Abstract
Granulocyte colony-stimulating factor (G-CSF)-producing tumors are known for their aggressive behavior. Only four cases of G-CSF-producing colorectal carcinoma have been previously reported. Herein, we present a case of an undifferentiated carcinoma of the descending colon showing G-CSF production and giant cell carcinoma morphology in a 93-year-old woman. A tumor with a diameter of 80 mm was identified in the descending colon via computed tomography. Descending colectomy was performed involving the abdominal wall where tumor invasion was observed. The white blood cell count, which was elevated before resection, decreased to normal levels after intervention. However, local recurrence at the resected site was detected 39 days after surgery. Upon recurrence, increased white blood cell counts and serum G-CSF were seen. The patient died because of respiratory failure 98 days after colectomy. By using immunohistochemistry, G-CSF expression was detected in tumor cells in the resected specimen, along with overexpression of CD44 and highly proliferating nestin-positive tumor vessels. The poor clinical outcome of this patient is consistent with previous reports that the expression of these three molecules predict poor prognosis. While G-CSF can be a therapeutic target considering its auto/paracrine function to induce tumor growth via the G-CSF receptor, CD44 and nestin may also be possible candidate therapeutic targets. Further studies are required to assess the efficacy of treatments targeting these three molecules.
Keywords: Colon, granulocyte colony-stimulating factor, undifferentiated carcinoma, giant cell carcinoma, CD44, nestin
Introduction
Granulocyte colony-stimulating factor (G-CSF)-producing tumors, associated with peripheral leukocytosis, have been documented in various organs and were first described by Robinson [1]. Lung carcinoma is one of the most frequent tumors producing G-CSF [2], although CSF-producing tumors of the urinary bladder are also relatively frequent [3]. Such tumors are largely confined to the digestive tract and have been reported in the gingiva [4], hypopharynx [5], esophagus [6], stomach [7], colon [8], and rectum [9].
Giant cell carcinoma (GCC) is classified as a variant of sarcomatoid carcinoma according to the recent World Health Organization histological classification of lung tumors [10]. GCC, sometimes producing G-CSF, is histologically characterized by diffuse and discohesive proliferation of pleomorphic giant cells, abundant neutrophilic infiltration, and emperipolesis of neutrophils [10], the latter presenting as intact neutrophils within the cytoplasm of tumor cells.
Both G-CSF-producing tumors and GCC have an aggressive clinical course [3,10]. Herein, we present the case of an undifferentiated carcinoma mimicking pulmonary GCC with confirmation of G-CSF production via immunohistochemistry (IHC) in the surgically resected specimen from a 93-year-old woman. Tumor recurrence was accompanied by elevated G-CSF in serum. The patient died of the disease within 3 months. This is the fifth reported case of G-CSF-producing colorectal carcinoma (CRC) in the English literature and the first with GCC-like morphology.
Other molecules that predict poor prognosis in CRC were also assessed, possibly indicating colorectal cancer stem cells (CSCs), such as CD44 [11-13] and CD133 [14], as G-CSF is associated with expansion of CSC-like carcinoma cells [15], and also stimulates angiogenesis to promote tumor growth [16]. Nestin, a marker for tumor vessels, was also investigated, because a high density of nestin positive vessels has been considered a poor prognostic indicator [17-19]. Overexpression of CD44 in tumor cells and highly proliferating tumor vessels positive for nestin were observed by using IHC.
Clinical summary
A 93-year-old woman was transferred to our hospital for intensive examination for bloody stool with an undetermined origin of bleeding. As she had been receiving antiplatelet therapy, she had previously undergone upper endoscopy for possible upper gastrointestinal bleeding following withdrawal of the antiplatelet drug at the previous institution, although the results were negative. Her medical history was otherwise unremarkable. Laboratory examination showed an elevated white blood cell (WBC) count of 20,030 cells/μl (reference range: 3,500-9,800), neutrophils of 16,480 cells/μl (1,830-7,250), and a slightly elevated C-reactive protein (CRP) of 0.81 mg/dL (0-0.3). Carcinoma antigen 19-9 was 3.5 U/mL (0-37) and carcinoma epithelial antigen was 3.7 ng/mL (0-2.5). Bcr-Abl chimeric messenger RNA was negative in peripheral blood cells, as detected via reverse transcription polymerase chain reaction. Lower endoscopy revealed a protruding lesion along with a white layer at the descending colon. The lesion caused intestinal stenosis and observation from the adoral direction was thus not possible. Contrast enema identified a tumor protruding into the lumen at the descending colon in a direction opposite to the root of the mesentery. Contrast computed tomography (CT) revealed an enhanced solid mass that was 80 mm in maximum diameter with a mildly enhanced internal area (Figure 1A). No enhancement or swelling of the lymph nodes, ascites, or pleural fluid was detected. Subsequently, descending colectomy was performed. During surgery, a tumor was observed at the descending colon near the sigmoid-descending junction. After the splenic flexure was mobilized, the left colonic artery was ligated. As the tumor showed stromal invasion into the abdominal wall, a portion of the abdominal wall was resected together with the descending colon. The intestine was reconnected by end-to-end anastomosis. At pathological diagnosis, the tumor was staged as pT4aN0M0 stage II, according to the 7th UICC-TMN system.
