Abstract
Background: Disrupting the physical structure of pulses by blending them or by using a digestive supplement (α-galactosidase) to reduce intestinal discomfort could potentially negate the previously observed beneficial effects of whole pulses of lowering appetitive and glycemic responses because of more rapid digestion.
Objective: We hypothesized that blended lentils, α-galactosidase, or both increase postprandial appetite and blood glucose responses vs. whole lentils.
Methods: Men and women [n = 12; means ± SDs body mass index (kg/m2): 23.3 ± 3.1; aged 28 ± 10 y] consumed breakfast meals containing whole (W), blended (B), or no lentils [control (C)], each with 3 α-galactosidase or placebo capsules in a randomized, crossover, double-blind placebo-controlled trial. Between each test day there was a 3- to 5-d washout period.
Results: Mixed-model ANOVA showed effects of meal on postprandial appetite and glucose (P = 0.0001–0.031). The B meal resulted in higher postprandial appetite ratings than did the W meal but not the C meal for hunger, desire to eat, and prospective consumption (Δ = 0.4–0.5 points; P = 0.002–0.044). Postprandial glucose concentration was 4.5 mg/dL lower for the B meal than for the C meal (P < 0.0001) but did not differ from the W meal. There were no main effects of α-galactosidase, but there were meal × α-galactosidase interaction effects, with a greater postprandial desire to eat and lower postprandial fullness with the B meal than with the 2 other meals in the placebo condition but not in the α-galactosidase condition.
Conclusions: Blending lentils increased appetite (∼6%), but not glycemic response, compared with whole lentils, whereas α-galactosidase did not. Both B and W meals may be consumed (with or without an α-galactosidase supplement) with little impact on appetite, without increasing glycemic response. This trial was registered at clinicaltrials.gov as NCT02110511.
Keywords: Lentils, α-galactosidase, appetite, glucose, humans
Introduction
Pulses are defined as dry seeds of leguminous plants that are distinguished from leguminous oil seeds by their low-fat content (e.g., lentils, beans, and chickpeas) (1). In short-term studies, pulse-containing meals may aid in reducing appetite (2–4) and result in lower postprandial glycemic responses (3, 5) than meals that do not contain pulses. Cross-sectional studies showed that pulse consumption is associated with lower BMI (2) and chronic disease risk (5, 6). Consistent with the epidemiologic studies, intervention studies showed that pulses can facilitate weight loss (2) and improve biomarkers of chronic disease risk (7). In the United States, pulse consumption is markedly below the recommended amount (2, 8). There is therefore a need to promote pulses more widely and address barriers that prevent their consumption.
One potential barrier to pulse consumption is the lack of knowledge about different serving options for pulses. A variety of sensory properties in food are known to promote greater consumption (9). Therefore, increasing awareness about different ways to prepare and serve pulses may help to increase pulse consumption in the United States. Blended pulses from whole forms, such as in hummus [a traditional food made from blended chickpeas consumed in the Middle East and which in recent years has gained popularity in the United States (10)], are alternative ways to serve pulses. Refried beans and many bean dips also contain a form of blended pulses. Although blending pulses disrupts their physical structure, potentially allowing more rapid digestion and absorption, it is not known if blending pulses will result in attenuated appetite- or glycemia-reducing effects. In previous studies on blended pulses, although appetite was not measured, there were mixed effects on the glucose response (11, 12). However, the consumption of other foods in their blended form, such as rice, wheat, carrots, and potatoes, compared with their whole form, resulted in greater postprandial appetite (13–15) and glycemia (16–18).
A second barrier to pulse consumption, for some individuals, is their potential adverse gastrointestinal effects (19). The degree of discomfort experienced among individuals varies but depends on factors such as the amount consumed (19), pulse type (20), and differences in gut biology (e.g., sensitivity to gastrointestinal distension) (21). Pulses contain oligosaccharides, which humans lack the enzyme to digest. Oligosaccharides are normally fermented in the colon by bacteria and produce gases. Digestive aids containing the enzyme α-galactosidase are available that enable oligosaccharide digestion in the small intestine, thereby preventing gas production and associated gastrointestinal discomfort. Potentially, however, taking α-galactosidase may result in an increase in glucose production from oligosaccharide digestion in the small intestine. In only 1 study in type 2 diabetics has the effects of α-galactosidase on blood glucose changes been assessed (22). In that study, although blood glucose concentration at 2 h after intake was higher compared with the control, appetite was not measured. Therefore, the effects of α-galactosidase on appetite and glucose responses to a pulse-containing meal in nondiabetic individuals are uncertain.
The purpose of this study was to assess the effects of consumption of whole or blended lentils with and without taking α-galactosidase on appetite and blood glucose responses. Fasting and postprandial energy expenditure (FEE and PPEE, respectively)8 and substrate oxidation were secondary outcomes. We hypothesized that postprandial appetite would be higher (i.e., greater hunger, desire to eat, and prospective consumption and lower fullness) and glucose responses would be higher after consumption of blended lentils compared with whole lentils and after taking the α-galactosidase enzyme supplement vs. a placebo. We further hypothesized that the combination of blended lentils and the enzyme supplement would result in even higher appetite and glycemic responses than either one alone.
