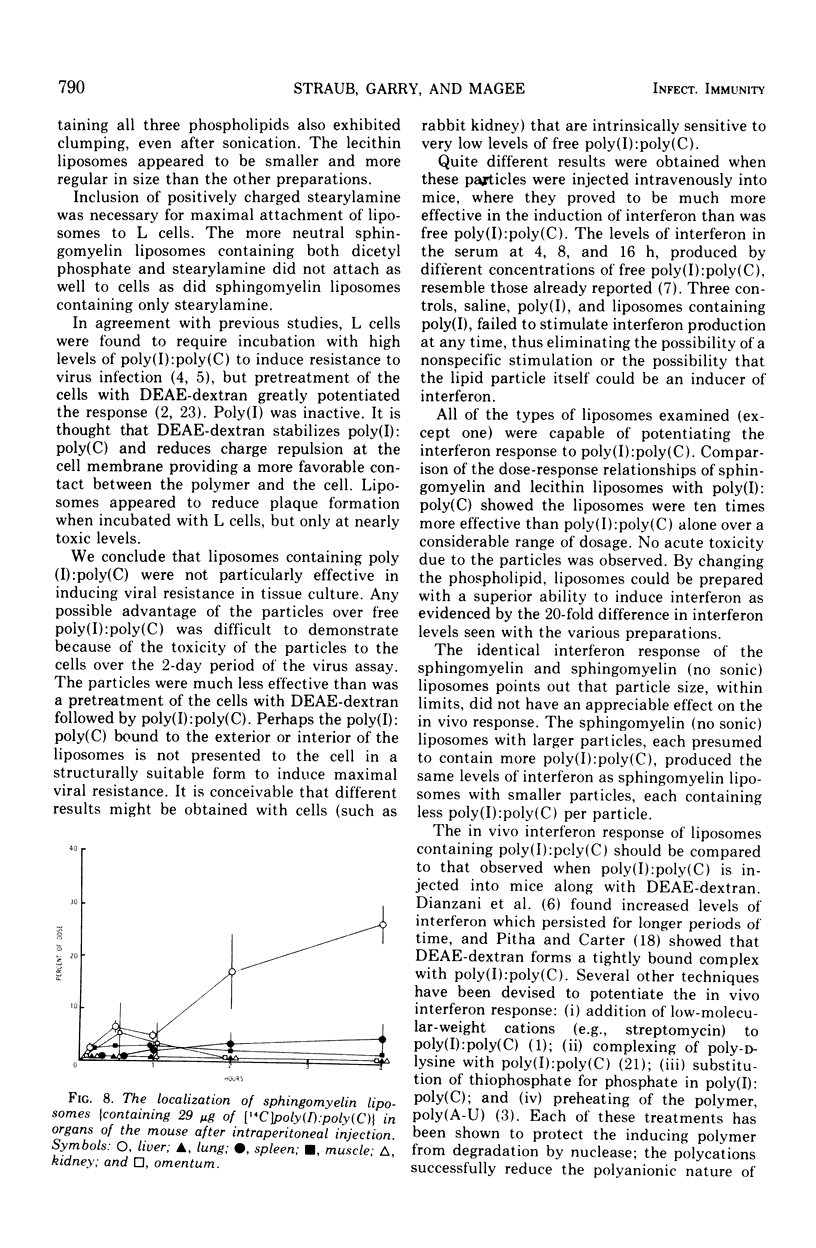
Abstract
Liposomes were prepared with phospholipids (sphingomyelin, lecithin, and phosphatidylethanolamine) in combination with cholesterol and charged lipids (dicetyl phosphate and stearylamine) and contained either poly(I):poly(C) or poly(I). Neutral and positively charged liposomes attached much better to L-929 cells in tissue culture than did negatively charged particles. Liposomes were toxic to L cells at relatively low concentrations, making the determination of antiviral activity induced by particles containing poly(I):poly(C) difficult to measure by the plaque reduction assay. When injected into mice, all of the liposomes containing poly(I):poly(C), except phosphatidylethanolamine liposomes, greatly potentiated and extended the serum interferon response of poly(I):poly(C). Lecithin and sphingomyelin liposomes given intravenously were ten times more effective than free poly(I):poly(C) in stimulating production of serum interferon. Sphingomyelin liposomes containing [14C]poly(I):poly(C) were 88% cleared from the bloodstream of mice by 3 min after intravenous injection. Most of the radioactivity (70%) was captured by the liver and remained there for at least 4 h. By 2 h, 7% of the radioactivity could be found in the spleen. Five percent of the radioactivity was found in the lungs at 30 min, with decreasing amounts thereafter. Small amounts of radioactivity were found in the muscle and kidneys. The spleen was shown to contain appreciable levels of interferon at 4 h, and low levels were found in the liver. Radioactivity accumulated slowly in the liver following an intraperitoneal injection of sphingomyelin liposomes containing [14C]poly(I):poly(C). By 4 h, 26% of the dose was recovered from the liver and 4.9% from the spleen, with small amounts in the lung, kidney, and omentum.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billiau A., Buckler C. E., Dianzani F., Uhlendorf C., Baron S. Induction of the interferon mechanism by single-stranded RNA: potentiation by polybasic substances. Proc Soc Exp Biol Med. 1969 Nov;132(2):790–796. doi: 10.3181/00379727-132-34310. [DOI] [PubMed] [Google Scholar]
- Colby C., Chamberlin M. J. The specificity of interferon induction in chick embryo cells by helical RNA. Proc Natl Acad Sci U S A. 1969 May;63(1):160–167. doi: 10.1073/pnas.63.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Merigan T. C. Requirement of a stable secondary structure for the antiviral activity of polynucleotides. Nature. 1969 Jun 21;222(5199):1148–1152. doi: 10.1038/2221148a0. [DOI] [PubMed] [Google Scholar]
- Dianzani F., Cantagalli P., Gagnoni S., Rita G. Effect of DEAE-dextran on production of interferon induced by synthetic double-stranded RNA in L cell cultures. Proc Soc Exp Biol Med. 1968 Jul;128(3):708–710. doi: 10.3181/00379727-128-33105. [DOI] [PubMed] [Google Scholar]
- Dianzani F., Rita G., Cantagalli P., Gagnoni S. Effect of DEAE-dextran on interferon production and protective effect in mice treated with the double-stranded polynucleotide complex polyinosinic-polycytidylic acid. J Immunol. 1969 Jan;102(1):24–27. [PubMed] [Google Scholar]