Figure 1.
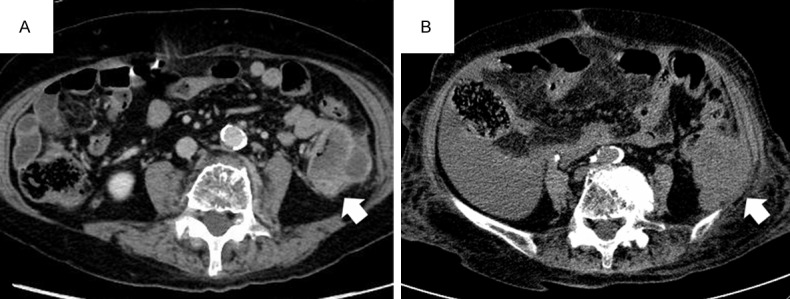
Computed tomography (CT) images. A. Preoperative contrast-enhanced CT revealed a mildly enhanced tumor in the descending colon (arrow). B. Postoperative plain CT on recurrence depicted a large recurrent tumor on the abdominal wall consistent with local recurrence (arrow).
The postoperative course was uneventful, and the patient was discharged on day 14 after intervention. Laboratory data on day 22 showed an elevated WBC count of 13,680 cells/μL with neutrophils of 11,260 cells/μl, and elevated serum CRP (5.14 mg/dL). The patient was re-admitted for respiratory failure and accumulation of ascites at day 39. Blood culture was negative for microorganisms. Abdominal and pelvic CT revealed peritoneal dissemination and recurrence of the tumor at the abdominal wall where tumor invasion was detected during surgery (Figure 1B). Chest radiography on day 57 showed the presence of pleural fluids and elevation of the diaphragm due to ascites. Laboratory data on day 88 revealed an increased inflammatory response; WBC count, proportion of neutrophils, and serum CRP was 38,090 cells/μL, 92.6%, and 15.4 mg/dL, respectively. Serum levels of G-CSF (89.0 pg/mL; 0-39.0) and interleukin 6 (IL-6) were also elevated (44.0 pg/mL; 0-4.0). The patient’s condition further deteriorated and she died 98 days after surgery owing to respiratory failure.
Pathological findings
The surgically resected specimen was fixed in 10% buffered formalin for approximately 24 h, and 5 mm-thick tissue slices were embedded in paraffin. Sections (2.5 μm thick) were cut from each block for hematoxylin and eosin (HE) staining; 4 μm sections were made for IHC. An automated slide stainer (Bench-Mark TX; Ventana Medical Systems, Tucson, AZ, USA) was used for IHC, except for epithelial growth factor receptor (EGFR) which was carried out manually. The primary antibodies used are listed in Table 1. Possible KRAS mutation of codon 12 and 13 was investigated via direct sequencing by using sections from the same paraffin block used for IHC.
Table 1.