Methods
Screening and participants
Healthy, nonsmoking, weight-stable individuals aged 18–55 y with a BMI (kg/m2) of 18.5–40 were recruited by local advertisement. Screening for inclusion and exclusion criteria took place initially by telephone, then by a detailed questionnaire and an in-person medical screening visit at the Purdue Clinical Research Center (PCRC). Individuals were excluded if they were taking medications or had chronic diseases or conditions known to affect energy regulation or appetite, were pregnant or lactating within the past year, had gained or lost >2.3 kg in the past 3 mo, performed exercise or physical activity at moderate or vigorous intensity >12 h/wk, were allergic or sensitive to or disliked the foods and ingredients used for the study meals and α-galactosidase or placebo capsules, were vegetarians or had problems digesting pulses, had a systolic blood pressure ≥140 mm Hg, a diastolic pressure ≥90 mm Hg, or a fasting blood glucose concentration ≥126 mg/dL. During the medical screening visit, height, body weight and composition, blood pressure, and fasting blood glucose measurements were made. Enrollment took place between January and October 2012. The study, which was in accord with the Helsinki Declaration of 1752 as revised in 1983, was approved by the Purdue University Institutional Review Board, and written informed consent was obtained from all individuals. After completing the study, individuals received monetary compensation.
Study design
A randomized, double-blind, placebo-controlled crossover trial design was used. Participants completed a baseline visit and 6 4-h test day visits to the laboratory. Each test day was separated by 3–5 d. During each test day, participants consumed, in random order, 1 of 6 breakfast meal/enzyme supplement capsule combinations. The meals contained either blended lentils (B), whole lentils (W), or no lentils [control (C)], whereas the capsules contained either the α-galactosidase enzyme or a placebo. Immediately before meal consumption and serially at selected time points for 3 h after the start of the meal, measurements of appetite, blood glucose, and energy expenditure were made. The evening before each test day visit, participants were provided with a standard dinner. Both participants and researchers were blinded to the enzyme supplement treatment order, and the meal composition order was concealed from participants. The main study procedures took place at the PCRC in the Department of Nutrition Science at Purdue University. The consumption of the standard dinner took place in the community setting while participants carried out their usual lives.
Study and test day protocol
After screening was complete, participants reported to the PCRC ∼2 or 3 d before the start of the first test day for measurement of body weight, body composition, and waist circumference. They were also given a standard dinner to consume at home the evening before each test day. On the morning of each test day, participants reported to the PCRC after a 12-h fast. Upon arrival they rested in a supine position and appetite was assessed. After ∼15 min, FEE was measured for 30–40 min. A blood sample was then taken (time point: 0 min), and immediately afterward the test breakfast and the α-galactosidase or placebo capsules were provided. Participants were instructed to take the capsules with water just before the meal and to consume the entire meal with 8 fluid ounces (240 mL) of water (provided) within 15 min. Participants could consume water ad libitum for the remainder of the test period. Participants were asked to rate the palatability of each meal at the first bite. Finger-stick blood samples were taken at 20, 40, 60, 90, 120, and 180 min. Appetite was assessed 2 min before each blood draw, whereas PPEE was assessed for 13–15 min before each blood sample. After the last blood draw, participants were dismissed and returned 3–5 d later for the next visit until a total of 6 visits were completed.
Study meals
Test day breakfasts.
The test breakfasts were served at 20% of each individual’s estimated energy requirement (23) rounded to the nearest 50 kcal/d. The resulting sizes of the breakfasts served in the study ranged from 400 to 800 kcal/d. For the B and W meals, each 200-kcal serving contained 0.25 cups (42 g) of canned lentils (Table 1), so that the total amount of lentils consumed per treatment was ≥0.5 cups (84 g) depending on the individual serving amount. The 3 meals were similar in macronutrient composition (Table 1), except that the B and W meals were higher in fiber than the C meal. To achieve this composition, the meals were designed to contain the same ingredients, with the exception of lentils. Meals were planned with ProNutra software, version 3.3.0.10 (2000–2009; Viocare Technologies). Pilot testing was conducted to ensure that the meals were matched for taste, texture, appearance, and aroma before study implementation.
TABLE 1.
Ingredients and nutrient composition of lentil and no-lentil burritos1
| Blended- and whole-lentil meals | No-lentil meal | |
| Ingredients, g | ||
| Lentils, canned (Eden organic, no sodium), drained and rinsed | 84.6 | 0 |
| 100% liquid egg white | 0 | 66.0 |
| Liquid egg | 15.0 | 17.0 |
| Rice, cooked, white, long-grain | 8.0 | 59.0 |
| Cheddar cheese, shredded | 16.0 | 17.0 |
| Sour cream | 16.0 | 17.0 |
| Celery, raw | 24.0 | 4.0 |
| Salsa | 48.0 | 48.0 |
| Whole-wheat tortilla | 52.4 | 0 |
| 100% Whole-wheat tortilla | 0 | 52.4 |
| Nutrient composition | ||
| Energy, kcal | 404 | 408 |
| Carbohydrate | ||
| g | 49.6 | 47.0 |
| % of energy | 48.4 | 47.3 |
| Protein | ||
| g | 20.4 | 20.0 |
| % of energy | 20.0 | 19.6 |
| Fat | ||
| g | 14.6 | 14.4 |
| % of energy | 31.8 | 32.5 |
| Fiber | ||
| g | 15.4 | 5.2 |
| g/1000 kcal | 37.7 | 12.7 |
Meals were served as 50-kcal pieces, but values are shown for a 400-kcal meal.
