Abstract
Background
Several scoring systems have tried to determine the severity of coronary artery disease (CAD) to investigate the connection between CAD severity and laboratory parameters.
Methods
In total, 189 male (mean age: 61.86±10.77 years) and 75 female CAD patients (mean age: 67.84±7.70 years) were recruited and underwent angiography, which determined stenosis grade, of 17 coronary segments: no points for each nonstenosed segment or only calcified segments, one point for each stenosis from <30% to <50%, two points for each stenosis from 50% to <70%, and three points for each stenosis >70%. The points were added and should represent the severity of patients’ CAD.
Results
The coronary score correlated positively with systolic blood pressure, creatinine, blood urea nitrogen, lipase, glucose, glycated hemoglobin, triglycerides, C-reactive protein, fibrinogen Clauss, and leukocytes, and correlated negatively with Cl−, iron, and high-density lipoprotein cholesterol. Stepwise multiple regression analysis with backward elimination revealed diabetes status, sex, and fibrinogen Clauss as significant predictors of coronary score.
Conclusion
The coronary score delivers a quite simple but very precise tool for the quantification of CAD severity. These results show plainly the connection between CAD severity and the lipid, glucose, coagulation, and immunologic status of CAD patients, and substantiate the importance of sufficient treatment in this group of patients – in particular, CAD patients suffering from type 2 diabetes mellitus. The coronary score would offer a suitable tool for the investigation of the connection between CAD and new biomarkers. Further studies are needed to investigate the correlation of the coronary score with outcome parameters (eg, death).
Keywords: coronary artery disease, grading system, angiography, diabetes mellitus, atherosclerosis
Introduction
For scientists and clinicians who carry out research about the genesis of atherosclerosis, it has always been compelling to somehow quantify the grade of severity of coronary artery calcification and stenosis. Since the late 1960s, the severity of coronary stenosis was suspected to be a prognostic factor for patients with coronary artery disease (CAD),1–3 and this hypothesis was proven in several clinical studies with long follow-up periods.4–7 However, the quantification of CAD poses a problem due to the lack of consistent and universally valid scoring systems. For example, in one of their studies, Proudfit et al3 concentrated on the number of severe stenoses and correlated them to clinical characteristics such as duration of the history of angina pectoris, distribution of pain, and serum cholesterol. In another study,1 they used a more precise system by classifying the coronary vessels as non/slightly/moderately/severely/totally obstructed depending on the grade of obstruction in percental gradations from no to total stenosis, but they concentrated on the major arteries and branches. Parker et al2 used a similar system. As basis for their quantification, they measured the remaining lumina in the right coronary artery, main left coronary artery and its anterior descending and circumflex branches. However, in case there were multiple lesions in a vessel, they counted the most severe. Humphries et al5 used a scoring system that included the three major coronary arteries and determined the severity of CAD by measuring “narrowing(s)” in them. Further studies that used coronary scoring systems were performed by Reardon et al,8 Jenkins et al,9 Sullivan et al,10 Brandt et al,11 Leaman et al,12 Sianos et al,13 and Hamsten et al,14 who all more or less concentrated on the grade of stenosis in different coronary arteries. A work by Neeland et al15 in 2012 compared ten angiographic scoring systems in about 3,600 patients and found a strong correlation of the systems with each other and with atherosclerotic plaque burden.
All mentioned methods, although some of them are nearly half a century old, are mostly simple and reasonable, and their application provided an enormous increase in knowledge concerning the connection between the severity of CAD and clinical and prognostic correlations. A further method to investigate the genesis of atherosclerosis in CAD is the determination of coronary calcification by means of electron beam computed tomography and multidetector computed tomography. The scoring system used (Agaston Score) has again provided important information about the development of atherosclerosis.16,17
In the present explorative study, we investigated the connection between a new score grading severity of stenosis (“coronary score”) determining the severity of CAD in 17 segments and anamnestic and clinical circumstances, as well as routine laboratory parameters in patients with CAD.
