Abstract
Epidermal growth factor receptor (EGFR) is an effective molecular target of anti-cancer therapies. Curcumin is known to inhibit growth, invasion and metastasis by downregulating EGFR expression in some cancer cells. However, the mechanism underlying the effect of curcumin in human oral squamous cell carcinoma (OSCC) remains unclear. In this study, we investigated the efficacy of curcumin on proliferation and invasion in SCC-25 cell line. We also explored the effect of curcumin on the activition of EGFR and its downstream signaling molecules Akt, ERK1/2 and STAT3. Furthermore, we examined the inhibition effect of curcumin on EGF-induced EGFR phosphorylation and SCC-25 cells invasion. Our results showed that curcumin inhibited SCC-25 cells proliferation and induced G2/M phase arrest in a dose-dependent manner. Curcumin also inhibited SCC-25 cells invasion and downregulated MMP-2, MMP-9, uPA and uPAR expression. We further revealed that curcumin regulated the p-EGFR and EGFR downstream signaling molecules including Akt, ERK1/2 and STAT3. Finally, our data showed that crucumin reduced the EGF-induced phosphorylation of EGFR and suppressed EGF-triggered SCC-25 cells invasion. Taken together, our results suggest that curcumin reduced SCC-25 cells proliferation and invasion through inhibiting the phosphorylation of EGFR and EGFR downstream signaling molecules Akt, ERK1/2 and STAT3.
Keywords: OSCC, curcumin, EGFR, proliferation, invasion
Introduction
Epidermal growth factor receptor (EGFR) is a 170-kDa transmembrane protein which belongs to ErbB family of receptor tyrosine kinases. It contains an extracellular ligand-binding domain, a transmembrane domain and an intracellular domain with tyrosine kinase activity. Upon binding of ligands such as epidermal growth factor (EGF), EGFR forms homodimers or heterodimers with other members of the ErbB family of receptor tyrosine kinases such as ErbB2, ErbB3 and ErbB4, resulting in autophosphorylation and could further activate downstream signaling pathways, including PI3K/Akt/mTOR, Ras/Raf/MAPK/ERK and JAK2/STAT3, which play important roles in regulating cell proliferation, differentiation, migration, invasion and apoptosis [1].
Oral squamous cell carcinoma (OSCC) is the most frequent malignant tumor in the oral cavity, which is the sixth most common cancer worldwide [2]. Unfortunately, the prognosis and survival of OSCC patients is still poor. 50% patients die within 5 years, and most of the patients die from recurrent or metastatic tumor [3]. EGFR high expression has been detected in OSCC [4] and is significantly association with high histological grading [5,6]. The increased expression and activity of EGFR is associated with tumor proliferation, invasion and metastasis [7,8]. Positive pEGFR tumors are associated with poor prognosis and a poor response to chemotherapy [9]. Therefore, EGFR has become an important target in the research of cancer therapy [10].
Two classic EGFR inhibitors, anti-EGFRs monoclonal antibodies (cetuximab, panitumumab and trastuzumab) and tyrosine kinases inhibitors (gefitinib, erlotinib and lapatinib), have now been approved for the treatment of some cancer that originated from epithelium, including advanced colorectal cancer [11], squamous cell carcinoma of the head and neck [12], advanced lung cancer [13] and breast cancer [14]. However, the benefits of these inhibitors treatment to most cancer patients are very limited due to complications of drug-resistant and side effects.
Curcumin, a plant polyphenol isolated from the rhizome of the turmeric plant, has been well known as a cancer chemopreventive and chemotherapeutic agent in recent years. In vitro and in vivo studies indicated that curcumin was able to treat some types of cancer, such as lung cancer [15], colon cancer [16], breast cancer [17] and prostate carcinoma [18]. It has been revealed that curcumin had a diverse range of molecular targets, such as PKB/Akt, NF-κB and MAPK. Recently, some studies indicate that the anti-cancer effects of curcumin are also involved in regulation EGFR expression and EGFR downstream signaling pathways [19,20]. It has been shown that curcumin inhibit human colon cancer cell growth by suppressing gene expression of EGFR through reducing the trans-activation activity of Egr-1 [21]. Treatment of breast cancer cells with curcumin induces cell apoptosis by inhibition of EGFR expression [22]. However, whether curcumin has effects on oral cancer cells such as OSCC cell line, as well as the underlying mechanism remain unknown to people.
In this study, we investigated the efficacy of curcumin on proliferation and invasion in SCC-25 cell line. We further explored whether curcumin could inhibit the activition of EGFR and its downstream signaling molecules Akt, ERK1/2 and STAT3. In addition, we also examined the inhibitory effect of curcumin on EGF-induced EGFR phosphorylation and SCC-25 cells invasion.
