Abstract
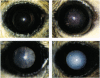
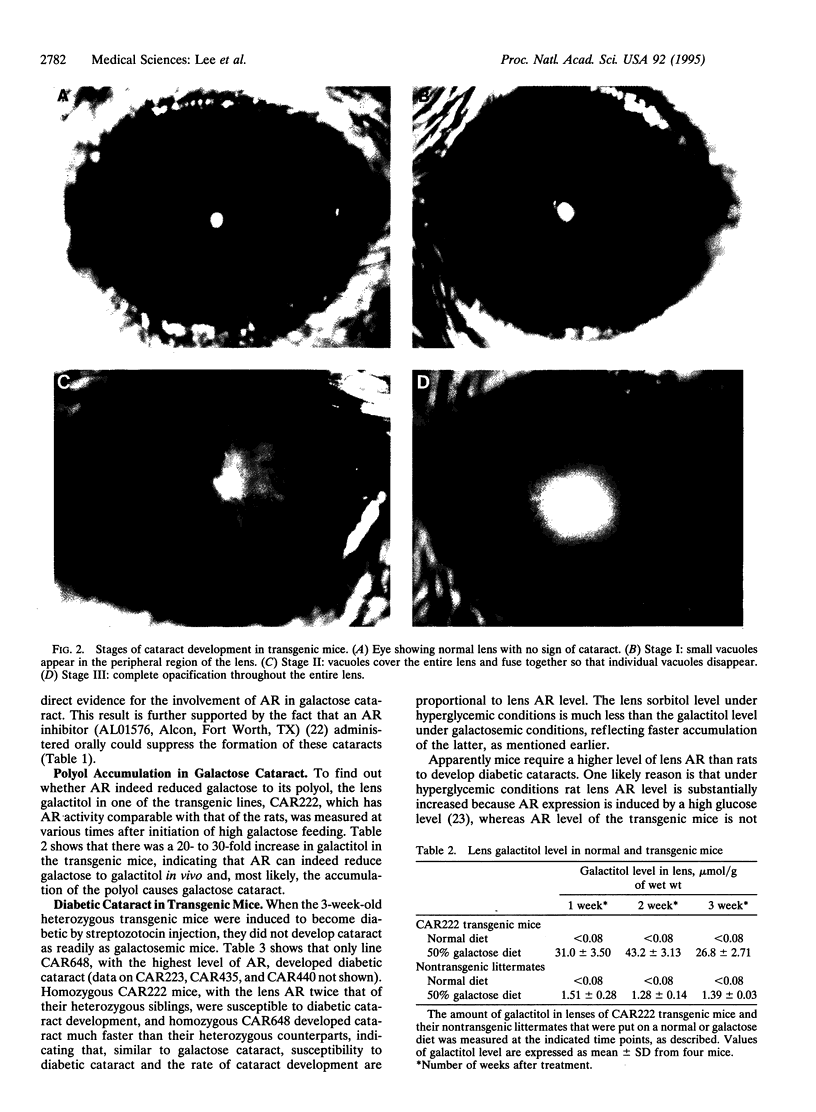
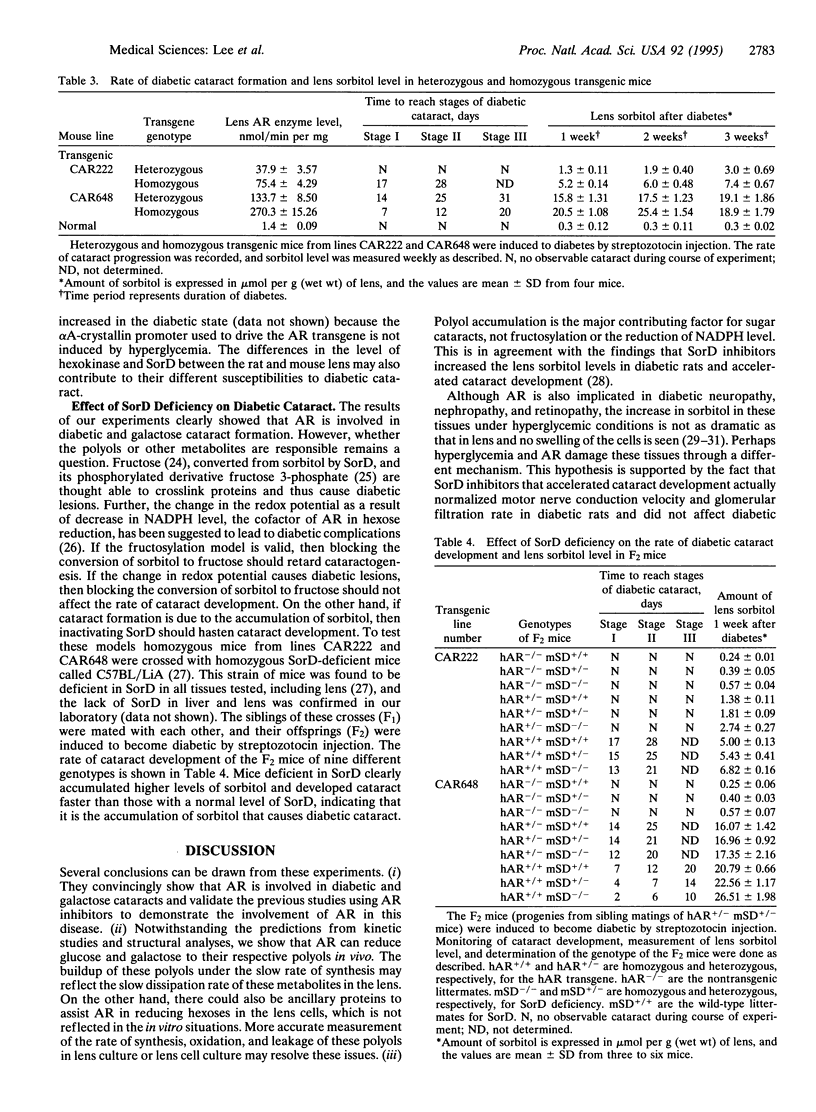
Aldose reductase (AR) has been implicated in the etiology of diabetic cataract, as well as in other complications. However, the role of AR in these complications remains controversial because the strongest supporting evidence is drawn from the use of AR inhibitors for which specificity in vivo cannot be ascertained. To settle this issue we developed transgenic mice that overexpress AR in their lens epithelial cells and found that they become susceptible to the development of diabetic and galactose cataracts. When the sorbitol dehydrogenase-deficient mutation is also present in these transgenic mice, greater accumulation of sorbitol and further acceleration of diabetic cataract develop. These genetic studies demonstrated convincingly that accumulation of polyols from the reduction of hexose by AR leads to the formation of sugar cataracts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi Y., Kador P. F., Kinoshita J. H. Immunohistochemical localization for aldose reductase in diabetic lenses. Invest Ophthalmol Vis Sci. 1987 Jan;28(1):163–167. [PubMed] [Google Scholar]
- Beyer-Mears A., Ku L., Cohen M. P. Glomerular polyol accumulation in diabetes and its prevention by oral sorbinil. Diabetes. 1984 Jun;33(6):604–607. doi: 10.2337/diab.33.6.604. [DOI] [PubMed] [Google Scholar]
- Cheng H. M., González R. G. The effect of high glucose and oxidative stress on lens metabolism, aldose reductase, and senile cataractogenesis. Metabolism. 1986 Apr;35(4 Suppl 1):10–14. doi: 10.1016/0026-0495(86)90180-0. [DOI] [PubMed] [Google Scholar]
- Chung S., LaMendola J. Cloning and sequence determination of human placental aldose reductase gene. J Biol Chem. 1989 Sep 5;264(25):14775–14777. [PubMed] [Google Scholar]