Materials and methods
In total, 189 male (mean age: 61.86±10.77 years) and 75 female (mean age: 67.84±7.70 years) CAD patients were recruited, whereby 45.3% had never had an ischemic cardiac event before and 54.7% had had at least one ischemic event (ST-segment elevation myocardial infarction [STEMI], non-ST-segment elevation myocardial infarction [NSTEMI], or acute coronary syndrome [ACS]) before. Patients with thrombotic occlusions were excluded from the study. They underwent a coronary angiography for diagnostic and/or therapeutic reasons on grounds of their underlying disease. The coronary artery system was divided into 17 segments, and stenosis grade for each segment was measured. The coronary arteries were divided into the following 17 segments – left main, proximal/medial/distal left anterior descending, intermediate branch, first and second marginal branch, posterolateral branch, first and second diagonal branch, proximal/medial/distal left circumflex, proximal/medial/distal right coronary artery, and ramus interventricularis posterior – and stenosis grade for each segment was measured. A simple three-point grading per stenosis was used to develop a score considering both frequency and severity of CAD stenoses: no points for each nonstenosed segment or only calcified segments, one point for each stenosis from <30% to <50%, two points for each stenosis from 50% to <70%, and three points for each stenosis >70%. The points were added over all stenoses per patient and should represent the severity of patients’ CAD (score).
Anthropometric and anamnestic data, including age, body mass index (BMI), systolic and diastolic blood pressure, heart rate, risk factor assessment (type 2 diabetes mellitus [T2DM] status, hypertension, hypercholesterolemia, family history, smoking, physical activity), and routine laboratory parameters were gathered. Blood samples for determination of routine laboratory parameters were taken before or at least 48 hours after invasive therapies/acute events (eg, angiography/STEMI, NSTEMI) to avoid a strong distortion. Statistical analysis was done with SPSS 20.0 (IBM Corporation, Armonk, NY, USA). Continuous and normally distributed data are described by mean ± standard deviation. Ordinal data or continuous data with skew distribution or outliers are described by median and first and third quartiles. Simple associations of the coronary score with continuous variables were assessed by Spearman’s correlation coefficient, and point biserial correlation coefficients were calculated for dichotomous variables. Those variables that correlated significantly with the coronary score were then entered in a stepwise multiple regression analysis with backward elimination to assess the variables that predict the coronary score best. Simple tests were performed two-sided, and P-values ≤0.05 were considered significant. To adjust for multiple testing, only parameters correlating with P<0.01 were used for the multiple regression analysis. The study protocol, which is in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments, was approved by the Ethical Commission of the Medical University of Vienna, Vienna, Austria, and informed consent was obtained from patients.
Results
The present study aimed to investigate the connection between the severity of CAD determined by the coronary score (as described in the “Materials and methods” section) and routine laboratory parameters, as well as CAD risk factors such as T2DM or physical activity in 264 CAD patients. The distribution of score points is shown in Figure 1. Table 1 shows anthropometric and hemodynamic data of the population, and Table 2 shows median levels (first and third quartile) of routine laboratory parameters, correlation coefficient (Spearman’s correlation), and P-value of the correlation with the coronary score.
Figure 1.
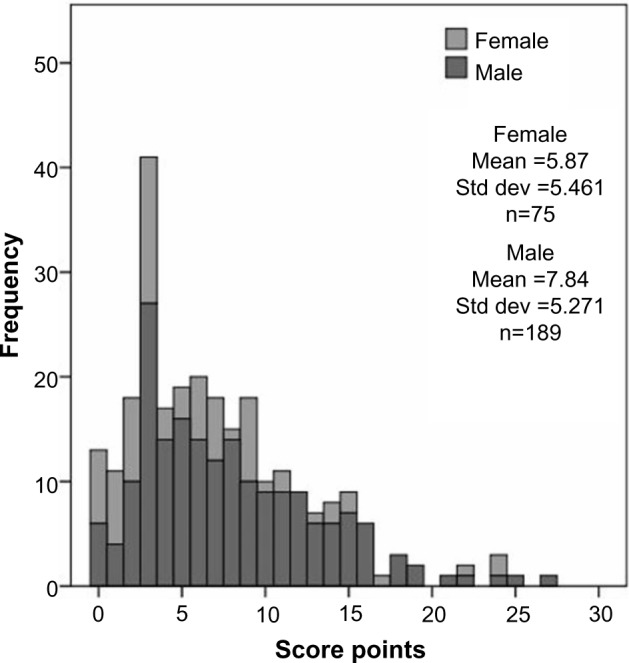
Distribution of the score points within the study population.
Abbreviation: Std dev, standard deviation.
Table 1.