Materials and methods
SCC-25 cell culture
SCC-25 cell line were purchased from ATCC (Manassas, VA, USA), derived from patients with OSCC. The SCC-25 cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco BRL, USA) supplemented with 10% fetal bovine serum (FBS), 100 IU/ml penicillin, and 100 μg/ml streptomycin (Gibco BRL) at 37°C under the humidified atmosphere of 95% air and 5% CO2. SCC-25 cell grows as monolayer and cells are ready to treat when they reach about 80% confluency in all experiments.
Cell proliferation assay
The proliferation of SCC-25 cells under the treatment of curcumin (Sigma-Aldrich, MO, USA) was detected by MTT assay. Specifically, cells were seeded into 96-well plates (Corning, USA) at 5 × 103 cells/well. Then they were treated with 0, 10, 20, 40 and 80 μmol/L curcumin for 24 hours and 48 hours, respectively. MTT dye (5 mg/mL, Sigma, USA) was added to each well for at least 4 hours of treatment. The reaction was stopped by the addition of DMSO (Sigma), and optical density was measured at 570 nm wavelength on a multiwell plate reader. Background absorbance of the medium in the absence of cells was subtracted and results were normalized to blank control. All samples were performed in triplicates and results were calculated as mean ± SD.
Cell cycle analysis
Cells were seeded at a density of 1.5 × 105 cells per well into 6-well plates. Following treated with 0, 10 and 40 μmol/L curcumin for 24 hours, cells were fixed for 30 minutes on ice with 100% cold ethanol, then washed and resuspended in a staining buffer (BD, USA) content with 0.05 mg/ml propidium iodide and RNase A for 30 minutes at room temperature in the dark. Cell samples were analyzed in the flow cytometer. Percentages of cells in different phases of cell-cycle were calculated using Multicycle software provided by the manufacturer.
Transwell cell matrigel invasion assay
Cell invasion assay were performed using a 24-well Transwell chamber (Corning, 6.5 mm; 8 μm pore size). For invasion assay, the filter of the Transwell chamber was coated with Matrigel (20 μg/ml, Becton Dickinson Labware, USA) for 90 minutes. After treatment with 0, 10 and 40 μmol/L curcumin for 24 hours, cells (5.0 × 105 cells/ml) were resuspended in serum-free DMEM. 100 μl cell suspension was added into the upper chamber. DMEM with or without 10% FBS and with 100 ng/ml EGF (Sigma) was then placed into the lower chamber as a chemoattractant. Cells were incubated at 37°C in incubator and allowed to migrate through the chemotaxis chamber for 24 hours. The non-migratory cells on the upper membrane surface of the insert were completely removed with a cotton tip, and the migratory cells attached to the membrane surface were fixed with 4% paraformaldehyde and stained with hematoxylin and Eosin. The number of migrated cells was counted under a light microscope (magnification 200×) with five different fields per filter. The experiments were repeated in triplicates.
Real-time PCR
Total RNA was isolated from different treatment SCC-25 cells with Trizol agent under the instructions of manufacture (Invitrogen, USA). Synthesis of first-strand cDNA was carried out using RT-PCR first-strand cDNA synthesis kit (Invitrogen). 1 μg cDNA was used for real-time PCR with a Bio-Rad iQ5 thermal cycler. Several genes which were related with cell invasion were analyzed. GAPDH was used as the control. The primer sequences used for real-time RT-PCR are shown as follows: GAPDH: forward 5’-ACAGTCAGCCGCATCTTCTT-3’, reverse 5’-GACAAGCTTCCCGTTCTCAG-3’; uPA: forward 5’-GCCTTGCTGAAGATCCGTTCCAAGGAGGGC-3’, reverse 5’-CAGGCCAT TCTCTTCCTTGGTGTGACTGCG-3’; uPAR: forward 5’-CTCCAATGGTTTCCACAACAACGACACCTT-3’, reverse 5’-TGGTTACAGCCACTTTTAGTACAGCAGGAG-3’; MMP-2: forward 5’-GAGAACCAAAGTCTGAAGAG-3’, reverse 5’-GGAGTGAGAATGCTGATTAG-3’; MMP-9: forward 5’-GTAACCCTGGTCACCGGACTT-3’, reverse 5’-CAGATACGTTCCCGGCTGAT-3’.