Standard dinner.
The standard dinner was provided at 40% of each subject’s estimated energy requirement (23) and consisted of chicken and broccoli with pasta (110 g) and macaroni and cheese (211 g) (both were Stouffer’s; Nestlé USA), French-style green beans (61 g; Kroger), chocolate chip cookies (58 g; Nabisco Chips Ahoy Thin Crisps 100 Calorie Packs; Kraft Foods Global), and 2% milk (138 g; Kroger) for a 400-kcal serving. Participants were instructed to consume all of the dinner at home and to return the containers the next morning. The containers and any uneaten food were weighed upon return. All participants reported consuming all the foods given as evidenced by return of empty containers.
Enzyme supplement (α-galactosidase) and placebo capsules
The α-galactosidase enzyme supplement was purchased in tablet form over the counter (Beano food enzyme dietary supplement; GlaxoSmithKline Consumer Healthcare). For the purpose of this study, the enzyme tablets (150 galactosidase units each) were ground into powder form, weighed to the nearest milligram, and put into capsules. Placebo capsules were made to look, smell, and feel identical to the active enzyme capsules by filling capsules with an equal amount of lactose. On test days, participants took 3 capsules of either the active enzyme or placebo immediately before consuming each study meal, consistent with the manufacturer’s suggested dosage. The capsules were provided in marked containers to personnel at the PCRC who were otherwise unaffiliated with the study for dispersion into smaller containers labeled with a code; this code, which corresponded to the active and placebo capsules, was concealed from the investigators and only known to those personnel. The code was not revealed to the investigators until the last participant completed the study.
Measurements
Anthropometric and body composition measurements.
Height was measured to the nearest 0.1 cm by using a wall-mounted stadiometer (Holtain). After a 12-h overnight fast, participants reported the following morning to the PCRC for anthropometric and body composition measurements. Participants were asked to arrive well hydrated, to refrain from exercise or vigorous physical activity, and not to shower or bathe on the morning of these measurements. Body weight and composition were assessed by using air displacement plethysmography (BOD POD Gold Standard Body Composition Tracking System 2009, software version 2007A; Cosmed USA) with measured thoracic gas volume as previously described (24). Waist circumference was measured at the natural waist (narrowest point of the midsection) with the use of an anthropometric tape measure (Gulick II, model 67020; Country Technology) as previously described (25).
Meal palatability, appetite, and gastrointestinal symptoms.
A standard 1–9 rating scale anchored with the words “not at all” (at 1) and “extremely” (at 9) was used to assess palatability ratings of each breakfast meal and appetitive sensations. Participants circled the number that corresponded with their perception at the moment. For palatability, participants rated the taste of the food at the first bite. For appetite, the following sensations were assessed: hunger, desire to eat, prospective consumption, and fullness.
Blood glucose.
Blood glucose was measured with a glucometer (FreeStyle Lite; Abbott) by using finger-stick blood samples collected at each time point. This method was previously shown to be valid and reliable compared with glucose measured from venous blood samples under fasting conditions and up to 6 h after intake (26).
FEE and PPEE and substrate oxidation.
Fasting and postprandial rates of oxygen consumption (˙O2) and carbon dioxide production (˙CO2) were measured by indirect calorimetry (ParvoMedics TruOne 2400 Metabolic Measurement System) by using a ventilated hood. After the study meal was consumed, PPEE and gas exchange data were collected for 13–15 min before each finger stick collection. FEE and PPEE in kcal/min were estimated by using the Weir equation (27). The respiratory exchange ratio was calculated as ˙CO2:˙O2. The average of the final 10 min of data for each interval of readings was used for the calculations.
Statistical analyses and calculations
Values are expressed as means ± SDs for subject characteristics and means ± SEMs for all other variables. Total AUC over the 3-h test period was calculated as recommended by Blundell et al. (28) by using the trapezoidal rule. For variables for which there was an overall statistically significant effect, the data were further analyzed by breaking them down to the first 90 min of data vs. the last 90 min. Data were analyzed by using Statistical Analysis Software (SAS, version 9.3; SAS Institute) and Statistical Package for Social Sciences (IBM SPSS Statistics, version 20; SPSS). The main effects of meal (B, W, or C), supplement (α-galactosidase or placebo), time, and their interaction effects were assessed by using a mixed-model (PROC MIXED in SAS) 3-factor repeated-measures ANOVA with the factors meal, enzyme supplement, and time as the main effects, with all of the 2-factor and 3-factor interaction effects included in the model, controlling for treatment order. To additionally examine the effects of meal, enzyme supplement, and meal × enzyme supplement on postprandial AUC and meal palatability ratings, a mixed-model 2-factor repeated-measures ANOVA was used, controlling for treatment order and fasting value (i.e., value at 0 min). For post hoc multiple comparisons, the Tukey-Kramer test was used for the main effects and the unadjusted paired comparison tests were used for interaction effects. Graphically, least squares mean (adjusted) responses are shown as bar graphs and reported as means ± SEMs. Postprandial responses over time are shown as change from the fasting value and were calculated as the value at each time point minus the value at fasting. The sample size calculation was based on the primary outcome of fullness. Although postprandial appetite sensations in general predict later ad libitum energy intake in test meals (29), some studies have shown postprandial fullness to be the strongest predictor (30, 31). A preliminary analysis of the data from the first 7 participants indicated that 12 subjects would provide adequate power for our study. This was not an interim analysis and therefore no adjustments to P values were made. For all statistical tests, P < 0.05 was considered significant.