Antibodies used in the present study
| Antibody to | Clone | Dilution | Antigen Retrieval | Source |
|---|---|---|---|---|
| G-CSF | 4-12-2 | 1:100 | HIER | Immuno-Biological Laboratories |
| G-CSFR | Polyclonal | 1:200 | HIER | Bioss |
| AE1/AE3 | Cocktail, AE1 and AE3 | 1:100 | HIER | Novocastra |
| HNF4α | K9218 | 1:100 | HIER | Perseus |
| CK7 | OV-TL 12/30 | 1:50 | HIER | Dako |
| CK20 | Ks 20.8 | 1:50 | HIER | Dako |
| CDX2 | AMT28 | 1:100 | HIER | Novocastra |
| Vimentin | V9 | Prediluted | HIER | Ventana |
| Nestin | N1602 | 1:20 | HIER | Immuno-Biological Laboratories |
| E-cadherin | 36 | Prediluted | HIER | Ventana |
| CD44 | DF1485 | 1:50 | HIER | Novocastra |
| CD133 | AC133 | 1:50 | HIER | Miltenyi |
| Ki-67 | MIB-1 | 1:100 | HIER | Dako |
| p53 | DO-7 | Prediluted | HIER | Ventana |
| EGFR | EGFR PharmDx™ Kit | Dako |
G-CSF: granulocyte colony-stimulating factor; G-CSFR: granulocyte colony-stimulating factor receptor; CK: cytokeratin; EGFR: epidermal growth factor receptor; HIER: heat-induced epitope retrieval; Immuno-Biological Laboratories, Gunma, Japan; Bioss, Massachusetts, USA; Novocastra Laboratories, Newcastle upon Tyne, UK; Perseus Proteomics, Tokyo, Japan; Dako, Glostrup, Denmark; Ventana Medical Systems, Arizona, USA; Miltenyi, California, USA.
Macroscopic examination revealed a Borrmann type 2 tumor, measuring 65 × 55 mm (Figure 2). The cut surface revealed a whitish solid neoplasm (Figure 2, inset); the invasive area in the parietal peritoneum had been resected en bloc. Histopathologically, the tumor was composed of pleomorphic large and giant cells with loose or no cohesion to each other (Figure 3A). There were abundant neutrophils both surrounding and within tumor cells (emperipolesis) (Figure 3A, inset). No other carcinomatous component was found. A diagnosis of undifferentiated carcinoma resembling pulmonary GCC was rendered. Mitotic figures were observed in a high proportion of cells. Neither vascular invasion nor lymph node metastasis was detected on routine examination. The surgical margins did not show the presence of the tumor.
Figure 2.
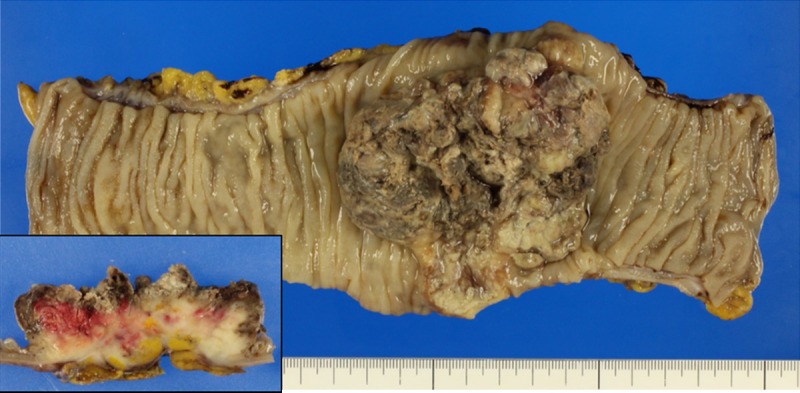
Macroscopic findings. The tumor was a Borrmann type 2 tumor measuring 65 × 55 mm. The cut surface revealed a deeply invasive whitish tumor (inset). A part of the abdominal wall was also included in the invasive area.
Figure 3.