Western blot
The SSC-25 cells were treated with or without curcumin and western blot analyses were performed as previously described [23]. Briefly, cells were lysed on ice for 30 minutes with RIPA lysis buffer including protease and phosphatase inhibitors (Sigma). Proteins were then extracted by centrifuging for 10 minutes at 10000 g at 4°C. 10 μg of each sample protein were resuspended in a loading buffer and electrophoresed on 8%-10% SDS-polyacrylamide gels. Fractionated proteins were transferred onto polyvinylidene difluoride membranes (Invitrogen). After the membranes were blocked with 5% milk for 1 hour, they were incubated with primary antibodies over night at 4°C. The primary antibodies used in this study including anti-MMP-2, anti-MMP-9, anti-uPA, anti-uPAR, anti-EGFR, anti-p-EGFR, anti-Akt, anti-pAkt, anti-ERK1/2, anti-pERK1/2, anti-STAT3 and anti-pSTAT3 (Santa Cruz), which concentrations ranged from 1:500 to 1:2000. Washing 3 times with PBS after primary antibody and followed by secondary antibodies (goat anti-rabbit IgG conjugated with the horseradish peroxidase, Santa Cruz) added on the membranes and incubated for 1 hour at room temperature. After another 3 times of PBS washing, reactive protein bands were detected using West Dura Luminol Reagent (Thermo Scientific, USA).
Statistical analysis
The SPSS 17.0 software was applied to complete data processing. All data were represented as mean ± SD of three independent experiments. Statistical significance was analyzed by Student’s t-test. Results were considered statistically significant when P < 0.05.
Results
Curcumin inhibits proliferation of SCC-25 cell line
Firstly, we examined the inhibition effects of curcumin on the proliferation of SCC-25 cells. The cells were exposed to curcumin at different concentrations for 24 and 48 hours, and cell viability was measured by MTT assay. The dose-dependent inhibition of curcumin on cells proliferation was observed (Figure 1).
Figure 1.
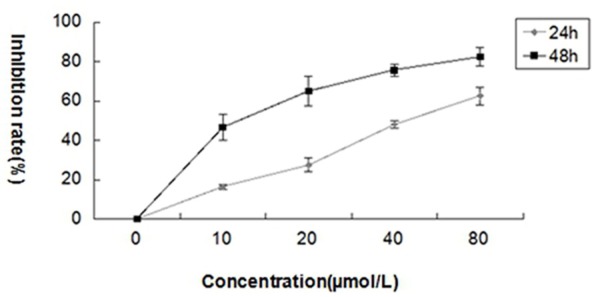
Curcumin inhibits the proliferation of SCC-25 cells. The cells were treated with different concentrations of curcumin (0, 10, 20, 40 and 80 μmol/L) for 24 and 48 hours. The cell viability was evaluated by MTT assay and the inhibitory effect was expressed as the percentage of viable cells. The values are mean ± SD at three independent experiments (each conducted in triplicate).
Curcumin induces cell cycle arrest in SCC-25 cells
Effects of curcumin on cell-cycle progression of SCC-25 cells were investigated by DNA content analysis. Cells were treated with curcumin (0, 10 and 40 μmol/L) for 24 hours. Curcumin treatment increased the proportion of cells in the G2/M phase of SCC-25 cell line compared to the control cells (Figure 2).
Figure 2.
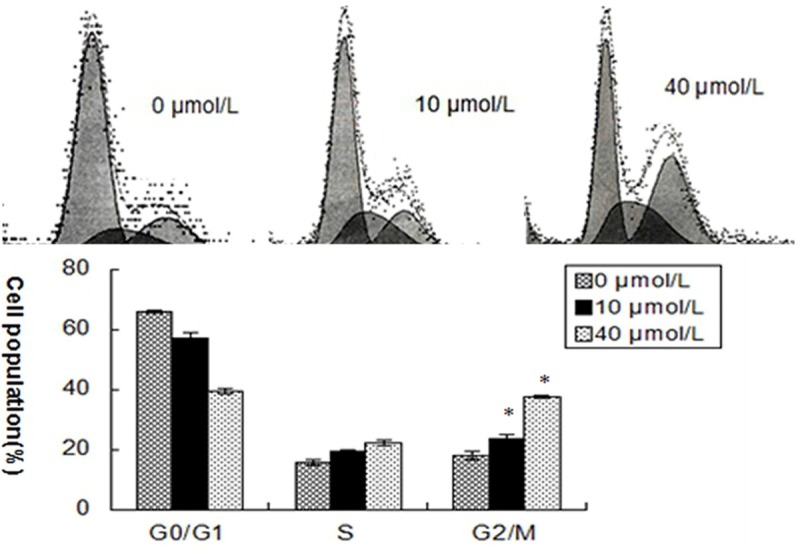
Curcumin induces G2/M cell cycle arrest in SCC-25 cells. SCC-25 cells were exposed to various concentrations of curcumin (0, 10 and 40 μmol/L) for 24 hours. Cell cycle distributions were analyzed by flow cytometry with PI staining. Results are mean ± SD from at least three independent experiments. *P < 0.05 as compared with the control cells.
Curcumin inhibits invasion of SCC-25 cells
To detect the impact of curcumin on the ability of SCC-25 cells invasion, transwell matrigel invasion assays were performed. As precautions to minimize the contribution of cell proliferation, the experiments were performed in serum free medium. After treatment with different concentrations curcumin for 24 hours, the number of cells migrating through the membrane showed a dose-dependent decrease compared to untreated cells (Figure 3).