Results
Participants.
Of the 14 participants who enrolled in the study, 12 (8 women, 4 men) completed the study. One participant discontinued because of time conflicts and another was lost to follow-up. Participants were aged 28 ± 10 y (means ± SDs) [20–55 y (minimum–maximum)], of normal weight (BMI: 23.3 ± 3.1; 20.4–30.9), and had a normal fasting glucose concentration (80.8 ± 6.3 mg/dL; 70.0–94.0 mg/dL). Approximately 17% of participants were overweight or obese, and 8% of the participants had a fasting glucose concentration >90 mg/dL.
Palatability of study meals.
The matching of study meals for taste pleasantness was successful, with taste ratings not differing significantly [B meal: 5.5 ± 0.2; W meal: 5.8 ± 0.2; C meal: 6.2 ± 0.2 (means ± SEMs)].
Appetite.
There was a main effect of time on all 4 appetite variables (P < 0.0001), with hunger (Figure 1D), desire to eat (Figure 1E), and prospective consumption (data not shown) decreasing immediately after the meal was consumed and slowly increasing over the remainder of the 3-h measurement period but not returning to premeal values. Fullness (Figure 1F) mirrored the other appetite variable responses, increasing immediately after the meal then steadily decreasing until the 3-h time point. Furthermore, there were main effects of meal on hunger (P = 0.003) (Figure 1A, D), desire to eat (P = 0.007) (Figure 1B, E), and prospective consumption (P = 0.031), with the differences averaging ∼0.5 on the 1–9 scale. Tukey-Kramer post hoc analyses indicated higher ratings in these 3 appetite variables for the B meal compared with the W meal (P = 0.003–0.031) but not the C meal, and no differences in ratings between the W and C meals. There was no main effect of meal on fullness. There were also no main effects of the enzyme supplement observed on any of the appetite variables. However, there were interaction effects of meal × enzyme supplement on desire to eat (P = 0.008) and fullness (P = 0.039) and a marginal effect on prospective consumption (P = 0.059). Unadjusted paired comparisons showed that for desire to eat in the placebo condition, the B meal resulted in higher ratings than did the W (P = 0.0004) and C (P = 0.002) meals, but the W and C meals did not differ from each other, whereas in the α-galactosidase condition, the desire to eat was lower for the W meal than for the C meal (P = 0.020). Also, with the B meal only, α-galactosidase resulted in lower desire to eat than did the placebo (P = 0.009) (Figure 1B, E). For fullness, in the placebo condition, the unadjusted paired comparisons showed that the B meal resulted in lower ratings than both the W (P = 0.009) and C (P = 0.008) meals, but the W and C meals did not differ from each other (Figure 1C, F).
FIGURE 1 .
Appetite responses [hunger (A, D); desire to eat (B, E); fullness (C, F)] of participants consuming B, W, or C burritos with α-gal or placebo capsules. Values are least-square means ± SEMs (A, B, and C) with corresponding changes over time (D, E, and F), n = 12. Letters above the bars represent pooled meal means, whereas those below the bars represent the individual meal/supplement means. Labeled means without a common letter differ, P < 0.05. All analyses were controlled for fasting value and visit order. B, blended lentil; C, control (no lentil); W, whole lentil; α-gal, α-galactosidase.
Concerning the appetite AUC outcomes, hunger was the only sensation in which there were any effects (Supplemental Table 1). In particular, there was a main effect of meal (P = 0.019), with the B meal resulting in a higher AUC than the W meal (Tukey-Kramer, P = 0.015) but not the C meal. However, there was no difference in hunger AUC between the W and C meals. Further analyses of the AUCs over the first and second halves of the postprandial period showed that the meal effect on hunger occurred only during the first 90 min (ANOVA P = 0.030; B vs. W meals: Tukey-Kramer P = 0.023).