Anthropometric and hemodynamic data and score points within the population
| Age (years) | 63.55±10.34 |
| Height (cm) | 171.78±9.34 |
| Weight (kg) | 83.41±18.57 |
| Body mass index (kg/m2) | 28.14±5.26 |
| Systolic blood pressure (mmHg) | 130.56±16.24 |
| Diastolic blood pressure (mmHg) | 76.02±10.95 |
| Heart rate (bpm) | 70.02±13.35 |
| Score points | 7.28 (3.00–10.00) |
Note: Data are given as mean ± standard deviation or median (first and third quartile), respectively.
Table 2.
Routine laboratory parameters within the study population
| Median (first and third quartiles) | Correlation coefficient | P-value | |
|---|---|---|---|
| Na+ (mmol/L) | 139 (137–141) | −0.090 | 0.144 |
| K+ (mmol/L) | 4.07 (3.80–4.30) | 0.043 | 0.486 |
| Cl− (mmol/L) | 104 (102–106) | −0.143 | 0.020 |
| Ca++ (mmol/L) | 2.39 (2.28–2.47) | −0.012 | 0.853 |
| Mg++ (mmol/L) | 0.85 (0.79–0.90) | −0.031 | 0.618 |
| Inorganic phosphate (mmol/L) | 0.98 (0.86–1.11) | 0.070 | 0.257 |
| Iron (μg/dL) | 65 (44–90) | −0.138 | 0.044 |
| Creatinine (mg/dL) | 1.02 (0.89–1.19) | 0.189 | 0.002 |
| Blood urea nitrogen (mg/dL) | 17.30 (13.40–23.20) | 0.156 | 0.011 |
| Uric acid (mg/dL) | 6.30 (5.15–7.60) | 0.113 | 0.071 |
| Alkaline phosphatase (kU/L) | 76 (61–94) | −0.004 | 0.950 |
| ASAT (GOT, U/L) | 30 (24–45) | −0.032 | 0.604 |
| ALAT (GPT, U/L) | 29 (20–43) | −0.032 | 0.609 |
| Gamma-GT (U/L) | 36 (23–63) | 0.038 | 0.543 |
| LDH (U/L) | 231 (194–313) | −0.005 | 0.933 |
| Alpha-amylase (U/L) | 58 (42–73) | 0.098 | 0.115 |
| Lipase (U/L) | 27 (18–40) | 0.135 | 0.030 |
| Cholinesterase (U/L) | 7.49–8.61) | 0.027 | 0.665 |
| Glucose (mg/dL) | 107 (93–133) | 0.138 | 0.026 |
| Glycated hemoglobin (relative%) | 6.0 (5.5–6.5) | 0.224 | 0.001 |
| Cholesterol (mg/dL) | 168 (140–202) | −0.105 | 0.091 |
| High-density lipoprotein cholesterol (mg/dL) | 44 (37–54) | −0.208 | 0.002 |
| Low-density lipoprotein cholesterol (mg/dL) | 99 (74–126) | −0.092 | 0.177 |
| Triglycerides (mg/dL) | 130 (95–199) | 0.176 | 0.005 |
| TSH (μU/mL) | 1.65 (0.97–2.49) | −0.008 | 0.905 |
| C-reactive protein (mg/dL) | 0.48 (0.15–1.66) | 0.142 | 0.023 |
| Transferrin (mg/dL) | 250 (221–291) | 0.142 | 0.531 |
| Ferritin (μg/L) | 131 (61–216) | −0.075 | 0.234 |
| Combined prothrombin time (%) | 101 (85–116) | 0.140 | 0.712 |
| aPTT (sec) | 34.2 (31.6–37.8) | 0.024 | 0.880 |
| Fibrinogen Clauss (mg/dL) | 395 (340–478) | 0.252 | <0.001 |
| Erythrocytes (Terra/liter) | 4.5 (4.2–4.9) | 0.028 | 0.657 |
| Hemoglobin (g/dL) | 13.3 (12.0–14.4) | −0.058 | 0.353 |
| Hematocrit (%) | 39.9 (36.2–42.7) | −0.059 | 0.347 |
| Thrombocytes (Giga/liter) | 7.17 (5.95–8.80) | 0.011 | 0.859 |
Note: Data are given as median (first and third quartiles), correlation coefficient of Spearman correlation, and P-value.
Abbreviations: LDH, lactate dehydrogenase; TSH, thyroid-stimulating hormone; GOT, glutamic oxaloacetic transaminasis; ASAT, aspartate aminotransferases; aPTT, activated partial thormboplastin time.
