Abstract
Background: Biotin functions as a cofactor for several carboxylase enzymes with key roles in metabolism. At present, the dietary requirement for biotin is unknown and intake recommendations are provided as Adequate Intakes (AIs). The biotin AI for adults and pregnant women is 30 μg/d, whereas 35 μg/d is recommended for lactating women. However, pregnant and lactating women may require more biotin to meet the demands of these reproductive states.
Objective: The current study sought to quantify the impact of reproductive state on biotin status response to a known dietary intake of biotin.
Methods: To achieve this aim, we measured a panel of biotin biomarkers among pregnant (gestational week 27 at study entry; n = 26), lactating (postnatal week 5 at study entry; n = 28), and control (n = 21) women who participated in a 10- to 12-wk feeding study providing 57 μg of dietary biotin/d as part of a mixed diet.
Results: Over the course of the study, pregnant women excreted 69% more (vs. control; P < 0.001) 3-hydroxyisovaleric acid (3-HIA), a metabolite that accumulates during the catabolism of leucine when the activity of biotin-dependent methylcrotonyl–coenzyme A carboxylase is impaired. Interestingly, urinary excretion of 3-hydroxyisovaleryl-carnitine (3-HIA-carnitine), a downstream metabolite of 3-HIA, was 27% lower (P = 0.05) among pregnant (vs. control) women, a finding that may arise from carnitine inadequacy during gestation. No differences (P > 0.05) were detected in plasma biotin, urinary biotin, or urinary bisnorbiotin between pregnant and control women. Lactating women excreted 76% more (vs. control; P = 0.001) of the biotin catabolite bisnorbiotin, indicating that lactation accelerates biotin turnover and loss. Notably, with respect to control women, lactating women excreted 23% less (P = 0.04) urinary 3-HIA and 26% less (P = 0.05) urinary 3-HIA-carnitine, suggesting that lactation reduces leucine catabolism and that these metabolites may not be useful indicators of biotin status during lactation.
Conclusions: Overall, these data demonstrate significant alterations in markers of biotin metabolism during pregnancy and lactation and suggest that biotin intakes exceeding current recommendations are needed to meet the demands of these reproductive states. This trial was registered at clinicaltrials.gov as NCT01127022.
Keywords: 3-hydroxyisovaleric acid, biotin, bisnorbiotin, lactation, pregnancy
See corresponding commentary on page 1885
Introduction
Biotin is an essential water-soluble B-vitamin with many key roles in human metabolism. It is a required cofactor for 5 enzymes that catalyze carboxylation reactions integral to FA synthesis and oxidation (i.e., acetyl-CoA carboxylase isoforms 1 and 2) and mitochondrial carbohydrate, lipid, and amino acid metabolism (i.e., pyruvate carboxylase, propionyl-CoA carboxylase, and methylcrotonyl-CoA carboxylase) (1, 2) (Supplemental Figure 1). In addition, biotin has a role in cellular processes including gene regulation and genome stability (3–5).
The dietary requirement for biotin is unknown; therefore, intake recommendations are provided as Adequate Intakes (AIs) . The current AI for healthy adults and pregnant women is 30 μg biotin/d and was derived by extrapolating data from infants exclusively fed human milk (6). However, pregnant women may require more biotin to meet the demands of pregnancy. Suboptimal biotin status is common during pregnancy and occurs in 50% of pregnant women (7–9). Biotin deficiency during pregnancy may have an adverse impact on biotin supply to the fetus and impair fetal development (10–12). An upward adjustment in the biotin AI to 35 μg/d was made for lactating women after accounting for milk biotin content and infant milk consumption (6). However, to the best of our knowledge, no studies have examined the relations between maternal biotin intake, biotin status, and breast-milk biotin content during human lactation.
Dietary biotin intakes in adults are estimated to range from 40 to 60 μg/d (6). The accuracy of these estimates, however, requires further investigation, because food composition tables for biotin are incomplete (13). The biotin content of most foods has not been determined; therefore, dietary intake in humans cannot be reliably assessed. Notably, pregnant and lactating women must rely on food sources to meet their biotin requirements, because biotin is 1 of few essential nutrients not included in most prenatal vitamins.
This study sought to quantify the effect of reproductive state on a panel of biotin status indicators (Supplemental Figure 1) and to assess biotin status response to a known dietary biotin intake. To achieve these aims, we used biologic samples collected from a randomized choline intervention study (14) in which pregnant, lactating, and control women consumed a controlled diet for 10–12 wk (14, 15). The dietary biotin content of the meals provided throughout the controlled feeding period was also quantified.
Methods
Study participants
Pregnant, lactating, and control (nonpregnant, nonlactating) women aged 21–40 y were recruited from the Ithaca, NY, region between January 2009 and October 2010 as described by Yan et al. (14) and West et al. (15). During the screening stage, interested women provided a blood sample for a blood chemistry profile and a complete blood count and completed a health history and demographics questionnaire. Inclusion criteria for study enrollment were as follows: 1) general healthiness as determined by the questionnaire, blood chemistry profile, and complete blood count; 2) normal kidney and liver function; and 3) willingness to comply with the study protocol—specifically, agreement to eat 1 meal per day at least 3 times per wk at the onsite location and only consume food and beverages provided by the study. Exclusion criteria included the following: 1) use of prescription medications known to affect liver function; 2) tobacco, drug, or alcohol use; 3) nonsingleton pregnancy (where applicable); 4) pregnancy-associated complications (e.g., pre-eclampsia, gestational diabetes), where applicable; and 5) lack of intention to exclusively breastfeed for the study period (where applicable). Eligible pregnant women were admitted to the study on a rolling basis at 26–29 wk of gestation; eligible lactating women were admitted to the study on a rolling basis at the start of 5 wk postpartum; and eligible women of reproductive age (nonpregnant, nonlactating) were added as scheduling and space constraints allowed until the desired number of participants completed the study (14, 15).