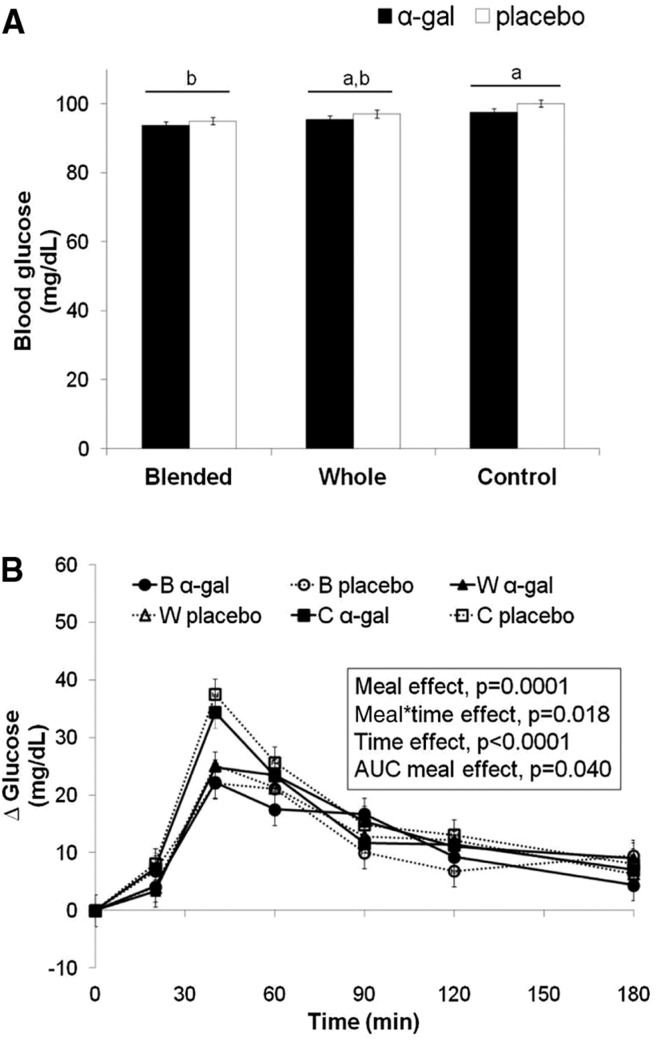
Blood glucose.
There was a main effect of meal (P = 0.0001) on postprandial glucose concentration, with glucose for the B meal being lower than that for the C meal (Tukey-Kramer P < 0.0001), but there were no differences between the W and either the B or C meals (Figure 2A, B). There was no main effect of the enzyme supplement and no meal × enzyme supplement interaction effect on glucose response. However, there were effects of time (P < 0.0001) and meal × time (P = 0.018) on blood glucose. With regard to the latter, unadjusted paired comparisons of the different meals were carried out only at preselected time points (0, 20, 40, 120, and 180 min) to reduce type 2 error. We found differences only at 40 min, with both the B and W meals resulting in lower blood glucose concentrations than the C meal (P < 0.0001) but no difference between B and W meals.
FIGURE 2 .
Glucose responses of participants consuming B, W, or C burritos with α-gal or placebo capsules. Values are least-square means ± SEMs (A) with corresponding changes over time (B), n = 12. Letters above the bars represent pooled meal means, whereas those below the bars represent the individual meal/supplement means. Labeled means without a common letter differ, P < 0.05. All analyses were controlled for fasting value and visit order. B, blended lentil; C, control (no lentil); W, whole lentil; α-gal, α-galactosidase.
Similar to the result in the time course analysis, there was a main effect of meal on glucose AUC (P = 0.010) (Supplemental Table 1), with that for the B meal being lower than for the C meal (Tukey-Kramer P = 0.008); however, there were no differences between the W meal and either the B or C meals. Further analyses of the AUCs over the first and second halves of the postprandial period showed that the meal effect occurred primarily during the first 90 min (P = 0.016), with the B meal resulting in lower glucose AUC than the C meal (Tukey-Kramer P = 0.012). No meal effect occurred in the second 90 min. There were no effects of enzyme supplement or meal × supplement on glucose AUC.
FEE and PPEE and substrate oxidation.
Results for energy expenditure and respiratory exchange ratio are shown in Supplemental Figure 1 and Supplemental Table 1.
Discussion
Our main findings were that, compared with whole lentils, blended lentils increased postprandial appetite by ∼6% but did not increase postprandial glucose response. There were no effects of the α-galactosidase enzyme supplement on appetite or glycemic responses, but there was a meal × enzyme supplement effect on postprandial desire to eat and fullness, with greater desire to eat and lower fullness sensations with the B meals in the placebo but not the α-galactosidase condition. These findings suggest that blended lentils and α-galactosidase supplement have very little, if any, effects on glucose and appetite responses. Thus, the consumption of both blended and whole lentils may be encouraged as approaches to increasing pulse consumption, and further testing is warranted with other types of pulses.
Consistent with our hypothesis, blended lentils resulted in slight but significant increases in postmeal appetite (greater hunger, desire to eat, and prospective consumption) compared with whole pulses. Furthermore, there was an interaction effect of lentil form with enzyme supplement condition so that these effects on appetite occurred only in the placebo condition, with the blended lentils resulting in significant changes in 2 of the 4 appetite variables: a small increase in postprandial desire to eat and a small decrease in postprandial fullness than whole lentils. Studies on food form effects on appetite generally showed increases in appetite with the administration of blended/pureed or liquid/juice forms compared with solid forms (13–15, 32) and when meal replacement beverages were compared with meal replacement bars (33, 34). Potential mechanisms explaining these food form effects include but are not limited to the following: the presence or absence of fiber (14, 33, 35); more rapid gastric emptying and/or motility, which may result in a lower satiety value of beverages, leading to weaker compensatory dietary responses (36, 37); less stimulation of cholecystokinin release, which elicits less satiation (reduced meal size) (38); less stimulation for the release of other appetite-related hormones such as glucagon-like peptide 1 and greater release of ghrelin; and more rapid glucose absorption, which may relate to an increase in appetite (39–41). Thus, the finding that blended lentils resulted in greater appetite sensations than whole lentils was consistent with our expectations. However, the average difference in appetite rating between the blended and the whole lentils was ∼0.47 or 6% on a 1–9 scale, and this may not be clinically relevant because it is far less than the 15% previously reported as necessary on a 100-mm visual analog scale to affect subsequent energy intake in controlled feeding trials (42). In addition, in the free-living situation, hunger ratings may be a weak predictor of energy intake (43, 44) in light of other environmental influences. To our knowledge, no other studies exist in which the effect of taking α-galactosidase on appetite was investigated. Taken together, we conclude that ingesting blended lentils may slightly increase appetite over whole lentils but the effect, although significant, is very small and is not likely to be clinically meaningful.