Figure 3.
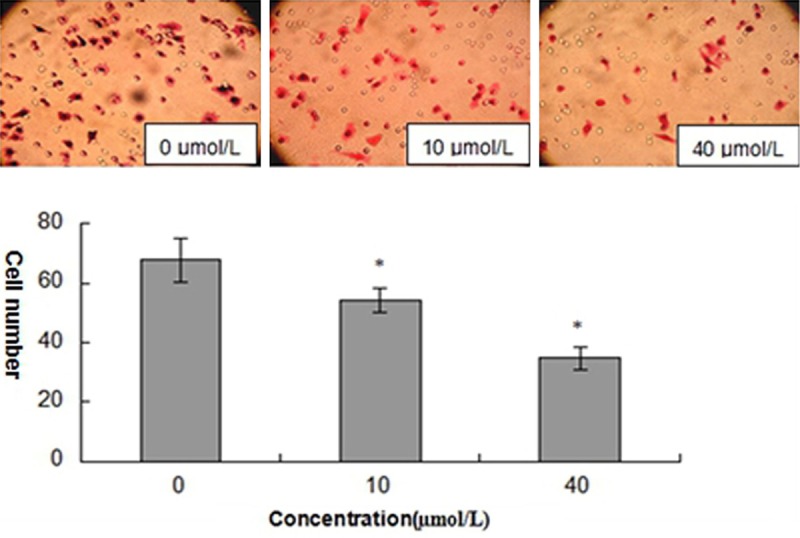
Curcumin inhibits the invasion in SCC-25 cells. The cells were exposed to curcumin for 24 hours at different concentrations (0, 10 and 40 μmol/L). Results are mean ± SD from at least three independent experiments. *P < 0.05 as compared with the control cells.
Curcumin inhibits the expression of MMP-2, MMP-9, uPA and uPAR
Carcinomas are characterized by the invasion of malignant cells into the underlying connective tissue and their migration to form metastases at distant sites. In particular, MMP-2 and MMP-9 play a significant proteolytic role in both migration and invasion. So we observed whether curcumin could down-regulate the expression of MMP-2 and MMP-9. Our results indicated that curcumin inhibited the expression of MMP-2 and MMP-9 in SCC-25 cells (Figure 4). Since the activity of proliferation and invasion in tumor cells has been shown to be related to the urokinase-type plasminogen activator (uPA) signaling, we also evaluated the expression levels of uPA and its receptor uPAR. Our results showed that curcumin significantly downregulated expressions of uPA and uPAR (Figure 4).
Figure 4.
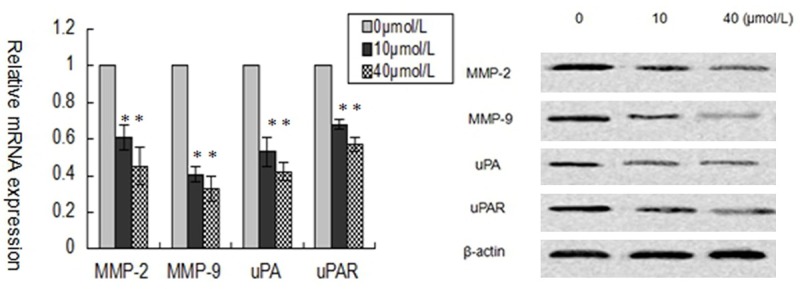
Curcumin inhibits MMP2/9 and uPA/uPAR expression in SCC-25 cells. A: RT-PCR assay of MMP2/9 and uPA/uPAR mRNA level of SCC-25 cells treated with different concentrations of curcumin (0, 10, and 40 μmol/L) for 24 hours. B: Western blot analysis of MMP2/9 and uPA/uPAR protein level of SCC-25 cells treated with different concentrations of curcumin (0, 10, and 40 μmol/L) for 24 hours. Results are mean ± SD from at least three independent experiments. *P < 0.05 as compared with the control cells.
Curcumin inhibits the activition both EGFR and EGFR downstream signaling in SCC-25 cells
It has been reported that curcumin could reduce EGFR expression in several cancer cell lines, such as prostate cancer cells, lung cancer cells and bladder cancer cells. In this study, the effect of curcumin on EGFR activition in SCC-25 cells was observed. Cells were treated with curcumin at different concentrations for 24 hours. Western blot analyses demonstrated that curcumin could not affect total EGFR expression, but significantly inhibited protein expression of p-EGFR in SCC-25 cells. Furthermore, phosphorylation of Akt, phosphorylation of ERK1/2 and phosphorylation of STAT3, the crucial downstream signaling molecules of EGFR, were also reduced (Figure 5).
Figure 5.
