Abstract
Background
Bacterial resistance against the classic antibiotics is posing an increasing challenge for the prevention and treatment of infections in health care environments. The introduction of antimicrobial nanocoatings with active ingredients provides alternative measures for active killing of microorganisms, through a preventive hygiene approach.
Purpose
The purpose of this study was to investigate the antimicrobial activity of a panel of antimicrobial coatings available on the European market.
Methods
A comparative, biased selection of commercially available antimicrobial coatings was tested for antimicrobial efficiency. Suppliers were contacted to deliver their coatings on glass and/or stainless steel substrates. In total, 23 coatings from eleven suppliers were received, which were investigated for their effect on the growth of Escherichia coli, using the International Organization for Standardization (ISO) 22196 protocol.
Results
The majority of nanomaterial-containing coatings (n=13) contained nanosilver (n=12), while only one had photocatalytic TiO2 as the active particle. The differences in antimicrobial activity among all of the coatings, expressed as log reduction values, varied between 1.3 and 6.6, while the variation within the nanomaterial-based group was between 2.0 and 6.2. Although nanosilver coatings were on average very effective in reducing the number of viable bacteria after challenge, the strongest log reduction (6.6) was seen with a coating that has immobilized, covalently bound quaternary ammonium salt in its matrix. Besides these two compounds, coatings containing TiO2, poly(dimethylsiloxane), triclosan, or zinc pyrithione evoked 100% killing of E. coli.
Conclusion
Our findings indicate that nanosilver dominates the nanoparticle-based coatings and performs adequately. However, considering the unknowns in relation to ecotoxicological emission and effects, it needs further consideration before widespread application into different environments.
Keywords: coatings, nanotechnology, nanosilver, biocides
Introduction
Bacterial infections increasingly burden society since pathogenic microbes can be ever more resistant to antibacterial agents. Poorly regulated global use of antibiotic drugs and genetic survival mechanisms of the bacteria underlie this trend. For certain bacterial species, treatment options have become so sparse that a growing number of infections are very dangerous to patients or lead to poorly controllable health care costs.1,2
Novel antibiotic drugs are scarce, and preventive innovations are needed to minimize microbial pressure in bacterial “hotspots”, like hospitals, nursing homes, or daycare centers. For example, bacteria can persist for many months on inanimate surfaces, forming an ongoing source of transmission.3 Health care workers can transfer these microbes to the patient after touching contaminated surfaces.4,5 Hand hygiene is thus a weak link in the prevention of bacterial transmission from environmental surfaces. Also, biofilms harboring pathogenic bacteria can form on the surface of medical implants after surgery and are a source of poorly treatable recurrent infections.6–8 A state-of-the-art innovation to combat pathogenic bacteria is the creation of self-disinfecting surfaces through the application of coatings with antibiofouling and/or bactericidal properties.9 Particularly bactericidal coatings are interesting in health care because of the capability of these coatings to kill pathogens on contact. Many different chemical strategies and technologies for antibacterial coatings have been described. Antibacterial coatings may contain active eluting agents (eg, ions or nanoparticles of silver, copper, zinc, or antibiotics, chloride, iodine, etc), immobilized molecules that become active upon contact (eg, quaternary ammonium polymers or peptides), or light-activated molecules (eg, TiO2 or photosensitizers).10,11
As depicted in a recent market analysis (source: marketsandmarkets.com), the global market for these coatings is growing. With an estimated market worth of $1.5 billion, the global antimicrobial coating demand is expected to reach $2.9 billion in 2018.12
Here, we investigated the antibacterial activity of 23 commercially available antimicrobial coatings based on nanomaterials (n=13) or other active ingredients (n=10). Using the International Organization for Standardization (ISO) test ISO 22196, a direct, 24-hour inoculation method, we assessed activity against the common model organism Escherichia coli.
Although many independent studies8–10 have previously shown that antibacterial coatings aid in reducing bacterial loads on surfaces, a comparative study in which multiple substrates were evaluated using one standard protocol, has been lacking. In this study, we evaluated the outgrowth of E. coli under the standardized conditions of the ISO 22196 test.
Material and methods
Antimicrobial coatings
European manufacturers were approached by email and by phone to recruit their participation in the current study. Interviews with the participating supplier were undertaken, explaining the aims, timeline, and conditions of the current study. A Material Transfer Agreement (MTA) was subsequently signed, allowing exchange of materials between researchers and supplying manufacturers. Furthermore, a nondisclosure agreement was signed, which limits the details that can be provided in this manuscript regarding brand names or specific formulations. Manufacturers received a set of test substrates (discussed below) and were allowed to apply their coatings according to their own best practices. Coated stainless steel or glass surfaces (40×40 mm) were provided ready-to-use by manufacturers. Control substrates for this study included nontreated stainless steel and/or glass. In all, 23 coatings (total 32 samples) were tested for antibacterial efficacy, provided by the following suppliers: Nanoservices (Nunspeet, The Netherlands), Ag Polymer (Volpiano, Italy), Bionic Technology (Winschoten, The Netherlands), Sioen Industries (Mouscron, Belgium), Hexis (Zevenhuizen, The Netherlands), General Paints (Celbridge, Ireland), Millidyne Oyc (Tampere, Finland), PPG Europe BV (Uithoorn, The Netherlands), AM Coatings (Ede, The Netherlands), Sirec (Colorno, Italy), and van Wijhe (Zwolle, The Netherlands). The active antibacterial compound of each coating is provided in Table 1. Individual results were communicated in written form to suppliers before submission of this study. Aggregated data presented in this work cannot be traceable to individual producers.