The score points correlated positively with systolic blood pressure (P=0.018), creatinine, blood urea nitrogen (BUN), lipase, glucose, glycated hemoglobin (HbA1c), triglycerides (TGs), C-reactive protein (CRP), fibrinogen Clauss, and leukocytes, and correlated negatively with Cl−, iron, and high-density lipoprotein (HDL) cholesterol.
The score points independent of CAD risk factors are shown in Table 3. The coronary score correlated significantly only with sex (P<0.01) and T2DM status (P<0.01). There was no difference between T2DM with and without insulin therapy and patients without T2DM. Consequently, sex, T2DM status (yes/no), creatinine, HbA1c, HDL cholesterol, TGs, and fibrinogen Clauss were used for multiple regression analysis. As can be seen in Table 4, only T2DM, sex, and fibrinogen Clauss were significant but moderate predictors of the coronary score (r2adj =0.14; F=13.59; P<0.01). The presence or absence of the other risk factors (BMI, hypertension/statin therapy, ex-smoking status, positive family anamnesis, physical inactivity) did not have a significant impact on the height of the coronary score.
Table 3.
Coronary score (points) dependent on cardiovascular risk factors
| Type 2 diabetes mellitus | |
| No diabetes (n=191) | 5 (3–9) |
| Type 2 diabetes mellitus (n=73) | 9 (3–13.5) |
| Sex | |
| Male | 7 (3.5–11) |
| Female | 4 (2–9) |
| Physical inactivity | |
| No (n=183) | 6 (3–9) |
| Yes (n=81) | 7 (3–12.50) |
| Smoking | |
| Never smoking (n=99) | 7 (3–11) |
| Ex-smoking (n=165) | 6 (3–10) |
| Hypertension therapy | |
| No (n=20) | 5 (3–10) |
| Yes (n=264) | 6 (3–10) |
| Statin therapy | |
| No (n=24) | 4 (3–11.75) |
| Yes (n=264) | 6 (3–10) |
| Coronary artery disease family anamnesis | |
| No (n=116) | 7 (3–11.75) |
| Yes (n=148) | 6 (3–9) |
| Body mass index <24.99 kg/m2 (n=78) | 5 (2.75–9) |
| Body mass index 25.00–29.99 kg/m2 (n=107) | 6 (3–10) |
| Body mass index >30.00 kg/m2 (n=79) | 6 (3–12) |
Table 4.
Results from stepwise multiple regression analysis with backward elimination
| Beta | T | P-value | Change in r2 | |
|---|---|---|---|---|
| Constant | −2.11 | −1.17 | 0.243 | |
| Type 2 diabetes mellitus (0= no; 1= yes) | 2.64 | 3.61 | <0.01 | 0.06 |
| Fibrinogen Clauss | 0.01 | 3.63 | <0.01 | 0.04 |
| Sex (1= female; 2= male) | 2.62 | 3.60 | <0.01 | 0.05 |
Discussion
It was the aim of the present study to test the connection between the severity of CAD represented by the coronary score, which is described in the “Materials and methods” section, and routine laboratory parameters, as well as cardiovascular risk factors. Considering data of 264 female and male CAD patients, this score showed a significant positive correlation with creatinine, BUN, lipase, glucose, HbA1c, TGs, CRP, fibrinogen Clauss, and leukocytes, and a negative correlation with Cl−, iron, and HDL cholesterol. The major part of the aforementioned factors has already been connected to CAD. It was shown that ferritin and transferring saturation levels were not associated with an increased extent of CAD.18 Our results confirm these findings, but we found a negative correlation between iron and CAD severity. Serum creatinine was shown to be an independent predictor of coronary heart disease mortality in normotensive and normal-weighted survivors of a myocardial infarction but with pre-existing renal disease or heart failure.19 In patients with chest pain undergoing angiography, creatinine correlated with the extent of CAD.20 Consequently, the tested score seems to confirm data from other study groups concerning creatinine.
Concerning BUN, Kirtane et al21 showed that it is associated with an increased mortality in patients with unstable coronary syndromes, and Saygitov et al22 even found BUN to be a more significant risk factor for ACS outcome compared with creatinine. However, available literature is rare. A correlation of BUN with severity of CAD has never been shown before, and the same holds true for lipase.