- Dvornik E., Simard-Duquesne N., Krami M., Sestanj K., Gabbay K. H., Kinoshita J. H., Varma S. D., Merola L. O. Polyol accumulation in galactosemic and diabetic rats: control by an aldose reductase inhibitor. Science. 1973 Dec 14;182(4117):1146–1148. doi: 10.1126/science.182.4117.1146. [DOI] [PubMed] [Google Scholar]
- Finegold D., Lattimer S. A., Nolle S., Bernstein M., Greene D. A. Polyol pathway activity and myo-inositol metabolism. A suggested relationship in the pathogenesis of diabetic neuropathy. Diabetes. 1983 Nov;32(11):988–992. doi: 10.2337/diab.32.11.988. [DOI] [PubMed] [Google Scholar]
- Geisen K., Utz R., Grötsch H., Lang H. J., Nimmesgern H. Sorbitol-accumulating pyrimidine derivatives. Arzneimittelforschung. 1994 Sep;44(9):1032–1043. [PubMed] [Google Scholar]
- Griffin B. W., McNatt L. G., Chandler M. L., York B. M. Effects of two new aldose reductase inhibitors, AL-1567 and AL-1576, in diabetic rats. Metabolism. 1987 May;36(5):486–490. doi: 10.1016/0026-0495(87)90048-5. [DOI] [PubMed] [Google Scholar]
- HAYMAN S., KINOSHITA J. H. ISOLATION AND PROPERTIES OF LENS ALDOSE REDUCTASE. J Biol Chem. 1965 Feb;240:877–882. [PubMed] [Google Scholar]
- HERS H. G. [The mechanism of the formation of seminal fructose and fetal fructose]. Biochim Biophys Acta. 1960 Jan 1;37:127–138. doi: 10.1016/0006-3002(60)90086-x. [DOI] [PubMed] [Google Scholar]
- Holmes R. S., Duley J. A., Hilgers J. Sorbitol dehydrogenase genetics in the mouse: a 'null' mutant in a 'European' C57BL strain. Anim Blood Groups Biochem Genet. 1982;13(4):263–272. doi: 10.1111/j.1365-2052.1982.tb01569.x. [DOI] [PubMed] [Google Scholar]
- Kador P. F., Robison W. G., Jr, Kinoshita J. H. The pharmacology of aldose reductase inhibitors. Annu Rev Pharmacol Toxicol. 1985;25:691–714. doi: 10.1146/annurev.pa.25.040185.003355. [DOI] [PubMed] [Google Scholar]
- Kinoshita J. H. Cataracts in galactosemia. The Jonas S. Friedenwald Memorial Lecture. Invest Ophthalmol. 1965 Oct;4(5):786–799. [PubMed] [Google Scholar]