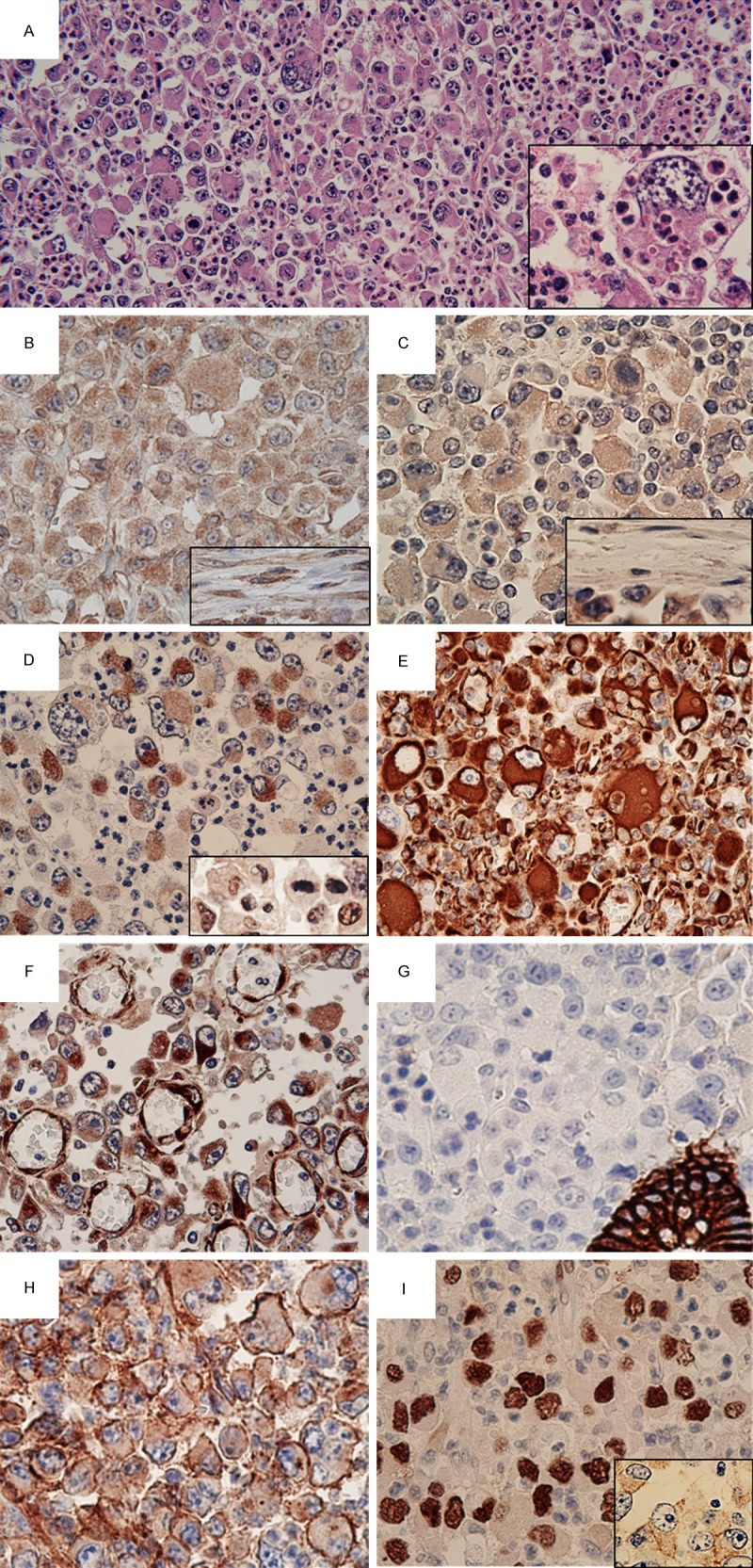
Microscopic findings with hematoxylin and eosin (HE) staining and immunohistochemistry. A. The tumor was composed of pleomorphic large and giant cells with loose or no cohesion to each other. There were abundant neutrophils surrounding the tumor (HE, ×200). Neutrophils were present within tumor cells (emperipolesis) (inset, ×600). B. Tumor cells stained positive for granulocyte colony-stimulating factor (×400). Note the positive reaction for stromal fibroblasts/myofibroblasts (inset, ×400). C. Immunoreactivity for granulocyte colony-stimulating factor receptor (G-CSFR) was observed in tumor cells (×400); stromal fibroblasts/myofibroblasts were negative for G-CSFR (inset, ×400). D. AE1/AE3 immunostaining demonstrated focal positivity in the tumor cells (×400). Inset: some tumor cells were positive for HNF4α (×400). E. Strong reactivity of the tumor cells for vimentin (×400). F. In addition to positive staining for nestin in tumor cells, endothelial cells in highly proliferating small blood vessels were highlighted via immunoreactivity for nestin (×400). G. Negativity of E-cadherin in tumor cells. Note the positive reaction to a pre-existing crypt (×400). H. Overexpression of CD44 was observed in tumor cells (×400). I. Diffuse nuclear accumulation of p53 was detected (×400). Membranous positivity of epidermal growth factor receptor was also present (inset, ×400).