To the contrary, much is known about glucose metabolism and CAD. Results from the Framingham Heart Study (>1,045 people)23 showed a significant association between coronary heart disease, stroke, and ischemic attack with HbA1c in women but not in men. These findings were consistent with results of former analysis of the Framingham Heart Study population24,25 indicating a stronger influence of diabetes and hyperglycemia on women compared with men. However, a recently published study by Pai et al26 using data from the Nurses’ Health Study and the Health Professionals Follow-Up Study, including 468 women and 454 men, suggested an association of HbA1c and coronary heart disease risk in apparently healthy nondiabetic men and women. A systematic search by Liu et al27 found HbA1c to be an independent risk factor for mortality in CAD patients without diabetes but not with established diabetes. However, another study showed that diabetes – in particular, in combination with albuminuria – is a powerful risk factor of CAD,28 and HbA1c was also correlated with severity of CAD,29 which can be confirmed by our results, because both HbA1c and blood glucose correlated positively with the coronary score. Within our study group, the 191 CAD patients who did not suffer from T2DM had a median coronary score of 5 compared with the CAD patient suffering from DM who had a score of 9. These results show plainly worse coronary status in diabetic CAD patients.
Apart from glucose metabolism, lipid metabolism plays a distractive role in the genesis of coronary atherosclerosis. TGs were shown to be a clinical marker of CAD to a greater extent in hypertensive patients30 and postmenopausal women;31 however, in the last mentioned study, patients were divided into only three groups (nonobstructive, one vessel stenosis, or multivessel stenosis), which is a quite common but also vague method of CAD severity quantification. A recently published study32 in which patients were again divided into three groups (single branch/double branch/multiple branch stenosis) showed that the TG/HDL cholesterol ratio is predictive for the severity of CHD. The connection between lipid profile and CAD severity was also observed and published for a Chinese population using a four-step grading system of CAD severity (single/double/triple/multiple vessel stenosis).33
Contrary to TGs, high HDL cholesterol levels have a protective effect on the cardiovascular system. A large study including about 5,600 individuals showed that those without CAD had higher levels of HDL cholesterol compared with those with CAD.34 Low levels of HDL cholesterol were shown to predict CHD mortality and the occurrence of new CHD events in persons aged >70 years. Interestingly, in the course of the same study, total cholesterol was not associated with CHD mortality in elderly men but was suggested as a CHD risk factor for elderly women.35 Concerning the present study, the CAD severity correlated positively with TGs and the TG/HDL cholesterol ratio (but not with total or low-density lipoprotein [LDL] cholesterol) and negatively with HDL cholesterol. Regarding our results and the results of the aforementioned studies, TG and HDL levels seem to be of greater relevance for the genesis of coronary artery stenosis compared with total or LDL cholesterol.
Serum fibrinogen was shown in the course of the Scottish Health Study to be a predictor for fatal and nonfatal CAD in both middle-aged women and men,36 and similar results were received for plasma levels of fibrinogen37 whereby in the aforementioned study plasma levels of fibrinogen were shown to correlate with CAD severity (using a four-step grading system). Using the more precise coronary score of our study, we can confirm these results because serum fibrinogen levels correlated significantly with the score in both female and male patients.
Concerning the connection between the immunologic system and CAD severity, the parameters CRP and leukocytes are of special interest. Individuals without CAD show significantly lower levels of CRP compared with CAD patients.34 A study by Auer et al38 including 100 individuals (about 60 CAD patients) suggested an association of CRP with the presence but not with the severity of CAD (using a quite precise zero- to three-point grading system). However, a study by Liu et al39 (including 418 patients with LDL cholesterol <3.37 mmol/L) and also our study, which included 264 CAD patients, found a significant correlation between CAD severity and CRP levels. Similar to CRP, the white blood cell count has earlier been associated with an increased risk for, and progression of, CHD, with a shorter survival time in CAD patients40 and also with the extent of CAD severity,41 which can be confirmed by our results and shows the importance of the immunological system in CAD. On the one hand, this score delivers interesting information on the connection between the severity of CAD and several routine laboratory parameters. On the other hand, further studies (eg, in younger and elderly groups of CAD patients) should be performed to obtain further results.