The study protocol was reviewed and approved by the Institutional Review Board for Human Study Participant Use at Cornell University and at Cayuga Medical Center (the hospital where pregnant participants delivered their infants; Ithaca, NY). Informed consent was obtained from all women during the screening period, and study participants were compensated for their participation.
Study design
This was a nonrandomized human feeding study that used biologic samples from a previous human feeding study in which pregnant (n = 26), lactating (n = 28), and control (n = 21) women were randomly assigned to choline intakes of 480 or 930 mg/d for 10–12 wk (14, 15). Throughout the study period, the sole source of biotin intake was the study diet because none of the study supplement preparations contained biotin (verified by our laboratory). Biologic samples, including blood, urine, and breast milk, were collected at study baseline (week 0) and study end (week 10 or 12).
Study diet and supplements
As previously described (14), the study diet provided ∼2000 kcal/d and could be individualized by the addition or subtraction of non-nutritive food items. Food was prepared by study personnel in the Human Metabolic Research Unit (HMRU) at Cornell University. Pregnant and control women consumed 1 meal per day of their choice in the HMRU under the supervision of study personnel on a weekly basis, whereas lactating women consumed 1 meal every other day under these same conditions. All other food and beverages were provided as take-away products.
Study participants consumed a daily 100- or 550-mg choline chloride supplement, prepared and dispensed as described by Yan et al. (14). To meet nutrient intake recommendations (13, 16), all study participants also consumed a daily over-the-counter prenatal multivitamin supplement (Pregnancy Plus; Fairhaven Health LLC), a 200-mg DHA supplement (Neuromins; Nature’s Way Products), and a thrice-weekly potassium and magnesium supplement (General Nutrition). The Pregnancy Plus prenatal multivitamin supplement used during this study did not contain biotin (verified by our laboratory). During meals consumed in the HMRU, participants consumed the day’s supplements under the supervision of study personnel. Otherwise, study supplements were packed in plastic bags and included with take-away foods and beverages, and participants were instructed to consume the supplements with a meal of their choice.
Compliance
The study regimen was well tolerated and >90% of enrolled participants completed the study (14, 15). Reasons for dropouts included nausea, early delivery, personal food challenges, and food dislikes (14).
Study participants completed daily checklists indicating that they received and consumed all menu items and supplements. Study personnel were able to directly monitor study compliance during meals consumed in the HMRU, and participants returned empty supplement bags and food containers of meals consumed off-site during their next visit to the HMRU. Study personnel had near daily contact with study participants throughout the study to maintain positive rapport and monitor compliance.
Sample collection and processing
Fasting (10 h) venous blood was collected at baseline (all participants), at study week 10 (lactating participants), and at study week 12 (pregnant and control participants) in the HMRU ward by a trained phlebotomist. Blood was collected in EDTA-coated and serum separator tubes, processed within 2 h, and stored in cryostat tubes at −80°C until analysis as previously described (14). Twenty-four-hour urine samples were collected at baseline (study week 0) and study end (i.e., study week 10 or 12) in opaque acid-washed 2-L plastic bottles (14, 15). Breast-milk samples were collected in the HMRU on the same day as the week’s corresponding blood draw at baseline and at study end (i.e., study week 10) with an electric breast pump (Medela) and processed as previously described (15). Lactating women provided a fasted breast-milk sample, which consisted of the full expression of 1 breast 2 h after the first feeding of the day (15).
Analytical measurement of dietary biotin and status markers
Plasma total biotin was quantified by using a commercial ELISA kit (catalog no. M046019; MD Bioproducts) in the Human Nutritional Chemistry Service Laboratory at Cornell University. LC coupled with tandem MS (LC-MS/MS) was used to measure the biotin content of the diet and all other indicators of biotin status and metabolism, including breast-milk biotin, urinary biotin, urinary 3-hydroxyisovaleric acid (3-HIA), urinary 3-hydroxyisovaleryl-carnitine (3-HIA-carnitine), and urinary bisnorbiotin. Reagents were purchased from Fisher or Sigma unless otherwise stated, and Milli-Q water was used for all preparations. All batches included baseline and study-end samples for 4–10 participants with at least 1 sample from each reproductive group. In addition, in-house control samples were included with each batch and used to calculate assay precision. Urinary creatinine was measured by using the Dimension Xpand clinical chemistry system (Siemens Healthcare Diagnostic) in the Human Nutritional Chemistry Service Laboratory at Cornell University.
Dietary biotin
Dietary biotin extraction.
The biotin content of the study diet was quantified from duplicate meal samples prepared and processed during implementation of the study as described by Yan et al. (14). Dietary biotin was extracted from meal samples by using the combined sulfuric acid hydrolysis/papain digestion method of Höller et al. (17). Briefly, 20 g of homogenized meal sample was combined with 40 mL 3N sulfuric acid and 150 μL d2-biotin (catalog no. 5023; IsoSciences) internal standard (40 μg/mL) and autoclaved for 3 h. Samples were adjusted to pH 5.7, and 600 μL glutathione (10 g/L), 600 μL Na-EDTA (10 g/L), 6 mL papain solution, and 60 mL citrate buffer were added. Samples were then incubated for 16 h at 37°C with gentle shaking in a horizontal shaker. After incubation, samples were filtered 3 times through Whatman 42 ashless filter paper; then filtered twice through 0.45-μm polyvinylidene difluoride filters attached to a BD 20-mL Luer-Lok tip syringe. Samples were cleaned and concentrated as described by Nelson et al. (18). Sep-Pak Vac 12-cc (2-g) tC18 SPE cartridges on a 12-port vacuum manifold (J.T. Baker) were conditioned with 3 mL each of methanol, water, and 1.5% formic acid. After loading the samples, cartridges were washed with 6 mL 1.5% formic acid, and biotin was eluted with 4 mL 1.5% formic acid in (1:1) methanol:water solution. The eluent was loaded onto a second set of cartridges [Sep-Pak Vac 3-cc (500 mg) Certified tC18] conditioned as above, and biotin was eluted with 1 mL 1.5% formic acid in (1:1) methanol:water solution.
LC-MS/MS parameters.