IHC demonstrated G-CSF positivity not only for tumor cells (Figure 3B), but also for stromal fibroblasts/myofibroblasts (Figure 3B, inset). Expression of G-CSF receptor (G-CSFR) was also seen only in tumor cells (Figure 3C and inset), which were focally positive for AE1/AE3 (Figure 3D) and P1-driven HNF4α (Figure 3D, inset); the tumor was negative for cytokeratin (CK) 7, CK20, and CDX2. Strong immunoreactivity for vimentin (Figure 3E) and nestin (Figure 3F) was detected, while immunoreactivity for E-cadherin (Figure 3G) was not identified. Nestin was also expressed in endothelial cells of small blood vessels that were highly proliferating within and near the tumor (Figure 3F). Overexpression of CD44 was shown in tumor cells (Figure 3H); CD133 was not detected. The Ki-67 index exceeding 90% of tumor cells indicated high proliferative activity. Diffuse accumulation of p53 in the nuclei of tumor cells was observed (Figure 3I), and membranous immunopositivity for EGFR was detected (Figure 3I, inset). A mutational analysis of KRAS revealed no mutation of codons 12 and 13.
Discussion
The primary colonic origin of the tumor was verified based on clinical findings such as CT, its macroscopic growth pattern (Borrmann type 2), and immunoreactivity for P1-driven HNF4α. In spite of immunonegativity for other markers such as CK20 and CDX2 that would indicate a primary colonic origin, the positive reaction for P1-driven HNF4α contributed in confining the primary site of origin to the small intestine, colon, hepatocyte, kidney, and epididymis, but not the lungs [20,21]. There are only four cases of G-CSF-producing tumor of the colorectum reported in the English literature. The site of neoplastic initiation in these cases was judged primarily on tumor location because IHC makers typically shown in CRCs were attenuated (Table 2) [8,9,22,23].
Table 2.
Clinicopathological features of granulocyte colony-stimulating factor (G-CSF)-producing colorectal carcinoma in patients reported in the literature
| Year | Age (y) | Sex | Location | G-CSF (pg/mL) | WBC (cells/mm3) | Histology | Outcome |
|---|---|---|---|---|---|---|---|
| 2002 [22] | 71 | M | A | NA | 29,400 | Moderately diff. adenoca | Died after 5 months |
| 2008 [9] | 57 | M | R | 840 | 81,000 | Undiff. ca. | Died after 2 months |
| 2009 [8] | 52 | M | A | 640 | 63,000 | Poorly diff. ca. | Died after 1 month |
| 2011 [23] | 81 | M | A | 334 | 17,620 | Poorly diff. ca. | Alive after 24 months |
| Present case | 93 | F | D | 38,090 | Undiff. ca. (GCC-like) | Died after 3 months |
A, ascending colon; R, rectum; D, descending colon; NA, not available; diff., differentiated; ca., carcinoma; GCC, giant cell carcinoma.
An unusually high WBC count without evidence of infection could be attributed to G-CSF secreted from the tumor. In the present case, the WBC count decreased to the normal range after tumor resection, although the count concomitantly exceeded the normal range upon tumor recurrence. Along with these observations, G-CSF production in the surgically resected specimen was confirmed via IHC, and serum G-CSF was elevated after recurrence. The diagnostic criteria for G-CSF producing tumor are as follows: (i) marked leukocytosis; (ii) increased G-CSF activity; (iii) drop in WBC count after tumor resection; or (iv) evidence of G-CSF production in the tumor [24]. Thus, diagnosis of G-CSF-producing tumor in this patient was appropriate.
Some G-CSF-producing tumors are known for simultaneous production of IL-6 and/or IL-6 receptor [25]. Concordantly, the present case also showed elevated levels of serum IL-6, a pleiotropic interleukin that is important in neoplastic growth and inflammation. Of note, the signal from G-CSF and IL-6 is transduced through the same pathway that involves Janus kinases and signal transducer and activator of transcription 3 [26], both of which are associated with the proliferation of colon cancer [27]. Thus, G-CSF and IL-6 may have contributed to tumor aggression in the present case.
The production of G-CSF by both tumor cells and tumor-associated fibroblasts/myofibroblasts along with the expression of G-CSFR in tumor cells suggest the existence of autocrine and paracrine loops associated with stimulation of tumor progression [15]. In this case, IHC confirmed the expression of G-CSF in both tumor cells and stromal fibroblasts/myofibroblasts, whereas expression of G-CSFR was observed only in tumor cells. Therefore, tumor growth in an autocrine and paracrine manner due to G-CSF production via the signal-transducing pathway described above can be postulated.
