.1016/j.amjsurg.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 40.Patil TB, Shrikhande SV, Kanhere HA, Saoji RR, Ramadwar MR, Shukla PJ. Solid pseudopapillary neoplasm of the pancreas: a single institution experience of 14 cases. HPB (Oxford) 2006;8:148–50. doi: 10.1080/13651820510035721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin RC, Klimstra DS, Brennan MF, Conlon KC. Solid-pseudopapillary tumor of pancreas: A surgical enigma? Ann Surg Oncol. 2002;9:35–40. doi: 10.1245/aso.2002.9.1.35. [DOI] [PubMed] [Google Scholar]
- 42.Yoon DY, Hines OJ, Bilchik AJ, Lewin K, Cortina G, Reber HA. Solid and papillary epithelial neoplasms of the pancreas: aggressive resection for cure. Am Surg. 2001;67:1195–9. [PubMed] [Google Scholar]
- 43.Fried P, Cooper J, Balthazar E, Fazzini E, Newall J. The role of radiotherapy in the treatment of solid and papillary neoplasms of the pancreas. Cancer. 1985;56:2783–5. doi: 10.1002/1097-0142(19851215)56:12<2783::aid-cncr2820561211>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 44.Matsunou H, Konishi F. Papillary-cystic neoplasm of the pancreas: a clinicopathologic study concerning the tumor aging and malignancy of nine cases. Cancer. 1990;65:283–91. doi: 10.1002/1097-0142(19900115)65:2<283::aid-cncr2820650217>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 45.Das G, Bhuyan C, Das BK, Sharma JD, Saikia BJ, Purkystha J. Spleen-preserving distal pancreatectomy following neoadjuvant chemotherapy for papillary solid and cystic neoplasm of pancreas. Indian J Gastroenterol. 2004;23:188–9. [PubMed] [Google Scholar]
- 46.Maffuz A, Bustamante Fde T, Silva JA, Torres-Vargas S. Preoperative gemcitabine for unresectable, solid pseudopapillary tumour of the pancreas. Lancet Oncol. 2005;6:185–6. doi: 10.1016/S1470-2045(05)01770-5. [DOI] [PubMed] [Google Scholar]
- 47.Krug S, Bartsch DK, Schober M, Librizzi D, Pfestroff A, Burbelko M, Moll R, Michl P, Gress TM. Successful selective internal radiotherapy (SIRT) in a patient with a malignant solid pseudopapillary pancreatic neoplasm (SPN) Pancreatology. 2012;12:423–7. doi: 10.1016/j.pan.2012.07.014. [DOI] [PubMed] [Google Scholar]