Concerning glycemic response, contrary to our hypothesis we observed no significant difference in glucose response between blended and whole lentils. Very few investigators examined the effect of disrupting the physical form of pulses (lentils) on postprandial blood glucose response (11, 12). Our glucose results are in agreement with findings from 1 of these studies (11) but not the other (12). Perhaps serving lentils as the only food in the test meal in the latter study (12) as opposed to serving the lentils as part of a meal as was done in the former study (11) as well as in our own may account for the equivocal results. The presence of other nutrients such as fats and protein in a mixed meal may form a complex with the carbohydrates present in the meal and thereby lower glucose response (45).
The fact that our B meal but not our W meal differed from the C meal in glucose response, such that the B meal resulted in a lower glucose response than the C meal, was not expected and is contrary to the general assumption that all pulses elicit lower glucose responses than most other carbohydrate-containing foods (46–48). Our B and W meals contained the same ingredients in the same proportions with the only difference being the physical structure of the lentils. Our C meal was matched to the lentil meals in macronutrient amounts, with the major difference being the proportions that came from different sources. Both the eggs and celery in our pulse-containing meals contributed very little or nothing to the glycemic index of our meals: we served between 24 and 48 g celery (110 g serving size of raw celery contains 2 g of sugar) (49), which will have a very negligible effect on glycemic index. Palatability may affect glycemic response, with meals having higher palatability resulting in a higher glycemic response (50). However, there were no differences between our meals in taste rating. Thus, blended lentils may be incorporated into a meal without significantly increasing glycemic response and the effects in terms of postprandial glycemic response are not different than whole lentils.
We hypothesized that taking α-galactosidase would increase blood glucose response because α-galactosidase enables the digestion of the oligosaccharides in lentils in the small intestine, with end products being simple sugars that can enter the bloodstream. The total oligosaccharide content of cooked lentils was determined to be 71.1 mg/g of cooked lentils (51), from which stachyose, raffinose, and verbascose constitute 38.2, 19.7, and 13.2 mg/g of cooked lentils, respectively. Complete hydrolysis of each of the 3 types of oligosaccharides could potentially increase the 50-g glucose content of cooked lentils (12) by ∼1.6 g. This amount constitutes only an ∼3% increment in glucose content and may not be large enough to affect blood glucose response. Both fructose and galactose can be converted to glucose but this is not their main fate. Thus, their contribution to blood glucose concentration may be very minimal, if any. Hence, although not surprising to us, our finding of no effect of α-galactosidase on blood glucose is in contrast to previous work on the effects of α-galactosidase on blood glucose (22). Discrepancies in study results might be explained by the use of older diabetic participants with a higher mean BMI in the previous study, whereas our participants were generally healthy, had a normal BMI, and were younger.
Some of the strengths of our study include the randomized, crossover, double-blind placebo-controlled design, which minimized bias, the utilization of study meals matched for nutrient content and palatability, and provision of meals catering to each participant’s specific energy need. Furthermore, we had a no-lentil control group in addition to the blended vs. whole-lentil group. Thus, our design allowed us to simultaneously study the effects of lentil consumption on our outcome variables as well as the effect of food form. Our study also had some limitations. These include not assessing ad libitum food intake after each treatment, potential physiologic mechanisms underlying effects on appetite, including appetite regulatory hormones or gastric emptying rate, or insulin response. In addition, we used lentils in our breakfast meals, but the effects of other pulse types on appetite and glucose responses may differ from those of lentils. There is still the need for both acute and long-term studies on the effects of blending different pulses and taking α-galactosidase on appetite and glucose because data in this area are scarce.
In conclusion, although our findings showed a slight increase in appetite with blended lentils compared with whole lentils, the increase appears to be clinically unimportant. Our data further showed a lack of adverse effects of blending lentils or using α-galactosidase on the postprandial glucose response. Considering that pulse consumption is far below the recommended amount in the U.S. population, there is a need to promote ways for consumers to incorporate pulses easily and frequently into their everyday meals that do not compromise the benefits observed with whole pulses on glucose and appetite responses.
Supplementary Material
Acknowledgments
We thank OH Maroney and GW Wehr for technical assistance and WE Gwin for the reformulation of α-galactosidase pills into capsules and making of the placebo capsules. KO-BA, BSW, and MAM designed the research; KO-BA and BSW conducted the research; KO-BA and GPM analyzed the data; KO-BA, WWC, and MAM interpreted the data; KO-BA and MAM wrote the manuscript; KO-BA, WWC, GPM, and MAM reviewed the manuscript for important intellectual content; and MAM had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: B, blended lentil; C, control (no lentil); FEE, fasting energy expenditure; PCRC, Purdue Clinical Research Center; PPEE, postprandial energy expenditure; W, whole lentil.