Other known functions of G-CSF in tumors are: 1) acquisition of migratory capacity; 2) expansion of CSC-like cells [15]; and 3) promotion of angiogenesis [16]. Concerning migration, epithelial-mesenchymal transformation (EMT) has been observed in G-CSF-producing tumors [24,28]. The focal weak expression of AE1/AE3, loss of E-cadherin expression, and strong expression of vimentin in this case are all consistent with the usual molecular signature of cells after EMT. However, we cannot dismiss the possibility that the low expression of E-cadherin is related only to progression of the tumor towards dedifferentiation [29], considering that the tumor morphology was not necessarily typical of that seen after EMT.
Regarding the expansion of CSC-like cells, the putative markers mainly used to detect colorectal CSC are CD44 and CD133 [30], and expression of molecule in CRC is indicative of poor prognosis [11-14]. In the present case, diffuse overexpression of CD44 was demonstrated, whereas CD133 was not expressed. As CD44 endows invasiveness of tumor cells [31], overexpression of CD44 might have been causal to the invasive character of the tumor and poor prognosis in the present patient.
Considering the role of G-CSF in angiogenesis, nestin is a useful marker to evaluate tumor vessels since it recognizes newly-formed vessels more efficiently than other classical markers such as CD31, CD34, and factor VIII related antigen [19]. Nestin has been detected in tumors of various organs and its expression in tumor cells is correlated with poor prognosis in some neoplasms [18]. Moreover, in CRC, microvessel density, evaluated according to the staining intensity of nestin in the endothelium, has been suggested to be an indicator of poor prognosis [17,18]. Considering that the present case showed strong expression of nestin in tumor cells, along with highly proliferating nestin-positive vessels, this suggests the importance of nestin as a prognostic indicator in CRC. It was recently reported that p53 has a role in suppressing the expression of nestin [32]. Considering that diffuse nuclear accumulation of p53 in IHC is indicative of mutation of the p53 gene, impaired p53 function may have caused overexpression of nestin in our case.
Anti-cancer therapy after recurrence was not performed in this patient owing to the rapid clinical course. If any therapy were possible, administration of an EGFR tyrosine kinase inhibitor would have been the most likely treatment. This is because membranous EGFR expression was positive and KRAS mutation in codon 12 and 13 was negative, which fulfills criteria for using an EGFR tyrosine kinase inhibitor in the presence of an unresectable recurrence. Regarding future therapies, G-CSF, CD44, and nestin could be considered as potential targets [15,18,33].
In conclusion, this is a unique case of undifferentiated colon carcinoma with G-CSF production with a morphology resembling that of pulmonary GCC. The poor clinical outcome may be explained by the overexpression of CD44 in tumor cells and nestin-positive, highly proliferating tumor vessels in addition to autocrine and paracrine growth due to G-CSF production. These three molecules could be potential targets for anti-cancer therapy against G-CSF producing tumors. Further studies are needed to investigate these molecules as potential targets of anti-cancer therapy for G-CSF producing CRC.
Disclosure of conflict of interest
None.
References
- 1.Robinson WA. Granulocytosis in neoplasia. Ann N Y Acad Sci. 1974;230:212–218. doi: 10.1111/j.1749-6632.1974.tb14451.x. [DOI] [PubMed] [Google Scholar]
- 2.Asano S, Urabe A, Okabe T, Sato N, Kondo Y. Demonstration of granulopoietic factor(s) in the plasma of nude mice transplanted with a human lung cancer and in the tumor tissue. Blood. 1977;49:845–852. [PubMed] [Google Scholar]