Dietary biotin extracts were purified by HPLC (Accela autosampler and pump with degasser; Thermo Fisher Scientific) before quantification to prevent clogging of the mass spectrometer. Specifically, 100 μL of extract was separated by using a Luna C18 column (250 × 4.6 mm, 5-μm bead; Phenomenex). The gradient mobile phase consisted of solution A (0.1% formic acid in water) and solution B [0.1% formic acid in (1:1) methanol:acetonitrile] with a flow rate of 500 μL/min. The gradient was 95% A and 5% B from minutes 0 to 9, then 1% A and 99% B from minutes 10 to 13 before returning to 95% A and 5% B at minute 14. Fractions were collected at ±0.5 min from the retention time, which was determined with injection of the biotin standard. Collected fractions from duplicate sample injections were combined and concentrated to 100 μL by centrifugation (SpeedVac Concentrator; Savant) under vacuum with no heat, then mixed with 100 μL (1:1) methanol:water solution and transferred to vials for LC-MS/MS quantification.
Biotin standard curves were generated by using D-biotin (Sigma 47868) in water and 15 μL d2-biotin internal standard (400 ng/μL). Biotin standard curves were validated by using National Institute of Standards and Technology standard reference material 3280 (multivitamin tablets) (18). The LC-MS/MS system used to quantify dietary biotin consisted of the HPLC with Luna C18 column and matching guard column, the gradient mobile phase described above and a TSQ Quantum MS (Thermo Fisher Scientific). The retention time for biotin was ∼8 min. The MS parameters were optimized by using direct infusion of D-biotin and d2-biotin, and the linear calibration range was 2–2000 μg/L. The mass spectrometer was operated with electrospray ionization in positive-ion mode with multiple-reaction monitoring of the following transitions: biotin m/z 245→m/z 227 and d2-biotin m/z 247→m/z 229. Total sequence time was 15 min, the injection volume was 100 μL, and the autosampler and column temperatures were set at 10°C and 25°C, respectively. Quantification was performed by using the XCalibur program by Thermo Fisher Scientific. Intra- and interassay CVs were 3% and 8%, respectively, based on in-house food control duplicates (n = 3 of differing biotin concentrations), as well as the meal sample duplicates measured over 14 d.
Breast-milk biotin
Breast-milk biotin extraction.
To quantify free biotin in breast milk as described by Mock et al. (19), duplicate 1-mL breast-milk samples were combined with 50 μL tricholoracetic acid (to precipitate protein) and 20 μL d2-biotin (1 ng/μL). Samples were mixed on a rocker (Thermolyne Vari-Mix; Thermo Fisher Scientific) for 30 min and then underwent centrifugation at 19,174 × g for 30 min. The skim milk portion of the sample (i.e., fluid located between the fat layer and precipitate pellet) was transferred to LC-MS/MS vials.
LC-MS/MS parameters.
Biotin standard curves were generated as described earlier for dietary biotin quantification. Biotin standard curves were validated by using National Institute of Standards and Technology standard reference material 1849a (infant/adult nutritional formula). The LC-MS/MS system used to quantify breast-milk biotin was a Kinetix C18 column (150 × 4.6 mm, 2.6-μm bead; Phenomenex) with matching guard column, and the mass spectrometer assembly as described for dietary biotin quantification. The mobile phase consisted of solutions A and B with a flow rate of 500 μL/min, as described for dietary biotin quantification, and had the following gradient: 90% A and 10% B from minutes 0 to 12, then 1% A and 99% B from minutes 13 to 14 before returning to 90% A and 10% B during minutes 15 to 20. The retention time for biotin was ∼8 min. The mass spectrometer was operated with electrospray ionization in positive-ion mode with selected reaction monitoring (SRM) of the following transitions: biotin m/z 245→m/z 227 and d2-biotin m/z 247→m/z 229. Total sequence time was 20 min, the injection volume was 25 μL, and the autosampler and column temperatures were set at 10°C and 25°C, respectively. Quantification was performed by using the XCalibur program by Thermo. Intra- and interassay CVs were 9% and 7%, respectively, based on in-house control breast-milk duplicates (n = 3 of differing biotin concentrations), and the breast-milk sample duplicates measured over 4 d.
Urinary biotin
Extraction.
Biotin was extracted from urine by using a solid-phase extraction clean-up method adapted from Nelson et al. (18). Briefly, 70 μL d2-biotin (1 ng/μL) was added to duplicate 3-mL urine samples and brought to pH 5–6. Urine samples were cleaned with Sep-Pak Vac tC18 cartridges (3 cc/500 mg; Waters) on a 12-port vacuum manifold (J.T. Baker) conditioned with 1 mL each of methanol, water, and 1.5% formic acid. After samples were loaded, cartridges were washed with 2 mL 1.5% formic acid solution and biotin was eluted with 2 mL 1.5% formic acid in (1:1) methanol:water solution; a portion of the eluent was transferred to LC-MS/MS vials.
LC-MS/MS parameters.
The LC-MS/MS system and mobile phase used to quantify urinary biotin were the same as those described for breast-milk biotin quantification. Intra- and interassay CVs were 4% and 5%, respectively, based on in-house control urine duplicates (n = 3 of differing biotin concentrations), and the urine sample duplicates measured over 8 d.
Urinary 3-HIA
Extraction.
Urinary 3-HIA was extracted from urine by using the method of Horvath et al. (20). Briefly, triplicate urine samples were heated at 60°C (Fisher Scientific dry bath incubator) for 30 min and then underwent centrifugation at 2446 × g for 10 min. A 50-μL sample of supernatant was mixed with 150 μL water and 20 μL d8-3-HIA internal standard [0.1 μg/μL; synthesized by the Cornell Chemistry Department using the method of Mock et al. (21)] and transferred to LC-MS/MS vials.
LC-MS/MS parameters.