References
- 1.FAO. Definition and classification of commodities. 4. Pulses and derived products [cited 2013 Oct 5]. Available from: http://www.fao.org/WAICENT/faoinfo/economic/faodef04e.htm.
- 2.McCrory MA, Hamaker BR, Lovejoy JC, Eichelsdoerfer PE. Pulse consumption, satiety, and weight management. Adv Nutr 2010;1:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mollard RC, Zykus A, Luhovyy BL, Nunez MF, Wong CL, Anderson GH. The acute effects of a pulse-containing meal on glycaemic responses and measures of satiety and satiation within and at a later meal. Br J Nutr 2012;108:509–17. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson A, Johansson E, Ekstrom L, Bjorck I. Effects of a brown beans evening meal on metabolic risk markers and appetite regulating hormones at a subsequent standardized breakfast: a randomized cross-over study. PLoS ONE 2013;8:e59985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchins AM, Winham DM, Thompson SV. Phaseolus beans: impact on glycaemic response and chronic disease risk in human subjects. Br J Nutr 2012;108(Suppl 1):S52–65. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal S, Ebrahim S. Association between legume intake and self-reported diabetes among adult men and women in India. BMC Public Health 2013;13:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayat I, Ahmad A, Masud T, Ahmed A, Bashir S. Nutritional and health perspectives of beans (Phaseolus vulgaris L.): an overview. Crit Rev Food Sci Nutr 2014;54:580–92. [DOI] [PubMed] [Google Scholar]
- 8.USDA; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th ed. Washington: U.S. Government Printing Office; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCrory MA, Burke A, Roberts SB. Dietary (sensory) variety and energy balance. Physiol Behav 2012;107:576–83. [DOI] [PubMed] [Google Scholar]
- 10.Ferretti E. There is hummus among us [cited 2014 Apr 9]. Available from: http://www.foxnews.com/leisure/2010/04/05/theres-hummus/.
- 11.Jenkins DJ, Thorne MJ, Camelon K, Jenkins A, Rao AV, Taylor RH, Thompson LU, Kalmusky J, Reichert R, Francis T. Effect of processing on digestibility and the blood glucose response: a study of lentils. Am J Clin Nutr 1982;36:1093–101. [DOI] [PubMed] [Google Scholar]
- 12.O'Dea K, Wong S. The rate of starch hydrolysis in vitro does not predict the metabolic responses to legumes in vivo. Am J Clin Nutr 1983;38:382–7. [DOI] [PubMed] [Google Scholar]
- 13.Flood-Obbagy JE, Rolls BJ. The effect of fruit in different forms on energy intake and satiety at a meal. Appetite 2009;52:416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haber GB, Heaton KW, Murphy D, Burroughs LF. Depletion and disruption of dietary fibre: effects on satiety, plasma-glucose, and serum-insulin. Lancet 1977;2:679–82. [DOI] [PubMed] [Google Scholar]
- 15.Forde CG, van Kuijk N, Thaler T, de Graaf C, Martin N. Texture and savoury taste influences on food intake in a realistic hot lunch time meal. Appetite 2013;60:180–6. [DOI] [PubMed] [Google Scholar]
- 16.O'Dea K, Snow P, Nestel P. Rate of starch hydrolysis in vitro as a predictor of metabolic responses to complex carbohydrate in vivo. Am J Clin Nutr 1981;34:1991–3. [DOI] [PubMed] [Google Scholar]
- 17.Holt SH, Miller JB. Particle size, satiety and the glycaemic response. Eur J Clin Nutr 1994;48:496–502. [PubMed] [Google Scholar]
- 18.Geliebter A, Lee MI, Abdillahi M, Jones J. Satiety following intake of potatoes and other carbohydrate test meals. Ann Nutr Metab 2013;62:37–43. [DOI] [PubMed] [Google Scholar]
- 19.Veenstra JM, Duncan AM, Cryne CN, Deschambault BR, Boye JI, Benali M, Marcotte M, Tosh SM, Farnworth ER, Wright AJ. Effect of pulse consumption on perceived flatulence and gastrointestinal function in healthy males. Food Res Int 2010;43:553–9. [Google Scholar]
- 20.Winham DM, Hutchins AM. Perceptions of flatulence from bean consumption among adults in 3 feeding studies. Nutr J 2011;10:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomlin J, Lowis C, Read NW. Investigation of normal flatus production in healthy volunteers. Gut 1991;32:665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lettieri JT, Dain B. Effects of Beano on the tolerability and pharmacodynamics of acarbose. Clin Ther 1998;20:497–504. [DOI] [PubMed] [Google Scholar]
- 23.Institute of Medicine. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids: part I. Washington: National Academy of Sciences; 2005. [Google Scholar]
- 24.McCrory MA, Gomez TD, Bernauer EM, Molé PA. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med Sci Sports Exerc 1995;27:1686–91. [PubMed] [Google Scholar]
- 25. Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign (IL): Human Kinetics Books; 1988.