- 3.Kumar AK, Satyan MT, Holzbeierlein J, Mirza M, Van Veldhuizen P. Leukemoid reaction and autocrine growth of bladder cancer induced by paraneoplastic production of granulocyte colony-stimulating factor-a potential neoplastic marker: a case report and review of the literature. J Med Case Rep. 2014;8:147. doi: 10.1186/1752-1947-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi J, Miyazaki A, Yamamot T, Nakamori K, Suzuki R, Kaneko T, Suzuki N, Hiratsuka H. Granulocyte colony-stimulating factor-producing squamous cell carcinoma of the lower gingiva: a case report. Head Neck Oncol. 2012;4:35. doi: 10.1186/1758-3284-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamura K, Yoshinaga T, Tanino M, Kimura T, Yamada N, Nishimura M, Fukuda S, Nishihara H, Shindoh M, Tanaka S. Hypopharyngeal squamous cell carcinoma producing both granulocyte colony-stimulating factor and parathyroid hormone-related protein. Pathol Int. 2008;58:652–656. doi: 10.1111/j.1440-1827.2008.02285.x. [DOI] [PubMed] [Google Scholar]
- 6.Mayanagi S, Niihara M, Goto H, Yokota T, Tabuse H, Yasui H, Ogawa H, Nishimura T, Kusafuka K, Tsubosa Y. Granulocyte colony-stimulating factor-producing esophageal squamous cell carcinoma following chemoradiotherapy and bone marrow transplantation for acute lymphoblastic leukemia. Esophagus. 2013;10:258–263. doi: 10.1007/s10388-013-0387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endo K, Kohnoe S, Okamura T, Haraguchi M, Adachi E, Toh Y, Baba H, Maehara Y. Gastric adenosquamous carcinoma producing granulocyte-colony stimulating factor. Gastric Cancer. 2005;8:173–177. doi: 10.1007/s10120-005-0330-y. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda A, Sasajima K, Matsutani T, Maruyama H, Miyamoto M, Yokoyama T, Suzuki S, Suzuki H, Tajiri T. Aggressive undifferentiated colon carcinoma producing granulocyte-colony stimulating factor: report of a case. Surg Today. 2009;39:990–993. doi: 10.1007/s00595-008-3941-1. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi H, Yasuda A, Ochi N, Sakamoto M, Takayama S, Wakasugi T, Funahashi H, Sawai H, Satoh M, Akamo Y, Takeyama H. Granulocyte-colony stimulating factor producing rectal cancer. World J Surg Oncol. 2008;6:70. doi: 10.1186/1477-7819-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press, International Agency for Research on Cancer; 2004. [Google Scholar]
- 11.Visca P, Del Nonno F, Botti C, Marandino F, Sebastiani V, Di Tondo U, Donnorso RP, Trombetta G, Filippi S, Alo PL. Role and prognostic significance of CD44s expression in colorectal cancer. Anticancer Res. 2002;22:2671–2675. [PubMed] [Google Scholar]
- 12.Huh JW, Kim HR, Kim YJ, Lee JH, Park YS, Cho SH, Joo JK. Expression of standard CD44 in human colorectal carcinoma: association with prognosis. Pathol Int. 2009;59:241–246. doi: 10.1111/j.1440-1827.2009.02357.x. [DOI] [PubMed] [Google Scholar]
- 13.Al-Maghrabi J, Gomaa W, Buhmeida A, Al-Qahtani M, Al-Ahwal M. Decreased immunoexpression of standard form of CD44 is an independent favourable predictor of nodal metastasis in colorectal carcinoma. Anticancer Res. 2012;32:3455–3461. [PubMed] [Google Scholar]
- 14.Wang K, Xu J, Zhang J, Huang J. Prognostic role of CD133 expression in colorectal cancer: a meta-analysis. BMC Cancer. 2012;12:573. doi: 10.1186/1471-2407-12-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris KT, Khan H, Ahmad A, Weston LL, Nofchissey RA, Pinchuk IV, Beswick EJ. G-CSF and G-CSFR are highly expressed in human gastric and colon cancers and promote carcinoma cell proliferation and migration. Br J Cancer. 2014;110:1211–1220. doi: 10.1038/bjc.2013.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M. G-CSF stimulates angiogenesis and promotes tumor growth: potential contribution of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun. 2002;297:1058–1061. doi: 10.1016/s0006-291x(02)02335-5. [DOI] [PubMed] [Google Scholar]
- 17.Teranishi N, Naito Z, Ishiwata T, Tanaka N, Furukawa K, Seya T, Shinji S, Tajiri T. Identification of neovasculature using nestin in colorectal cancer. Int J Oncol. 2007;30:593–603. [PubMed] [Google Scholar]