A limiting factor was that some routine laboratory parameters such as CRP and glucose are influenced by numerous factors and circumstances. Although we tried to avoid strong distortions (eg, from invasive examinations such as the angiography) by taking blood samples with distance to invasive events, an influence of noncontrolled factors cannot be excluded. We included patients with chronic CAD (n=144) and patients who had just suffered their first event (n=120), which might have led to distortions because chronic CAD patients had already received stents in former times; however, most of the correlation was also present when analyzing just the patients who had never had a CAD event.
The coronary score delivers quite a simple tool for the quantification of CAD severity. In 264 CAD patients, the severity of CAD quantified by this score correlated positively with creatinine, BUN, lipase, glucose, HbA1c, TGs, TG/HDL cholesterol ratio, CRP, fibrinogen Clauss, and leukocytes, and correlated negatively with Cl−, iron, and HDL cholesterol. The results show plainly the connection between CAD severity and the lipid, glucose, coagulation, and immunologic status of CAD patients, and substantiate the importance of sufficient treatment in this group of patients – in particular, CAD patients suffering from T2DM. Furthermore, the coronary score would offer a suitable tool for the investigation of the connection between CAD and new biomarkers. Further studies are needed to investigate its correlation with clinical outcome parameters (eg, death).
Acknowledgments
The authors give special thanks to the angiography team of the Medical University of Vienna and Heidi Kieweg. The study was funded by means of the Medical University of Vienna.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Proudfit WL, Shirey EK, Sones FM., Jr Selective cine coronary arteriography. Correlation with clinical findings in 1,000 patients. Circulation. 1966;33(6):901–910. doi: 10.1161/01.cir.33.6.901. [DOI] [PubMed] [Google Scholar]
- 2.Parker JO, Challis TW, West RO. Selective coronary arteriography. Can Med Assoc J. 1966;94(9):425–430. [PMC free article] [PubMed] [Google Scholar]
- 3.Proudfit WL, Shirey EK, Sheldon WC, Sones FM., Jr Certain clinical characteristics correlated with extent of obstructive lesions demonstrated by selective cine-coronary arteriography. Circulation. 1968;38(5):947–954. doi: 10.1161/01.cir.38.5.947. [DOI] [PubMed] [Google Scholar]
- 4.Proudfit WL, Bruschke AV, Sones FM., Jr Natural history of obstructive coronary artery disease. Supplement to a 10-year study. Cleve Clin Q. 1978;45(4):293–298. doi: 10.3949/ccjm.45.4.293. [DOI] [PubMed] [Google Scholar]
- 5.Humphries JO, Kuller L, Ross RS, Friesinger GC, Page EE. Natural history of ischemic heart disease in relation to arteriographic findings: a twelve year study of 224 patients. Circulation. 1974;49(3):489–497. doi: 10.1161/01.cir.49.3.489. [DOI] [PubMed] [Google Scholar]
- 6.Brymer JF, Buter TH, Walton JA, Jr, Willis PW., 3rd A natural history study of the prognostic role of coronary arteriography. Am Heart J. 1974;88(2):139–143. doi: 10.1016/0002-8703(74)90002-7. [DOI] [PubMed] [Google Scholar]
- 7.Friesinger GC, Page EE, Ross RS. Prognostic significance of coronary arteriography. Trans Assoc Am Physicians. 1970;83:78–92. [PubMed] [Google Scholar]
- 8.Reardon MF, Nestel PJ, Craig IH, Harper RW. Lipoprotein predictors of the severity of coronary artery disease in men and women. Circulation. 1985;71(5):881–888. doi: 10.1161/01.cir.71.5.881. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins PJ, Harper RW, Nestel PJ. Severity of coronary atherosclerosis related to lipoprotein concentration. Br Med J. 1978;2(6134):388–391. doi: 10.1136/bmj.2.6134.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan DR, Marwick TH, Freedman SB. A new method of scoring coronary angiograms to reflect extent of coronary atherosclerosis and improve correlation with major risk factors. Am Heart J. 1990;119(6):1262–1267. doi: 10.1016/s0002-8703(05)80173-5. [DOI] [PubMed] [Google Scholar]