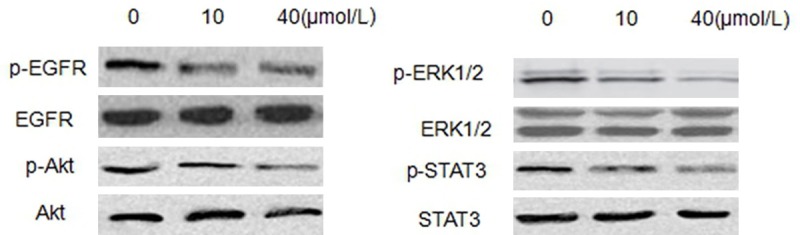
Curcumin inhibits the activition both EGFR and EGFR downstream signaling in SCC-25 cells. Western blot showing levels of total and phosphorylated EGFR, Akt, ERK1/2 and STAT3 in SCC-25 cells after different concentrations of curcumin (0, 10, and 40 μmol/L) treatment.
Curcumin reduces EGF-induced EGFR activition in SCC-25 cells
To determine whether the downregulation of EGFR activition by curcumin is involved in EGF/EGFR signaling pathway, we examined the effect of curcumin on EGF-induced EGFR phosphorylation. Our data showed that curcumin reduced the EGF-induced phosphorylation of EGFR (Figure 6A). It is well known that EGF/EGFR signaling pathway plays a critical role in OSCC invasion, so we further investigated the effects of curcumin on EGF-mediated SCC-25 cells invasion. We used 100 ng/ml EGF as chemoattractant in the transwell matrigel invasion assays. Our results showed that curcumin significantly suppressed EGF-triggered SCC-25 cells invasion (Figure 6B).
Figure 6.
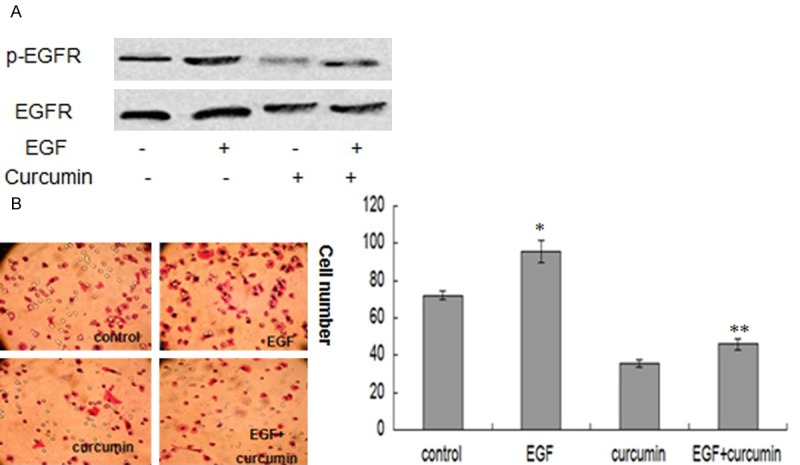
Curcumin reduces EGF-induced EGFR activition in SCC-25 cells. A: Western blot analysis indicates that curcumin inhibits the EGF-induced phosphorylation of EGFR in SCC-25 cells. B: Transwell matrigel invasion assays indicate that curcumin suppresses EGF-triggered SCC-25 cells invasion. Results are mean ± SD from at least three independent experiments. *P < 0.05 as compared with the control cells. **P < 0.05 as compared with the EGF treated cells.
Taken together, our findings suggest that curcumin reduces SCC-25 cells proliferation and invasion through inhibiting the phosphorylation of EGFR and EGFR downstream signaling including Akt, ERK1/2 and STAT3 (Figure 7).
Figure 7.
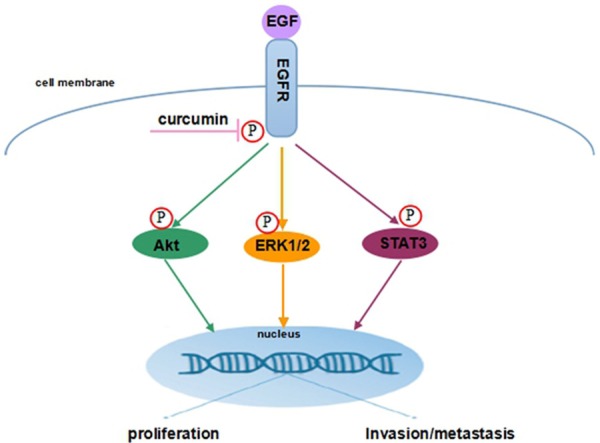
Plausible molecular mechanism of curcumin inhibits SCC-25 cells proliferation and invasion through EGFR signaling pathways.