The 3-HIA standard curves were generated by using β-3-HIA (Sigma 55453) in water and d8-3-HIA (0.1 μg/μL). The LC-MS/MS system used to quantify urinary 3-HIA was the same as that described for dietary biotin quantification. The gradient mobile phase consisted of solution A (acetonitrile) and solution B (0.1% formic acid in water) with a flow rate of 500 μL/min. The gradient was 10% A and 90% B from minutes 0 to 10, then 90% A and 10% B during minute 11 before returning to 10% A and 90% B during minutes 12–15. The retention time for 3-HIA was ∼8 min. MS parameters were optimized by direct infusion of β-3-HIA and d8-3-HIA, and the linear calibration range was 21–423 nmol/mL. The mass spectrometer was operated with electrospray ionization in negative-ion mode with SRM of the following transitions: 3-HIA m/z 117→m/z 59 and d8-3-HIA m/z 125→m/z 61. Total sequence time was 15 min, the injection volume was 10 μL, and the autosampler and column temperatures were set at 10°C and 25°C, respectively. Quantification was performed by using the XCalibur program by Thermo Fisher Scientific. Intra- and interassay CVs were 7% and 9%, respectively, based on in-house control urine triplicates (n = 2 of differing biotin concentrations), and the triplicate urine samples measured over 8 d.
Urinary 3-HIA-carnitine
Extraction.
Urinary 3-HIA-carnitine was extracted from urine by using the method of Horvath et al. (22). Briefly, triplicate urine samples were heated at 60°C (Fisher Scientific dry bath incubator) for 30 min and then underwent centrifugation for 10 min at 2446 × g. A 40-μL sample of supernatant was mixed with 680 μL water and 80 μL d3-3-HIA-carnitine (Cambridge Isotopes) internal standard (0.0265 ng/μL) and transferred to LC-MS/MS vials.
LC-MS/MS parameters.
The 3-HIA-carnitine standard curves were generated by using 3-HIA-carnitine (Cambridge Isotopes) in water and d3-3-HIA-carnitine internal standard (0.0265 ng/μL). The LC-MS/MS system used to quantify urinary 3-HIA-carnitine consisted of a Surveyor autosampler and pump HPLC (Thermo Finnigan) and LCQ Advantage MS (Thermo Fisher Scientific). 3-HIA-carnitine was separated by using the Luna C18 column (Phenomenex) and mobile phase solutions (with a flow rate of 500 μL/min) as described earlier for urinary 3-HIA quantification with the following gradient: 5% A and 95% B from minutes 0 to 10, then 100% A and 0% B during minute 11 before returning to 5% A and 95% B during minutes 12–16. The retention time for 3-HIA-carnitine was ∼5.5 min. MS parameters were optimized by direct infusion of 3-HIA-carnitine and d3-3-HIA-carnitine, and the linear calibration range was 0.2–6 nmol/mL. The mass spectrometer was operated with electrospray ionization in positive-ion mode with full-scan monitoring of the following transitions: 3-HIA-carnitine m/z 262→m/z 85 and d3-3-HIA-carnitine m/z 265→m/z 85. Total sequence time was 16 min, the injection volume was 30 μL, and the autosampler and column temperatures were set at 10°C and 25°C, respectively. Quantification was performed by using the XCalibur program by Thermo Fisher Scientific. Intra- and interassay CVs were both 7% based on in-house control urine triplicates (n = 3 of differing biotin concentrations), and triplicate urine samples measured over 16 d.
Urinary bisnorbiotin
Extraction.
Bisnorbiotin was extracted from urine (15 mL) by using the same modified solid-phase extraction clean-up method as described for urinary biotin extraction (18) but was performed on a separate extraction day.
LC-MS/MS parameters.
Bisnorbiotin standard curves were generated by using bisnorbiotin analytical standard (courtesy of Dr. Donald Mock) in (1:1) methanol:water and d2-biotin internal standard (40 ng/μL). The LC-MS/MS system used to quantify urinary bisnorbiotin was the same as described earlier for dietary biotin quantification with the mobile phase, gradient, and flow rate as described earlier for breast-milk and urinary biotin quantification. The retention time for bisnorbiotin was ∼6 min. MS parameters were optimized by direct infusion of bisnorbiotin analytical standard and d2-biotin, and the linear calibration range was 8–400 pmol/mL. The mass spectrometer was operated with electrospray ionization in positive-ion mode with SRM of the following transitions: bisnorbiotin m/z 217→m/z 199 and d2-biotin m/z 247→m/z 229. Total sequence time was 20 min, the injection volume was 5 μL, and the autosampler and column temperatures were set at 10°C and 25°C, respectively. Quantification was performed by using the XCalibur program by Thermo Fisher Scientific. Intra- and interassay CVs were 4.7% and 16.3%, respectively, based on in-house control urine duplicates (n = 3) measured over 8 d.
Statistical methods
To test for baseline (week 0) differences between the reproductive groups, Kruskal-Wallis (ethnicity) and ANOVA (age, BMI, and biotin biomarkers) tests were performed. When significant, Tukey’s honestly significant difference procedures were used for post hoc comparisons.
To compare reproductive groups and to assess biotin status response through time, linear mixed models (LMMs) were used. Baseline (week 0) measures of the dependent variables were included as a data point because reproductive status is a physiologic state, not a treatment. Natural log–transformed plasma biotin, urinary biotin, urinary 3-HIA, urinary 3-HIA-carnitine, and urinary bisnorbiotin were entered as dependent variables in LMMs. Reproductive state (pregnant, lactating, or control), time (baseline and study end), the interaction of reproductive state and time (reproductive state × time), and choline intake group (480 or 930 mg/d) were entered as fixed factors and participant identification was entered as random factor. When a significant time × reproductive state interaction occurred (plasma and urinary biotin), post hoc comparisons between the reproductive groups were conducted at study end. Bonferroni corrections were made for multiple comparisons within each model where applicable.
Ethnicity, age, and BMI were also entered in initial models, and nonsignificant predictors (P > 0.10) were progressively removed until final models were derived. BMI and ethnicity were predictors of plasma total biotin (P = 0.06) and urinary 3-HIA excretion (P = 0.02), respectively, and thus were retained in those models. For all other dependent variables, none of the covariates [choline intake group (P = 0.13–0.86), age (P = 0.12–0.91), ethnicity (0.41–0.88), and BMI (P = 0.14–0.91)] were significant predictors and thus were not retained in final models.