- 11.Brandt PW, Partridge JB, Wattie WJ. Coronary arteriography; method of presentation of the arteriogram report and a scoring system. Clin Radiol. 1977;28(4):361–365. doi: 10.1016/s0009-9260(77)80140-2. [DOI] [PubMed] [Google Scholar]
- 12.Leaman DM, Brower RW, Meester GT, Serruys P, van den Brand M. Coronary artery atherosclerosis: severity of the disease, severity of angina pectoris and compromised left ventricular function. Circulation. 1981;63(2):285–299. doi: 10.1161/01.cir.63.2.285. [DOI] [PubMed] [Google Scholar]
- 13.Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1(2):219–227. [PubMed] [Google Scholar]
- 14.Hamsten A, Walldius G, Szamosi A, Dahlen G, de Faire U. Relationship of angiographically defined coronary artery disease to serum lipoproteins and apolipoproteins in young survivors of myocardial infarction. Circulation. 1986;73(6):1097–1110. doi: 10.1161/01.cir.73.6.1097. [DOI] [PubMed] [Google Scholar]
- 15.Neeland IJ, Patel RS, Eshtehardi P, et al. Coronary angiographic scoring systems: an evaluation of their equivalence and validity. Am Heart J. 164(4):547–552. e541. doi: 10.1016/j.ahj.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann U, Brady TJ, Muller J. Cardiology patient page. Use of new imaging techniques to screen for coronary artery disease. Circulation. 2003;108(8):e50–e53. doi: 10.1161/01.CIR.0000085363.88377.F2. [DOI] [PubMed] [Google Scholar]
- 17.Qian Z, Anderson H, Marvasty I, et al. Lesion- and vessel-specific coronary artery calcium scores are superior to whole-heart Agatston and volume scores in the diagnosis of obstructive coronary artery disease. J Cardiovasc Comput Tomogr. 2010;4(6):391–399. doi: 10.1016/j.jcct.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Auer J, Rammer M, Berent R, Weber T, Lassnig E, Eber B. Body iron stores and coronary atherosclerosis assessed by coronary angiography. Nutr Metab Cardiovasc Dis. 2002;12(5):285–290. [PubMed] [Google Scholar]
- 19.Matts JP, Karnegis JN, Campos CT, Fitch LL, Johnson JW, Buchwald H. Serum creatinine as an independent predictor of coronary heart disease mortality in normotensive survivors of myocardial infarction. POSCH Group. J Fam Pract. 1993;36(5):497–503. [PubMed] [Google Scholar]
- 20.Korkmaz S, Demirkan B, Altay H, et al. Serum creatinine is independently associated with angiographic extent of coronary artery disease in patients with stable angina pectoris. Anadolu Kardiyol Derg. 2011;11(5):407–413. doi: 10.5152/akd.2011.107. [DOI] [PubMed] [Google Scholar]
- 21.Kirtane AJ, Leder DM, Waikar SS, et al. Serum blood urea nitrogen as an independent marker of subsequent mortality among patients with acute coronary syndromes and normal to mildly reduced glomerular filtration rates. J Am Coll Cardiol. 2005;45(11):1781–1786. doi: 10.1016/j.jacc.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 22.Saygitov RT, Glezer MG, Semakina SV. Blood urea nitrogen and creatinine levels at admission for mortality risk assessment in patients with acute coronary syndromes. Emerg Med J. 2010;27(2):105–109. doi: 10.1136/emj.2008.068155. [DOI] [PubMed] [Google Scholar]
- 23.Singer DE, Nathan DM, Anderson KM, Wilson PW, Evans JC. Association of HbA1c with prevalent cardiovascular disease in the original cohort of the Framingham Heart Study. Diabetes. 1992;41(2):202–208. doi: 10.2337/diab.41.2.202. [DOI] [PubMed] [Google Scholar]
- 24.Wilson PW, Kannel WB, Anderson KM. Lipids, glucose intolerance and vascular disease: the Framingham Study. Monogr Atheroscler. 1985;13:1–11. [PubMed] [Google Scholar]
- 25.Wilson PW, Cupples LA, Kannel WB. Is hyperglycemia associated with cardiovascular disease? The Framingham Study. Am Heart J. 1991;121(2 Pt 1):586–590. doi: 10.1016/0002-8703(91)90729-2. [DOI] [PubMed] [Google Scholar]