Discussion
Curcumin, the major pigment in turmeric powder, has been considered as potential source of drugs for the development of novel anti-tumor chemotherapeutics because of its effect on inhibiting various human cancer cells proliferation in vitro and in vivo. In this study, we found that curcumin could inhibit the proliferation of SCC-25 cell line with dose-dependent manner. For maintenance of a normal cell growth, the cell cycle is segregated into four phases: S phase (DNA replication), M phase (chromosome segregation), G1 phase (before DNA replication) and G2 phase (before mitosis). During DNA damage, cells are blocked in G2/M phase to provide time to repair damaged DNA or lead to apoptotic cell death in case of severe DNA damage [24]. Several studies have indicated that curcumin induces cell cycle arrest in various human cancer cells. To determine whether curcumin induces the cell cycle arrest of SCC-25 cells, the cell cycle distribution was analyzed by flow cytometry after a 24 hours exposure to curcumin. Our results confirmed that curcumin treatment can significantly blocks SCC-25 cells at G2/M phase of cell cycle. These results are in agreement with previous studies that curcumin caused a cell cycle arrest at the G2/M phase in lung cancer cells [25] and breast cancer cells [26]. Our study suggested that curcumin inhibited the SCC-25 cells proliferation by inducing G2/M cell cycle arrest.
Cell invasion into the underlying connective tissue is an important component of the malignant phenotype. Our transwell Matrigel invasion assay showed that curcumin evidently decreased cell number migrating through the Matrigel membrane. It is well known that the invasion processes of tumor cells require disruption of the interaction between cells and the extracellular matrix (ECM). Proteolytic enzymes MMPs and PAs are thought to be involved in the invasion and metastasis of tumor cells [27]. MMPs are necessary for tumor invasion and metastases by degradation the ECM [28]. The observed effect of curcumin on cell invasion may be partially caused by decreasing activity of extracellular matrix proteases such as MMP-2 and MMP-9, which play key roles in local invasion [29]. uPA/uPAR system plays an important role in destruction of ECM and degradation of the basement membrane. Previously studies have shown that the high expression of uPA/uPAR in OSCC correlated with tumor invasion and metastasis [30]. In this study, we tested the mRNA and protein level expression of MMP2/9 and uPA/uPAR by using real-time PCR and western blot. Our results revealed that curcumin could inhibit the motility of SCC-25 cell line, which result from its potential to downregulated the expressions of uPA/uPAR and MMP2/9.
An increase in EGFR activity has been correlated to a malignant evolution of cells [31]. Overexpression of EGFR is found in different types of human tumors and often correlates with the enhanced cellular proliferation and invasion [32]. Our results showed that curcumin could not affect total EGFR expression, but significantly inhibited the phosphorylation of EGFR in SCC-25 cells. Moreover, EGFR regulation of cell proliferation and survival has been shown to involve the Akt, ERK1/2 and STAT3 pathways [33]. In the present study, curcumin reduced the Akt, ERK1/2 and STAT3 activation in SCC-25 cells, which is of significance because the inactivation of the Akt, ERK1/2 and STAT3 signaling pathways suppresses cell proliferation and invasion. Furthermore, EGF is known as one of the major ligands that bind to EGFR to activate its downstream signaling molecules, which stimulates the proliferation and invasion of cancer cells [34]. In this study, our data showed that curcumin was able to inhibit EGF-induced EGFR phosphorylation migration and invasion of SCC-25 cells.
In conclusion, these results suggested that curcumin mediates the proliferation and invasion in SCC-25 cells through EGFR signaling pathways.
Acknowledgements
This study was supported by Shanghai Natural Science Foundation (No. 13ZR1436300), Young Scholars Project of Shanghai Health Bureau (No. 20124y034), Shanghai Yong Doctor Program (No. 2012-04-03) and Natural Science Foundation of Shanghai Stomatological Disease Center (No. SSDC-F2-2012-01, SSDC-2012-05).
Disclosure of conflict of interest
None.