Data points >2 SDs from their respective means that skewed residuals such that LMM assumptions were violated were deemed influential outliers and were excluded from analyses. Influential outliers included 1 urinary biotin value in each of the pregnant, lactating, and control groups and 1 plasma biotin value in the pregnant group; no other dependent variable was found to have influential outliers. Breast-milk biotin concentrations were natural log-transformed and analyzed with LMMs as described above without the reproductive group fixed factor. In addition, correlations between plasma biotin and breast-milk biotin were determined by using the Spearman correlation test.
All analyses were performed with IBM SPSS Statistics (version 21); P ≤ 0.05 was considered significant. Data are presented as means or geometric means (95% CIs) unless otherwise specified.
Results
Biotin content of the diet.
The biotin content of the study diet ranged from 13 to 101 μg/d and provided an average daily intake of 57 μg/d (Supplemental Table 1). This intake amount is approximately double the biotin AI for adults and pregnant women (30 μg/d) and 63% greater than the AI for lactating women (35 μg/d).
Participant characteristics.
Seventy-five women were included in the final analysis of the study. Twenty-one control women completed 12 wk of the study; 23 pregnant women completed 12 wk and 3 completed 10 wk of the study (n = 26 pregnant women total); 25 lactating women completed 10 wk, 2 completed 9 wk, and 1 completed 8 wk of the study (n = 28 lactating women total). The study-end time point reflects the last sample collected from each participant and was used in all statistical analyses. Reproductive groups did not differ in age or ethnicity. However, the self-reported prepregnancy BMI of lactating women was greater (P = 0.04) than the prepregnancy BMI of pregnant women and the baseline BMI of control women (Table 1).
TABLE 1.
Participant characteristics and concentrations of biotin biomarkers at study baseline among pregnant, lactating, and control women1
| Pregnant (n = 26) | Lactating (n = 28) | Control (n = 21) | P | |
| Ethnicity, n | 0.80 | |||
| White | 16 | 20 | 14 | |
| African American | 1 | 1 | 2 | |
| Hispanic | 4 | 3 | 2 | |
| Asian | 4 | 1 | 1 | |
| Other | 1 | 3 | 2 | |
| Age, y | 28 (27, 30) | 29 (27, 31) | 29 (26, 31) | 0.82 |
| Prepregnancy or baseline BMI,2 kg/m2 | 24 (22, 25)b | 27 (24, 30)a | 24 (22, 25)b | 0.04 |
| Plasma total biotin, ng/L | 144 (108, 194) | 186 (139, 248) | 146 (118, 182) | 0.32 |
| Urinary biotin, μmol/mol creatinine | 2.9 (2.0, 4.0) | 2.3 (1.6, 3.3) | 2.0 (1.3, 3.0) | 0.35 |
| Urinary 3-HIA, mmol/mol creatinine | 14.4 (11.8, 17.6)a | 6.6 (5.4, 8.1)b | 8.3 (6.9, 10.1)b | <0.001 |
| Urinary 3-HIA-carnitine, mmol/mol creatinine | 0.15 (0.11, 0.19)a,b | 0.13 (0.10, 0.16)b | 0.20 (0.15, 0.25)a | 0.04 |
| Urinary bisnorbiotin, μmol/mol creatinine | 6.9 (4.9, 9.6)b | 12.3 (8.5, 17.7)a | 6.2 (5.0, 7.7)b | <0.01 |
Age and BMI are means (95% CIs); all concentrations are geometric means (95% CIs). Differing letters indicate differences between the reproductive groups at study baseline. 3-HIA, 3-hydroxyisovaleric acid; 3-HIA-carnitine, 3-hydroxyisovaleryl-carnitine.
Self-reported prepregnancy BMI of pregnant and lactating groups and baseline weight measurement of control group.
Plasma total biotin (ng/L).
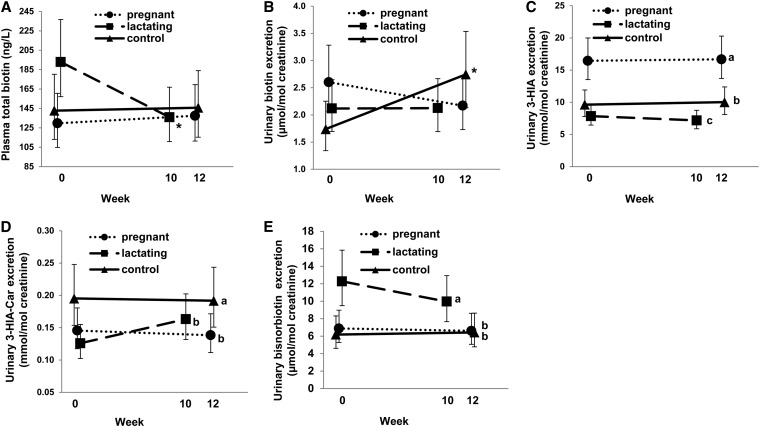
At baseline, no differences (P = 0.32) were detected in plasma total biotin between the reproductive groups (Table 1). Over the course of the study (i.e., baseline to study end), plasma total biotin response varied by reproductive group (reproductive group × time: P = 0.05). Among pregnant and control women, plasma total biotin did not change (P = 0.65–0.88) from baseline to study end (Figure 1A). However, among lactating women, plasma total biotin decreased (P < 0.01) by 30% from baseline to study end (Figure 1A). At study end, plasma total biotin did not differ (P = 0.90) by reproductive group (Figure 1A).
FIGURE 1.