- 26.Thomas LE, Kane MP, Bakst G, Busch RS, Hamilton RA, Abelseth JM. A glucose meter accuracy and precision comparison: the FreeStyle Flash Versus the Accu-Chek Advantage, Accu-Chek Compact Plus, Ascensia Contour, and the BD Logic. Diabetes Technol Ther 2008;10:102–10. [DOI] [PubMed] [Google Scholar]
- 27.Weir JBDV. New methods for calculating metabolic rate with special reference to protein. J Physiol 1949;109:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, Mela D, Salah S, Schuring E, van der Knaap H, et al. Appetite control: methodological aspects of the evaluation of foods. Obes Rev 2010;11:251–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 2000;24:38–48. [DOI] [PubMed] [Google Scholar]
- 30.Drapeau V, Blundell J, Therrien F, Lawton C, Richard D, Tremblay A. Appetite sensations as a marker of overall intake. Br J Nutr 2005;93:273–80. [DOI] [PubMed] [Google Scholar]
- 31.Drapeau V, King N, Hetherington M, Doucet E, Blundell J, Tremblay A. Appetite sensations and satiety quotient: predictors of energy intake and weight loss. Appetite 2007;48:159–66. [DOI] [PubMed] [Google Scholar]
- 32.Mattes RD, Campbell WW. Effects of food form and timing of ingestion on appetite and energy intake in lean young adults and in young adults with obesity. J Am Diet Assoc 2009;109:430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leidy HJ, Apolzan JW, Mattes RD, Campbell WW. Food form and portion size affect postprandial appetite sensations and hormonal responses in healthy, nonobese, older adults. Obesity (Silver Spring) 2010;18:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stull AJ, Apolzan JW, Thalacker-Mercer AE, Iglay HB, Campbell WW. Liquid and solid meal replacement products differentially affect postprandial appetite and food intake in older adults. J Am Diet Assoc 2008;108:1226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolton RP, Heaton KW, Burroughs LF. The role of dietary fiber in satiety, glucose, and insulin: studies with fruit and fruit juice. Am J Clin Nutr 1981;34:211–7. [DOI] [PubMed] [Google Scholar]
- 36.Mourao DM, Bressan J, Campbell WW, Mattes RD. Effects of food form on appetite and energy intake in lean and obese young adults. Int J Obes (Lond) 2007;31:1688–95. [DOI] [PubMed] [Google Scholar]
- 37.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord 2000;24:794–800. [DOI] [PubMed] [Google Scholar]
- 38.Apolzan JW, Leidy HJ, Mattes RD, Campbell WW. Effects of food form on food intake and postprandial appetite sensations, glucose and endocrine responses, and energy expenditure in resistance trained v. sedentary older adults. Br J Nutr 2011;106:1107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Dea K, Nestel PJ, Antonoff L. Physical factors influencing postprandial glucose and insulin responses to starch. Am J Clin Nutr 1980;33:760–5. [DOI] [PubMed] [Google Scholar]
- 40.Roberts SB. High-glycemic index foods, hunger, and obesity: is there a connection? Nutr Rev 2000;58:163–9. [DOI] [PubMed] [Google Scholar]
- 41.Bornet FR, Jardy-Gennetier AE, Jacquet N, Stowell J. Glycaemic response to foods: impact on satiety and long-term weight regulation. Appetite 2007;49:535–53. [DOI] [PubMed] [Google Scholar]
- 42.Diepvens K, Steijns J, Zuurendonk P, Westerterp-Plantenga MS. Short-term effects of a novel fat emulsion on appetite and food intake. Physiol Behav 2008;95:114–7. [DOI] [PubMed] [Google Scholar]
- 43.Mattes R. Hunger ratings are not a valid proxy measure of reported food intake in humans. Appetite 1990;15:103–13. [DOI] [PubMed] [Google Scholar]
- 44.McKiernan F, Hollis JH, McCabe GP, Mattes RD. Thirst-drinking, hunger-eating; tight coupling? J Am Diet Assoc 2009;109:486–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vosloo MC. Some factors affecting the digestion of glycaemic carbohydrates and the blood glucose response. J Fam Ecol Consum Sci 2005;33:1–9. [Google Scholar]
- 46.Jenkins DJ, Wolever TM, Taylor RH, Barker HM, Fielden H. Exceptionally low blood glucose response to dried beans: comparison with other carbohydrate foods. BMJ 1980;281:578–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akhtar MS, Asim AH, Wolever TMS. Blood glucose responses to traditional Pakistani dishes taken by normal and diabetic subjects. Nutr Res 1987;7:696–706. [Google Scholar]
- 48.Wong CL, Mollard RC, Zafar TA, Luhovyy BL, Anderson GH. Food intake and satiety following a serving of pulses in young men: effect of processing, recipe, and pulse variety. J Am Coll Nutr 2009;28:543–52. [DOI] [PubMed] [Google Scholar]
- 49.USDA National Nutrient Database for Standard Reference, Release 24 [Internet]. Beltsville (MD): USDA, Agricultural Research Service. c2011 [updated 2011 Dec 7]. Available from: http://www.ars.usda.gov/ba/bhnrc/ndl.
- 50.Sawaya AL, Fuss PJ, Dallal GE, Tsay R, McCrory MA, Young V, Roberts SB. Meal palatability, substrate oxidation and blood glucose in young and older men. Physiol Behav 2001;72:5–12. [DOI] [PubMed] [Google Scholar]
- 51.Han IH, Baik B. Oligosaccharide content and composition of legumes and their reduction by soaking, cooking, ultrasound, and high hydrostatic pressure. Cereal Chemistry 2006;4:428–33. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.