- 18.Ishiwata T, Matsuda Y, Naito Z. Nestin in gastrointestinal and other cancers: effects on cells and tumor angiogenesis. World J Gastroenterol. 2011;17:409–418. doi: 10.3748/wjg.v17.i4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuda Y, Hagio M, Ishiwata T. Nestin: a novel angiogenesis marker and possible target for tumor angiogenesis. World J Gastroenterol. 2013;19:42–48. doi: 10.3748/wjg.v19.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang S, Tanaka T, Iwanari H, Hotta H, Yamashita H, Kumakura J, Watanabe Y, Uchiyama Y, Aburatani H, Hamakubo T, Kodama T, Naito M. Expression and localization of P1 promoter-driven hepatocyte nuclear factor-4alpha (HNF4alpha) isoforms in human and rats. Nucl Recept. 2003;1:5. doi: 10.1186/1478-1336-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka T, Jiang S, Hotta H, Takano K, Iwanari H, Sumi K, Daigo K, Ohashi R, Sugai M, Ikegame C, Umezu H, Hirayama Y, Midorikawa Y, Hippo Y, Watanabe A, Uchiyama Y, Hasegawa G, Reid P, Aburatani H, Hamakubo T, Sakai J, Naito M, Kodama T. Dysregulated expression of P1 and P2 promoter-driven hepatocyte nuclear factor-4alpha in the pathogenesis of human cancer. J Pathol. 2006;208:662–672. doi: 10.1002/path.1928. [DOI] [PubMed] [Google Scholar]
- 22.Kojima K, Nakashima F, Boku A, Muroishi Y, Nakanishi I, Oda Y. Clinicopathological study of involvement of granulocyte colony stimulating factor and granulocyte-macrophage colony stimulating factor in non-lymphohematopoietic malignant tumors accompanied by leukocytosis. Histol Histopathol. 2002;17:1005–1016. doi: 10.14670/HH-17.1005. [DOI] [PubMed] [Google Scholar]
- 23.Fujiwara Y, Yamazaki O, Takatsuka S, Kaizaki R, Inoue T. Granulocyte colony-stimulating factor-producing ascending colon cancer as indicated by histopathological findings: report of a case. Osaka City Med J. 2011;57:79–84. [PubMed] [Google Scholar]
- 24.Takenaka M, Akiba J, Kawaguchi T, Niizeki T, Arinaga-Hino T, Sata M, Nakashima O, Yano H, Kage M. Intrahepatic cholangiocarcinoma with sarcomatous change producing granulocyte-colony stimulating factor. Pathol Int. 2013;63:233–235. doi: 10.1111/pin.12051. [DOI] [PubMed] [Google Scholar]
- 25.Masunaga A, Sato Y, Kadofuku T, Iwamoto S, Masuda M, Suzuki S, Suzuki T, Miyazaki A, Mitsuya T. A case of granulocyte colony-stimulating factor and interleukin 6 receptor-producing mediastinal mature cystic teratoma with somatic-type malignancy. Pathol Int. 2011;61:243–247. doi: 10.1111/j.1440-1827.2010.02641.x. [DOI] [PubMed] [Google Scholar]
- 26.Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 27.Corvinus FM, Orth C, Moriggl R, Tsareva SA, Wagner S, Pfitzner EB, Baus D, Kaufmann R, Huber LA, Zatloukal K, Beug H, Ohlschlager P, Schutz A, Halbhuber KJ, Friedrich K. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. 2005;7:545–555. doi: 10.1593/neo.04571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamasaki O, Shibata H, Suzuki N, Ikeda K, Takeshima C, Otsuka M, Aoyama Y, Iwatsuki K. Granulocyte colony-stimulating factor-producing squamous cell carcinoma of the skin associated with epithelial-mesenchymal transition. Eur J Dermatol. 2013;23:413–414. doi: 10.1684/ejd.2013.2025. [DOI] [PubMed] [Google Scholar]
- 29.van der Wurff AA, ten Kate J, van der Linden EP, Dinjens WN, Arends JW, Bosman FT. L-CAM expression in normal, premalignant, and malignant colon mucosa. J Pathol. 1992;168:287–291. doi: 10.1002/path.1711680308. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Xie J, Guo J, Manning HC, Gore JC, Guo N. Evaluation of CD44 and CD133 as cancer stem cell markers for colorectal cancer. Oncol Rep. 2012;28:1301–1308. doi: 10.3892/or.2012.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 32.Tschaharganeh DF, Xue W, Calvisi DF, Evert M, Michurina TV, Dow LE, Banito A, Katz SF, Kastenhuber ER, Weissmueller S, Huang CH, Lechel A, Andersen JB, Capper D, Zender L, Longerich T, Enikolopov G, Lowe SW. p53-Dependent Nestin Regulation Links Tumor Suppression to Cellular Plasticity in Liver Cancer. Cell. 2014;158:579–592. doi: 10.1016/j.cell.2014.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Misra S, Heldin P, Hascall VC, Karamanos NK, Skandalis SS, Markwald RR, Ghatak S. Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J. 2011;278:1429–1443. doi: 10.1111/j.1742-4658.2011.08071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