Functional and static biomarkers of biotin status in pregnant, lactating, and control women consuming 57 μg dietary biotin/d under controlled feeding conditions. Values are geometric means and 95% CIs; pregnant, n = 26; lactating, n = 28; control, n = 21. Plasma total biotin (A). *Change from week 0 to week 10 in the lactating group, P < 0.01. Urinary biotin excretion (B). *Change from week 0 to week 12 in the control group, P < 0.01. Urinary 3-HIA excretion (C). Means without a common letter (a > b > c) differ between the reproductive groups, P < 0.001. Urinary 3-HIA-Car excretion (D). Means without a common letter (a > b) differ between the reproductive groups, P = 0.03; Urinary bisnorbiotin excretion (E). Means without a common letter (a > b) differ between the reproductive groups, P < 0.001. 3-HIA, 3-hydroxyisovaleric acid; 3-HIA-Car, 3-hydroxyisovaleryl-carnitine.
Urinary biotin (μmol biotin/mol creatinine).
At baseline, no differences (P = 0.35) were detected in urinary biotin excretion between the reproductive groups (Table 1). Over the course of the study, urinary biotin excretion varied by reproductive group (reproductive group × time: P = 0.02). Among pregnant and lactating women, urinary biotin excretion did not change (P = 0.23–0.98) from baseline to study end (Figure 1B). However, among control women, urinary biotin excretion increased (P < 0.01) by 58% from baseline to study end (Figure 1B). At study end, urinary biotin excretion did not differ (P = 0.27) by reproductive group (Figure 1B).
Urinary 3-HIA (mmol 3-HIA/mol creatinine).
At baseline, pregnant women excreted 118% more (P < 0.001) 3-HIA than did lactating women and 73% more (P = 0.001) 3-HIA than did control women, and did not differ (P = 0.23) from each other (Table 1). Over the course of the study, 3-HIA excretion did not change (effect of time: P = 0.80); however, excretion varied (P < 0.001) by reproductive group (Figure 1C). On average, pregnant women excreted 69% more (P < 0.001) 3-HIA than did control women and 120% more (P < 0.001) 3-HIA than did lactating women; lactating women excreted 23% less (P = 0.04) 3-HIA than did control women (Figure 1C).
Urinary 3-HIA-carnitine (mmol 3-HIA-carnitine/mol creatinine).
At baseline, control women excreted 55% more (P = 0.03) 3-HIA-carnitine than did lactating women; no differences (P = 0.21–0.63) in urinary 3-HIA-carnitine excretion were observed between pregnant and lactating or control women (Table 1). Over the course of the study, 3-HIA-carnitine excretion did not change (effect of time: P = 0.39); however, excretion varied (P = 0.03) by reproductive group (Figure 1D). On average, pregnant and lactating women excreted 27% and 26% less (both P = 0.05) urinary 3-HIA-carnitine, respectively, than did control women and did not differ from each other (P = 1.0) (Figure 1D).
Urinary bisnorbiotin (μmol bisnorbiotin/mol creatinine).
At baseline, lactating women excreted 78% more (P = 0.03) bisnorbiotin than did pregnant women and 98% more bisnorbiotin (P = 0.01) than did control women, and did not differ (P = 0.89) from each other (Table 1). Over the course of the study, bisnorbiotin excretion did not change (effect of time: P = 0.45); however, excretion varied (P < 0.001) by reproductive state (Figure 1E). On average, l actating women excreted 64% more (P = 0.002) bisnorbiotin than did pregnant women and 76% more (P = 0.001) bisnorbiotin than did control women, which did not differ from each other (P = 1.0) (Figure 1E).
Breast-milk biotin (μg/L).
Breast-milk biotin decreased (effect of time: P = 0.02) by 41% from baseline (2.8 μg/L; 95% CI: 2.0, 4.0 μg/L) to study end (1.7 μg/L; 95% CI: 1.2, 2.4 μg/L). Breast-milk biotin was positively correlated (ρ = 0.70, P < 0.001) with maternal plasma total biotin at baseline but not (ρ = 0.16, P = 0.44) at study end, possibly due to a narrower range in plasma biotin concentrations among lactating women at the later time point.
Discussion
To the best of our knowledge, this is the first controlled feeding study to assess and compare biotin status response to a known biotin intake among third-trimester pregnant women, lactating women at 5–15 wk postpartum, and control (nonpregnant, nonlactating) women of childbearing age. Our findings suggest that 1) reproductive state alters biomarkers of biotin status and metabolism and 2) pregnant and lactating women require dietary biotin intakes that exceed current recommendations.
Reproductive state alters biomarkers of biotin status and metabolism.
Static and functional indicators of biotin status were measured to examine the influence of reproductive state on biotin metabolism. Of these, the functional markers (i.e., those reflecting alterations in biotin-dependent metabolic pathways) were most influenced by reproductive state. For example, as reported previously (7, 8, 23), third-trimester pregnant women excreted abundantly more 3-HIA than did control women (Figure 1C). The greater excretion of 3-HIA during pregnancy may arise from impaired activity of the biotin-dependent methylcrotonyl-CoA carboxylase enzyme under conditions of biotin inadequacy (Supplemental Figure 1). The impaired activity of methylcrotonyl-CoA carboxylase would also be expected to increase urinary 3-HIA-carnitine excretion (Supplemental Figure 1). However, in the present study, pregnant women excreted significantly less 3-HIA-carnitine than did control women (Figure 1D). This unexpected finding may be attributed to carnitine depletion, which appears to be common during pregnancy (24). Carnitine insufficiency may reduce the conversion of 3-HIA to 3-HIA-carnitine, thereby decreasing 3-HIA-carnitine excretion among pregnant women. Reduced conversion of 3-HIA to 3-HIA-carnitine may also contribute to the observed elevations in urinary 3-HIA in this reproductive state. However, quantitatively, 3-HIA excretion is ∼100 times higher than 3-HIA-carnitine excretion (Figure 1C, D), and the magnitude of increased 3-HIA excretion (+70%) vs. decreased 3-HIA-carnitine excretion (−27%) among pregnant (vs. control) women was greater. Thus, the increased urinary excretion of 3-HIA during pregnancy is most indicative of impaired activity of the biotin-dependent methylcrotonyl-CoA carboxylase enzyme and demonstrative of a higher biotin requirement among pregnant women.