References
- 1.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 2.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Brusevold IJ, Aasrum M, Bryne M, Christoffersen T. Migration induced by epidermal and hepatocyte growth factors in oral squamous carcinoma cells in vitro: role of MEK/ERK, p38 and PI-3 kinase/Akt. J Oral Pathol Med. 2012;41:547–58. doi: 10.1111/j.1600-0714.2012.01139.x. [DOI] [PubMed] [Google Scholar]
- 5.Nakata Y, Uzawa N, Takahashi K, Sumino J, Michikawa C, Sato H, Sonoda I, Ohyama Y, Okada N, Amagasa T. EGFR gene copy number alteration is a better prognostic indicator than protein overexpression in oral tongue squamous cell carcinomas. Eur J Cancer. 2011;47:2364–2372. doi: 10.1016/j.ejca.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Dai W, Li Y, Zhou Q, Xu Z, Sun C, Tan X, Lu L. Cetuximab inhibits oral squamous cell carcinoma invasion and metastasis via degradation of epidermal growth factor receptor. J Oral Pathol Med. 2014;43:250–257. doi: 10.1111/jop.12116. [DOI] [PubMed] [Google Scholar]
- 7.Doumiati S, Haupt K, Rossi C. Autophosphorylation activation and inhibition by curcumin of the epidermal growth factor receptor reconstituted in liposomes. J Mol Recognit. 2012;25:623–629. doi: 10.1002/jmr.2194. [DOI] [PubMed] [Google Scholar]
- 8.Lin MC, Huang MJ, Liu CH, Yang TL, Huang MC. GALNT2 enhances migration and invasion of oral squamous cell carcinoma by regulating EGFR glycosylation and activity. Oral Oncol. 2014;50:478–484. doi: 10.1016/j.oraloncology.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Aquino G, Pannone G, Santoro A, Liguori G, Franco R, Serpico R, Florio G, De Rosa A, Mattoni M, Cozza V, Botti G, Losito S, Longo F, Staibano S, Cuda G, Lo Muzio L, Sbordone C, Bufo P, Grimaldi A, Caraglia M, Di Domenico M. pEGFR-Tyr 845 expression as prognostic factors in oral squamous cell carcinoma: a tissue-microarray study with clinic-pathological correlations. Cancer Biol Ther. 2012;13:967–977. doi: 10.4161/cbt.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabinowits G, Haddad RI. Overcoming resistance to EGFR inhibitor in head and neck cancer: a review of the literature. Oral Oncol. 2012;48:1085–1089. doi: 10.1016/j.oraloncology.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Soeda H, Shimodaira H, Gamoh M, Ando H, Isobe H, Suto T, Takahashi S, Kakudo Y, Amagai K, Mori T, Watanabe M, Yamaguchi T, Kato S, Ishioka C. Phase II Trial of Cetuximab plus Irinotecan for Oxaliplatin- and Irinotecan-Based Chemotherapy-Refractory Patients with Advanced and/or Metastatic Colorectal Cancer: Evaluation of Efficacy and Safety Based on KRAS Mutation Status (T-CORE0801) Oncology. 2014;87:7–20. doi: 10.1159/000360989. [DOI] [PubMed] [Google Scholar]
- 12.Maseki S, Ijichi K, Nakanishi H, Hasegawa Y, Ogawa T, Murakami S. Efficacy of gemcitabine and cetuximab combination treatment in head and neck squamous cell carcinoma. Mol Clin Oncol. 2013;1:918–924. doi: 10.3892/mco.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sgambato A, Casaluce F, Maione P, Rossi A, Ciardiello F, Gridelli C. Cetuximab in advanced non-small cell lung cancer (NSCLC): the showdown? J Thorac Dis. 2014;6:578–580. doi: 10.3978/j.issn.2072-1439.2014.06.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nechushtan H, Vainer G, Stainberg H, Salmon AY, Hamburger T, Peretz T. A phase 1/2 of a combination of Cetuximab and Taxane for “triple negative” breast cancer patients. Breast. 2014;23:435–438. doi: 10.1016/j.breast.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Zhang J, Han J, Pan X, Cao Y, Guo H, Pan Y, An Y, Li X. Curcumin inhibits tumor proliferation induced by neutrophil elastase through the upregulation of α1-antitrypsin in lung cancer. Mol Oncol. 2012;6:405–417. doi: 10.1016/j.molonc.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu DJ, Chen XW, Wang JZ, Ju YL, Ou Yang MZ, Zhang WJ. Proteomic analysis identifies proteins associated with curcumin-enhancing efficacy of irinotecan-induced apoptosis of colorectal cancer LOVO cell. Int J Clin Exp Pathol. 2013;7:1–15. [PMC free article] [PubMed] [Google Scholar]
- 17.Lv ZD, Liu XP, Zhao WJ, Dong Q, Li FN, Wang HB, Kong B. Curcumin induces apoptosis in breast cancer cells and inhibits tumor growth in vitro and in vivo. Int J Clin Exp Pathol. 2014;7:2818–2824. [PMC free article] [PubMed] [Google Scholar]
- 18.Choi HY, Lim JE, Hong JH. Curcumin interrupts the interaction between the androgen receptor and Wnt/β-catenin signaling pathway in LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis. 2010;13:343–349. doi: 10.1038/pcan.2010.26. [DOI] [PubMed] [Google Scholar]