- Kinoshita J. H. Mechanisms initiating cataract formation. Proctor Lecture. Invest Ophthalmol. 1974 Oct;13(10):713–724. [PubMed] [Google Scholar]
- Krentz A. J., Honigsberger L., Ellis S. H., Hardman M., Nattrass M. A 12-month randomized controlled study of the aldose reductase inhibitor ponalrestat in patients with chronic symptomatic diabetic neuropathy. Diabet Med. 1992 Jun;9(5):463–468. doi: 10.1111/j.1464-5491.1992.tb01818.x. [DOI] [PubMed] [Google Scholar]
- MacGregor L. C., Rosecan L. R., Laties A. M., Matschinsky F. M. Altered retinal metabolism in diabetes. I. Microanalysis of lipid, glucose, sorbitol, and myo-inositol in the choroid and in the individual layers of the rabbit retina. J Biol Chem. 1986 Mar 25;261(9):4046–4051. [PubMed] [Google Scholar]
- Miwa I., Kanbara M., Wakazono H., Okuda J. Analysis of sorbitol, galactitol, and myo-inositol in lens and sciatic nerve by high-performance liquid chromatography. Anal Biochem. 1988 Aug 15;173(1):39–44. doi: 10.1016/0003-2697(88)90155-8. [DOI] [PubMed] [Google Scholar]
- Poulsom R. Inhibition of hexonate dehydrogenase and aldose reductase from bovine retina by sorbinil, statil, M79175 and valproate. Biochem Pharmacol. 1986 Sep 1;35(17):2955–2959. doi: 10.1016/0006-2952(86)90492-2. [DOI] [PubMed] [Google Scholar]
- Ranganathan S., Krempf M., Feraille E., Charbonnel B. Short term effect of an aldose reductase inhibitor on urinary albumin excretion rate (UAER) and glomerular filtration rate (GFR) in type 1 diabetic patients with incipient nephropathy. Diabete Metab. 1993 Mar-Apr;19(2):257–261. [PubMed] [Google Scholar]
- Sestanj K., Bellini F., Fung S., Abraham N., Treasurywala A., Humber L., Simard-Duquesne N., Dvornik D. N-[5-(trifluoromethyl)-6-methoxy-1-naphthalenyl]thioxomethyl]- N-methylglycine (Tolrestat), a potent, orally active aldose reductase inhibitor. J Med Chem. 1984 Mar;27(3):255–256. doi: 10.1021/jm00369a003. [DOI] [PubMed] [Google Scholar]