Lactation was also found to alter functional biomarkers of biotin status. Urinary excretion of 3-HIA and 3-HIA-carnitine was significantly lower among lactating than control women (Figure 1C, D). Importantly, 3-HIA and 3-HIA-carnitine are products of leucine catabolism (Supplemental Figure 1), and during lactation, large amounts of leucine (and other branched-chain amino acids) are taken up by the mammary gland where they are used to synthesize glutamine and aspartate in sows (25) and protein and lipid in rats (26). Thus, the reduced urinary excretion of 3-HIA and 3-HIA-carnitine among lactating women may arise from reduced catabolism of leucine due to its greater uptake (and utilization) by the mammary epithelium. As such, 3-HIA and 3-HIA-carnitine may not be useful biomarkers of biotin status among lactating women because factors other than biotin (e.g., greater partitioning of leucine to the mammary epithelium) may be influencing their production and metabolism.
Notably, lactating women excreted more bisnorbiotin than did control women (Figure 1E). Bisnorbiotin is a nonfunctional product of biotin degradation that represents a route of biotin loss (Supplemental Figure 1) (27). Induced biotin deficiency decreases urinary bisnorbiotin excretion among healthy adults, suggesting that biotin insufficiency reduces the rate of biotin catabolism (28). However, increased rates of biotin catabolism may arise in certain physiologic states that increase biotin turnover. Higher urinary concentrations of bisnorbiotin were reported during early pregnancy (7), in patients consuming anticonvulsant drugs (27) and in smokers exhibiting signs of biotin insufficiency (29). Thus, lactation may be a physiologic state that increases biotin turnover and results in greater urinary excretion of bisnorbiotin. Accelerated biotin catabolism during lactation is of concern because it can increase the risk of maternal biotin deficiency, which may have a negative impact on biotin supply to the nursing infant. In sum, the higher levels of biotin degradation (and loss) among lactating women would be expected to increase their dietary requirements for biotin and may increase susceptibility for suboptimal biotin status.
Pregnant and lactating women require dietary biotin intakes that exceed current recommendations.
The average daily intake of 57 μg biotin/d was supplied exclusively through a normal mixed diet (Supplemental Table 1) and provided a biotin intake approximately double the AI for adults and pregnant women (30 μg/d) and 63% greater than the AI for lactating women (35 μg/d) (6). This amount of biotin intake among pregnant women did not normalize urinary 3-HIA excretion to the nonpregnant control state (Figure 1C), indicating functional biotin deficiency in this reproductive group. Supplementation with 300 μg biotin/d was shown to decrease urinary 3-HIA excretion in pregnant women (8); however, with such supplementation, urinary biotin excretion also increased dramatically (8), suggesting that this intake amount (∼10 times the AI) exceeds cellular metabolic capacity and renal retention. In the present study, urinary biotin did not change over the course of the study among the pregnant women nor did it differ from that of control women (Figure 1B), suggesting that 57 μg/d did not exceed the metabolic requirements of pregnant women. Overall, these results suggest that pregnant women may require at least double the current AI to meet the metabolic demands of pregnancy. Additional studies designed to determine the biotin intake amount that normalizes biotin status markers during pregnancy, and improves fetal health, are warranted.
Among lactating women, several indicators suggest that they may benefit from intakes greater than the AI and perhaps greater than the intake amount (57 μg biotin/d) provided by the study diet. First, urinary bisnorbiotin excretion was substantially greater than in control women throughout the study, indicating that lactation increases biotin catabolism and loss, which was not accounted for during the formulation of the current AI (6). In addition, with an intake of 57 μg biotin/d (∼63% higher than the biotin AI), plasma and breast-milk biotin concentrations decreased over the course of the study, suggesting that a lower intake (i.e., the biotin AI) may accelerate these decreases.
Conclusions.
Results of this human feeding study that provided an average daily intake of 57 μg dietary biotin/d demonstrate that pregnancy and lactation increase the dietary requirement for biotin and suggest that intakes exceeding current dietary recommendations are needed to meet metabolic demands. The findings also indicate that physiologic processes associated with pregnancy and lactation may complicate the interpretation of biotin status indicators. Specifically, although urinary 3-HIA excretion has been validated as an indicator of biotin status during pregnancy (8, 9), the results of the present study imply that 3-HIA-carnitine should not be similarly used. In addition, the relatively low amounts of both 3-HIA and 3-HIA-carnitine excretion exhibited by lactating women suggest that amino acid metabolism during lactation obscures the connection between urinary excretion of these metabolites and biotin status. Thus, additional indicators of biotin status and metabolism, such as the abundance of biotinylated carboxylases in lymphocytes (30), should be investigated and validated for use in these reproductive states. Given the compelling animal data linking maternal biotin deficiency with birth defects (9), more longitudinal studies are also required to further elucidate the relation between maternal biotin status and child health outcomes in humans, and to determine the amount of biotin intake required to optimize biotin status among pregnant and lactating women.
Supplementary Material
Acknowledgments
The authors thank Donald M Mock and the Mock Laboratory at the University of Arkansas for Medical Sciences for providing the bisnorbiotin standard used to generate the calibration points and for advising on the analytical methodology. CAP and MAC designed the research; CAP, AAW, AG, LKL, JY, XJ, and OM conducted the research; AAW analyzed the data; CAP, AAW, and MAC wrote the manuscript; and MAC had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AI, Adequate Intake; HMRU, Human Metabolic Research Unit; LC-MS/MS, LC coupled with tandem MS; LMM, linear mixed model; SRM, selected reaction monitoring; 3-HIA, 3-hydroxyisovaleric acid; 3-HIA-carnitine, 3-hydroxyisovaleryl-carnitine.