- 19.Hung CM, Su YH, Lin HY, Lin JN, Liu LC, Ho CT, Way TD. Demethoxycurcumin modulates prostate cancer cell proliferation via AMPK-induced down-regulation of HSP70 and EGFR. J Agric Food Chem. 2012;60:8427–8434. doi: 10.1021/jf302754w. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Patmore DM, Jousma E, Eaves DW, Breving K, Patel AV, Schwartz EB, Fuchs JR, Cripe TP, Stemmer-Rachamimov AO, Ratner N. EGFR-STAT3 signaling promotes formation of malignant peripheral nerve sheath tumors. Oncogene. 2014;33:173–180. doi: 10.1038/onc.2012.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25:278–287. doi: 10.1038/sj.onc.1209019. [DOI] [PubMed] [Google Scholar]
- 22.Sun XD, Liu XE, Huang DS. Curcumin induces apoptosis of triple-negative breast cancer cells by inhibition of EGFR expression. Mol Med Rep. 2012;6:1267–1270. doi: 10.3892/mmr.2012.1103. [DOI] [PubMed] [Google Scholar]
- 23.Armanious H, Gelebart P, Mackey J, Ma Y, Lai R. STAT3 upregulates the protein expression and transcriptional activity of β-catenin in breast cancer. Int J Clin Exp Pathol. 2010;3:654–664. [PMC free article] [PubMed] [Google Scholar]
- 24.Huang M, Miao ZH, Zhu H, Cai YJ, Lu W, Ding J. Chk1 and Chk2 are differentially involved in homologous recombination repair and cell cycle arrest in response to DNA double-strand breaks induced by camptothecins. Mol Cancer Ther. 2008;7:1440–1449. doi: 10.1158/1535-7163.MCT-07-2116. [DOI] [PubMed] [Google Scholar]
- 25.Yang CL, Liu YY, Ma YG, Xue YX, Liu DG, Ren Y, Liu XB, Li Y, Li Z. Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3 signalling pathway. PLoS One. 2012;7:e37960. doi: 10.1371/journal.pone.0037960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang N, Wang MM, Wang YH, Zhang ZN, Cao HR, Lv YH, Yang Y, Fan PH, Qiu F, Gao XM. Tetrahydrocurcumin induces G2/M cell cycle arrest and apoptosis involving p38 MAPK activation in human breast cancer cells. Food Chem Toxicol. 2014;67:193–200. doi: 10.1016/j.fct.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 27.Shin HK, Kim J, Lee EJ, Kim SH. Inhibitory effect of curcumin on motility of human oral squamous carcinoma YD-10B cells via suppression of ERK and NF-kappaB activations. Phytother Res. 2010;24:577–582. doi: 10.1002/ptr.2989. [DOI] [PubMed] [Google Scholar]
- 28.Lee EJ, Whang JH, Jeon NK, Kim J. The epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 (Iressa) suppresses proliferation and invasion of human oral squamous carcinoma cells via p53 independent and MMP, uPAR dependent mechanism. Ann N Y Acad Sci. 2007;1095:113–128. doi: 10.1196/annals.1397.015. [DOI] [PubMed] [Google Scholar]
- 29.Dudás J, Fullár A, Romani A, Pritz C, Kovalszky I, Hans Schartinger V, Mathias Sprinzl G, Riechelmann H. Curcumin targets fibroblast-tumor cell interactions in oral squamous cell carcinoma. Exp Cell Res. 2013;319:800–809. doi: 10.1016/j.yexcr.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nozaki S, Endo Y, Kawashiri S, Nakagawa K, Yamamoto E, Yonemura Y, Sasaki T. Immunohistochemical localization of a urokinase-type plasminogen activator system in squamous cell carcinoma of the oral cavity: association with mode of invasion and lymph node metastasis. Oral Oncol. 1998;34:58–62. doi: 10.1016/s1368-8375(97)00028-6. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad N, Kalka K, Mukhtar H. In vitro and in vivo inhibition of epidermal growth factor receptor-tyrosine kinase pathway by photodynamic therapy. Oncogene. 2001;20:2314–2317. doi: 10.1038/sj.onc.1204313. [DOI] [PubMed] [Google Scholar]
- 32.Spaulding DC, Spaulding BO. Epidermal growth factor receptor expression and measurement in solid tumors. Semin Oncol. 2002;29:45–54. doi: 10.1053/sonc.2002.35647. [DOI] [PubMed] [Google Scholar]
- 33.Tsien CI, Nyati MK, Ahsan A, Ramanand SG, Chepeha DB, Worden FP, Helman JI, D’Silva N, Bradford CR, Wolf GT, Lawrence TS, Eisbruch A. Effect of erlotinib on epidermal growth factor receptor and downstream signaling in oral cavity squamous cell carcinoma. Head Neck. 2013;35:1323–1330. doi: 10.1002/hed.23128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brusevold IJ, Aasrum M, Bryne M, Christoffersen T. Migration induced by epidermal and hepatocyte growth factors in oral squamous carcinoma cells in vitro: role of MEK/ERK, p38 and PI-3 kinase/Akt. J Oral Pathol Med. 2012;41:547–558. doi: 10.1111/j.1600-0714.2012.01139.x. [DOI] [PubMed] [Google Scholar]