- 26.Pai JK, Cahill LE, Hu FB, Rexrode KM, Manson JE, Rimm EB. Hemoglobin a1c is associated with increased risk of incident coronary heart disease among apparently healthy, nondiabetic men and women. J Am Heart Assoc. 2013;2(2):e000077. doi: 10.1161/JAHA.112.000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Yang YM, Zhu J, Tan HQ, Liang Y, Li JD. Prognostic significance of hemoglobin A1c level in patients hospitalized with coronary artery disease. A systematic review and meta-analysis. Cardiovasc Diabetol. 2011;10:98. doi: 10.1186/1475-2840-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pajunen P, Syvanne M, Nieminen MS, et al. Serum homocysteine, creatinine, and glucose as predictors of the severity and extent of coronary artery disease in asymptomatic members of high-risk families. Eur J Clin Invest. 2002;32(7):472–478. doi: 10.1046/j.1365-2362.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 29.Ayhan SS, Tosun M, Ozturk S, et al. Glycated haemoglobin is correlated with the severity of coronary artery disease independently of traditional risk factors in young patients. Endokrynol Pol. 2012;63(5):367–371. [PubMed] [Google Scholar]
- 30.Chen CY, Hwu CM, Lin MW, Tsai CH, Yeh HI. High triglyceride level is associated with severe coronary artery disease in hypertensive subjects. Scand Cardiovasc J. 2008;42(2):146–152. doi: 10.1080/14017430701840325. [DOI] [PubMed] [Google Scholar]
- 31.Sposito AC, Mansur AP, Maranhao RC, Martinez TR, Aldrighi JM, Ramires JA. Triglyceride and lipoprotein (a) are markers of coronary artery disease severity among postmenopausal women. Maturitas. 2001;39(3):203–208. doi: 10.1016/s0378-5122(01)00223-7. [DOI] [PubMed] [Google Scholar]
- 32.Yunke Z, Guoping L, Zhenyue C. Triglyceride-to-HDL cholesterol ratio: predictive value for CHD severity and new-onset heart failure. Herz. 2014;39(1):105–110. doi: 10.1007/s00059-013-3788-0. [DOI] [PubMed] [Google Scholar]
- 33.Jin Z, Zhang Y, Chen J, et al. Study of the correlation between blood lipid levels and the severity of coronary atherosclerosis in a Chinese population sample. Acta Cardiol. 2006;61(6):603–606. doi: 10.2143/AC.61.6.2017958. [DOI] [PubMed] [Google Scholar]
- 34.Alber HF, Wanitschek MM, de Waha S, et al. High-density lipoprotein cholesterol, C-reactive protein, and prevalence and severity of coronary artery disease in 5641 consecutive patients undergoing coronary angiography. Eur J Clin Invest. 2008;38(6):372–380. doi: 10.1111/j.1365-2362.2008.01954.x. [DOI] [PubMed] [Google Scholar]
- 35.Corti MC, Guralnik JM, Salive ME, et al. HDL cholesterol predicts coronary heart disease mortality in older persons. JAMA. 1995;274(7):539–544. [PubMed] [Google Scholar]
- 36.Woodward M, Lowe GD, Rumley A, Tunstall-Pedoe H. Fibrinogen as a risk factor for coronary heart disease and mortality in middle-aged men and women. The Scottish Heart Health Study. Eur Heart J. 1998;19(1):55–62. doi: 10.1053/euhj.1997.0573. [DOI] [PubMed] [Google Scholar]
- 37.Kazmi RS, Lwaleed BA. Plasminogen and fibrinogen plasma levels in coronary artery disease. Rev Bras Hematol Hemoter. 2012;34(4):262–263. doi: 10.5581/1516-8484.20120067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Auer J, Rammer M, Berent R, Weber T, Lassnig E, Eber B. Relation of C-reactive protein levels to presence, extent, and severity of angiographic coronary artery disease. Indian Heart J. 2002;54(3):284–288. [PubMed] [Google Scholar]
- 39.Liu HH, Zhao D, Ma CS, et al. C-reactive protein predicts the severity of coronary artery disease beyond low-density lipoprotein cholesterol. Angiology. 2012;63(3):218–222. doi: 10.1177/0003319711411291. [DOI] [PubMed] [Google Scholar]
- 40.Amaro A, Gonzalez-Juanatey JR, Iglesias C, et al. Leukocyte count as a predictor of the severity ischaemic heart disease as evaluated by coronary angiography. Rev Port Cardiol. 1993;12(11):913–917. [PubMed] [Google Scholar]
- 41.Ates AH, Canpolat U, Yorgun H, et al. Total white blood cell count is associated with the presence, severity and extent of coronary atherosclerosis detected by dual-source multislice computed tomographic coronary angiography. Cardiol J. 2011;18(4):371–377. [PubMed] [Google Scholar]