References
- 1.Jitrapakdee S, Wallace JC. The biotin enzyme family: conserved structural motifs and domain rearrangements. Curr Protein Pept Sci 2003;4:217–29. [DOI] [PubMed] [Google Scholar]
- 2.Lynen F. New experiments of biotin enzymes. CRC Crit Rev Biochem 1979;7:103–19. [DOI] [PubMed] [Google Scholar]
- 3.Singh MP, Wijeratne SSK, Zempleni J. Biotinylation of lysine 16 in histone H4 contributes toward nucleosome condensation. Arch Biochem Biophys 2013;529:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Healy S, Perez-Cadahia B, Jia D, McDonald MK, Davie JR, Gravel RA. Biotin is not a natural histone modification. Biochim Biophys Acta 2009;1789:719–33. [DOI] [PubMed]
- 5.Kuroishi T, Rios-Avila L, Pestinger V, Wijeratne SSK, Zempleni J. Biotinylation is a natural, albeit rare, modification of human histones. Mol Genet Metab 2011;104:537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Institute of Medicine. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington: National Academies Press; 1998 [cited 2012 Feb 6]. Available from: http://www.nap.edu/openbook.php?record_id=6015&page=1. [PubMed]
- 7.Mock DM, Stadler DD, Stratton SL, Mock NI. Biotin status assessed longitudinally in pregnant women. J Nutr 1997;127:710–6. [DOI] [PubMed] [Google Scholar]
- 8.Mock DM, Quirk JG, Mock NI. Marginal biotin deficiency during normal pregnancy. Am J Clin Nutr 2002;75:295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mock DM. Marginal biotin deficiency is common in normal human pregnancy and is highly teratogenic in mice. J Nutr 2009;139:154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mock DM, Mock NI, Stewart CW, LaBorde JB, Hansen DK. Marginal biotin deficiency is teratogenic in ICR mice. J Nutr 2003;133:2519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe T, Endo A. Teratogenic effects of avidin-induced biotin deficiency in mice. Teratology 1984;30:91–4. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T, Nagai Y, Taniguchi A, Ebara S, Kimura S, Fukui T. Effects of biotin deficiency on embryonic development in mice. Nutrition 2009;25:78–84. [DOI] [PubMed] [Google Scholar]
- 13.Otten J, Pitzi H, Meyers L. National Research Council. Dietary reference intakes: the essential guide to nutrient requirements. Washington: The National Academies Press, 2006:196–201.
- 14.Yan J, Jiang X, West AA, Perry CA, Malysheva OV, Devapatla S, Pressman E, Vermeylen F, Stabler SP, Allen RH, et al. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am J Clin Nutr 2012;95:1060–71. [DOI] [PubMed] [Google Scholar]
- 15.West AA, Yan J, Perry CA, Jiang X, Malysheva OV, Caudill MA. Folate-status response to a controlled folate intake in nonpregnant, pregnant, and lactating women. Am J Clin Nutr 2012;96:789–800. [DOI] [PubMed] [Google Scholar]
- 16.Koletzko B, Cetin I, Brenna JT. Dietary fat intakes for pregnant and lactating women. Br J Nutr 2007;98:873–7. [DOI] [PubMed] [Google Scholar]
- 17.Höller U, Wachter F, Wehrli C, Fizet C. Quantification of biotin in feed, food, tablets, and premixes using HPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 2006;831:8–16. [DOI] [PubMed] [Google Scholar]
- 18.Nelson BC, Sharpless KE, Sander LC. Improved liquid chromatography methods for the separation and quantification of biotin in NIST standard reference material 3280: multivitamin/multielement tablets. J Agric Food Chem 2006;54:8710–6. [DOI] [PubMed] [Google Scholar]
- 19.Mock DM, Mock NI, Langbehn SE. Biotin in human milk: methods, location, and chemical form. J Nutr 1992;122:535–45. [DOI] [PubMed] [Google Scholar]
- 20.Horvath TD, Matthews NI, Stratton SL, Mock DM, Boysen G. Measurement of 3-hydroxyisovaleric acid in urine from marginally biotin-deficient humans by UPLC-MS/MS. Anal Bioanal Chem 2011;401:2805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mock DM, Jackson H, Lankford GL, Mock NI, Weintraub ST. Quantification of urinary 3-hydroxyisovaleric acid using deuterated 3-hydroxyisovaleric acid as internal standard. Biomed Environ Mass Spectrom 1989;18:652–6. [DOI] [PubMed] [Google Scholar]
- 22.Horvath TD, Stratton SL, Bogusiewicz A, Owen SN, Mock DM, Moran JH. Quantitative measurement of urinary excretion of 3-hydroxyisovaleryl carnitine by LC-MS/MS as an indicator of biotin status in humans. Anal Chem 2010;82:9543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mock DM, Stadler DD. Conflicting indicators of biotin status from a cross-sectional study of normal pregnancy. J Am Coll Nutr 1997;16:252–7. [DOI] [PubMed] [Google Scholar]
- 24.Ringseis R, Hanisch N, Seliger G, Eder K. Low availability of carnitine precursors as a possible reason for the diminished plasma carnitine concentrations in pregnant women. BMC Pregnancy Childbirth 2010;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li P, Knabe DA, Kim SW, Lynch CJ, Hutson SM, Wu G. Lactating porcine mammary tissue catabolizes branched-chain amino acids for glutamine and aspartate synthesis. J Nutr 2009;139:1502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viña J, Williamson D. Effects of lactation on L-leucine metabolism in the rat—studies invivo and invitro. Biochem J 1981;194:941–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mock DM, Dyken ME. Biotin catabolism is accelerated in adults receiving long-term therapy with anticonvulsants. Neurology 1997;49:1444–7. [DOI] [PubMed] [Google Scholar]
- 28.Mock NI, Malik MI, Stumbo PJ, Bishop WP, Mock DM. Increased urinary excretion of 3-hydroxyisovaleric acid and decreased urinary excretion of biotin are sensitive early indicators of decreased biotin status in experimental biotin deficiency. Am J Clin Nutr 1997;65:951–8. [DOI] [PubMed] [Google Scholar]
- 29.Sealey WM, Teague AM, Stratton SL, Mock DM. Smoking accelerates biotin catabolism in women. Am J Clin Nutr 2004;80:932–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eng WK, Giraud D, Schlegel VL, Wang D, Lee BH, Zempleni J. Identification and assessment of markers of biotin status in healthy adults. Br J Nutr 2013;110:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.