- Field A. K., Tytell A. A., Lampson G. P., Hilleman M. R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1004–1010. doi: 10.1073/pnas.58.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriadis G., Buckland R. A. Enzyme-containing liposomes alleviate a model for storage disease. Nature. 1973 Jul 20;244(5412):170–172. doi: 10.1038/244170a0. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G., Ryman B. E. Fate of protein-containing liposomes injected into rats. An approach to the treatment of storage diseases. Eur J Biochem. 1972 Jan 21;24(3):485–491. doi: 10.1111/j.1432-1033.1972.tb19710.x. [DOI] [PubMed] [Google Scholar]
- Ho M., Ke Y. H. The mechanism of stimution of interferon production by a complexed polyribonucleotide. Virology. 1970 Mar;40(3):693–702. doi: 10.1016/0042-6822(70)90214-x. [DOI] [PubMed] [Google Scholar]
- Johnson S. M. The effect of charge and cholesterol on the size and thickness of sonicated phospholipid vesicles. Biochim Biophys Acta. 1973 Apr 25;307(1):27–41. doi: 10.1016/0005-2736(73)90022-9. [DOI] [PubMed] [Google Scholar]
- Magee W. E., Griffith M. J. The liver as a site for interferon production in response to poly I:poly C. Life Sci II. 1972 Nov 22;11(22):1081–1086. doi: 10.1016/0024-3205(72)90216-0. [DOI] [PubMed] [Google Scholar]
- Magee W. E., Miller O. V. Liposomes containing antiviral antibody can protect cells from virus infection. Nature. 1972 Feb 11;235(5337):339–341. doi: 10.1038/235339a0. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Miller N. Phospholipid model membranes. I. Structural characteristics of hydrated liquid crystals. Biochim Biophys Acta. 1967 Sep 9;135(4):624–638. doi: 10.1016/0005-2736(67)90094-6. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Nir S., Oki S. Permeability properties of phospholipid membranes: effect of cholesterol and temperature. Biochim Biophys Acta. 1972 Jun 20;266(3):561–583. doi: 10.1016/0006-3002(72)90001-7. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Watkins J. C. Phospholipid model membranes. II. Permeability properties of hydrated liquid crystals. Biochim Biophys Acta. 1967 Sep 9;135(4):639–652. doi: 10.1016/0005-2736(67)90095-8. [DOI] [PubMed] [Google Scholar]
- Pitha P. M., Carter W. A. The DEAE dextran:polyriboinosinate-polyribocytidylate complex: physical properties and interferon induction. Virology. 1971 Sep;45(3):777–781. doi: 10.1016/0042-6822(71)90194-2. [DOI] [PubMed] [Google Scholar]
- Rahman Y. E., Rosenthal M. W., Cerny E. A. Intracellular plutonium: removal by liposome-encapsulated chelating agent. Science. 1973 Apr 20;180(4083):300–302. doi: 10.1126/science.180.4083.300. [DOI] [PubMed] [Google Scholar]
- Rice J. M., Turner W., Chirigos M. A., Rice N. R. Enhancement by poly-D-lysine of poly I: C-induced interferon production in mice. Appl Microbiol. 1970 May;19(5):867–869. doi: 10.1128/am.19.5.867-869.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice J. M., Turner W., Chirigos M. A., Spahn G. Dose responsiveness and variation among inbred strains of mice in production of interferon after treatment with poly (I)-poly (C) (poly-D-lysine) complexes. Appl Microbiol. 1971 Sep;22(3):380–386. doi: 10.1128/am.22.3.380-386.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Chan S. I. Effect of sonication on the structure of lecithin bilayers. Biochemistry. 1972 Nov 21;11(24):4573–4581. doi: 10.1021/bi00774a024. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Barmak S. L., Havell E. A. Control of interferon synthesis: effect of diethylaminoethyl-dextran on induction by polyinosinic-polycytidylic acid. J Virol. 1972 Oct;10(4):614–621. doi: 10.1128/jvi.10.4.614-621.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