- Sundkvist G., Armstrong F. M., Bradbury J. E., Chaplin C., Ellis S. H., Owens D. R., Rosén I., Sönksen P. Peripheral and autonomic nerve function in 259 diabetic patients with peripheral neuropathy treated with ponalrestat (an aldose reductase inhibitor) or placebo for 18 months. United Kingdom/Scandinavian Ponalrestat Trial. J Diabetes Complications. 1992 Apr-Jun;6(2):123–130. doi: 10.1016/1056-8727(92)90023-e. [DOI] [PubMed] [Google Scholar]
- Szwergold B. S., Kappler F., Brown T. R. Identification of fructose 3-phosphate in the lens of diabetic rats. Science. 1990 Jan 26;247(4941):451–454. doi: 10.1126/science.2300805. [DOI] [PubMed] [Google Scholar]
- Terashima H., Hama K., Yamamoto R., Tsuboshima M., Kikkawa R., Hatanaka I., Shigeta Y. Effects of a new aldose reductase inhibitor on various tissues in vitro. J Pharmacol Exp Ther. 1984 Apr;229(1):226–230. [PubMed] [Google Scholar]
- VAN HEYNINGEN R. Metabolism of xylose by the lens, 2. Rat lens in vivo and in vitro. Biochem J. 1959 Sep;73:197–207. doi: 10.1042/bj0730197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma S. D., Kinoshita J. H. The absence of cataracts in mice with congenital hyperglycemia. Exp Eye Res. 1974 Dec;19(6):577–582. doi: 10.1016/0014-4835(74)90095-5. [DOI] [PubMed] [Google Scholar]
- Wawrousek E. F., Chepelinsky A. B., McDermott J. B., Piatigorsky J. Regulation of the murine alpha A-crystallin promoter in transgenic mice. Dev Biol. 1990 Jan;137(1):68–76. doi: 10.1016/0012-1606(90)90008-7. [DOI] [PubMed] [Google Scholar]
- Wermuth B., Bürgisser H., Bohren K., von Wartburg J. P. Purification and characterization of human-brain aldose reductase. Eur J Biochem. 1982 Oct;127(2):279–284. doi: 10.1111/j.1432-1033.1982.tb06867.x. [DOI] [PubMed] [Google Scholar]
- Wilson D. K., Bohren K. M., Gabbay K. H., Quiocho F. A. An unlikely sugar substrate site in the 1.65 A structure of the human aldose reductase holoenzyme implicated in diabetic complications. Science. 1992 Jul 3;257(5066):81–84. doi: 10.1126/science.1621098. [DOI] [PubMed] [Google Scholar]