Table 1.
Overview of coatings tested
| Coating | Antimicrobial agent | Nanocompound | Reported effect | Substrate
|
Log reduction
|
% inhibition
|
|||
|---|---|---|---|---|---|---|---|---|---|
| SS | G | SS | G | SS | G | ||||
| AMC-01 | Titanium dioxide | Yes | Bactericidal | SS | G | 6.6 | 2.0 | 100 | 32 |
| AMC-02 | Silver | Yes | Bactericidal | G | 6.1 | 100 | |||
| AMC-03 | Silver | Yes | Bactericidal | G | 6.1 | 100 | |||
| AMC-04 | Polysilazane | No | Antifouling | SS | G | 4.4 | 1.3 | 93 | 23 |
| AMC-05 | Poly(dimethylsiloxane) | No | Antifouling | SS | G | 4.7 | 3.2 | 100 | 54 |
| AMC-06 | Silver | Yes | Bactericidal | SS | 5.5 | 100 | |||
| AMC-07 | Silver | Yes | Bactericidal | SS | 5.5 | 100 | |||
| AMC-08 | Silver | Yes | Bactericidal | SS | 5.5 | 100 | |||
| AMC-09 | Triclosan | No | Dose-dependent# | G | 6.2 | 100 | |||
| AMC-10 | Wood extract | No | Unknown | SS | G | 3.6 | 1.9 | 65 | 31 |
| AMC-11 | Cationic salts | No | Bactericidal | SS | G | 1.8 | 1.9 | 32 | 31 |
| AMC-12 | Wood extract | No | Unknown | SS | G | 1.7 | 1.9 | 32 | 31 |
| AMC-13 | Silver | Yes | Bactericidal | G | 6.1 | 100 | |||
| AMC-14 | Silver | Yes | Bactericidal | G | 5.8 | 95 | |||
| AMC-15 | Silver | Yes | Bactericidal | G | 5.8 | 95 | |||
| AMC-16 | Silver | Yes | Bactericidal | G | 6.1 | 100 | |||
| AMC-17 | Quaternary ammonium | No | Bactericidal | G | 6.1 | 100 | |||
| AMC-18 | Quaternary ammonium | No | Bactericidal | G | 6.1 | 100 | |||
| AMC-19 | Silver | Yes | Bactericidal | G | 6.0 | 100 | |||
| AMC-20 | Silver | Yes | Bactericidal | G | 6.0 | 100 | |||
| AMC-21 | CMIT/MIT | No | Bactericidal | SS | G | 3.4 | 5.7 | 99 | 93 |
| AMC-22 | Silver | Yes | Bactericidal | SS | G | 3.2 | 6.2 | 94 | 100 |
| AMC-23 | Zinc pyrithione | No | Bacteriostatic | SS | G | 3.4 | 6.1 | 100 | 100 |
Notes: We received 23 coatings from eleven manufacturers, which have been encrypted in the table. Listed in columns, from left to right are: the AMC numbers; the antimicrobial agent in each coating; whether based on nanotechnology; the substrates on which the coatings were provided (SS or G); and the log reduction and % inhibition values after testing according to the ISO 22196:2011 protocol.
Triclosan is bacteriostatic at a low dose and bactericidal at a higher dose (dose not given).
Abbreviations: AMC, antimicrobial coating; CMIT, chloromethylisothiazolinone; G, glass; ISO, International Organization for Standardization; MIT, methylisothiazolinone; SS, stainless steel.
Bacterial suspensions
In accordance to the ISO 22196:2011 protocol, E. coli (American Tissue Type Collection [ATCC] number 72002) was grown aerobically overnight (16–24 hours) on Lysogeny broth (LB) agar at 35°C, after which colonies were scraped and dissolved in 0.9% NaCl to an optical density (OD)600 at 600 nm of 0.35 absorption units. The suspended solution was diluted 1:1,000, generating a bacterial suspension with concentration between 2.5×105 CFU/mL and 10×105 CFU/mL. From this bacterial suspension, serial dilution was done to attain solutions containing 101-, 102-, 103-, 104-, and 105-fold dilutions. Using the pour plate method,13 the amount of CFU/mL of the suspension used for testing was assessed (data not shown). Briefly, 1 mL of diluted suspension was embedded in 15 mL liquid nutrient agar in a 10 cm petri dish. After overnight incubation at 37°C, colony counts were used to interpolate the amount of CFU/mL in the original suspension.
Antimicrobial testing according to ISO 22196:2011
An overview of the following method is depicted in Figure 1. Test strips containing antibacterial coatings and their corresponding stainless steel or glass control surfaces were tested in triplicate. Each test surface was quickly cleaned by slightly wiping with 70% EtOH prior to testing. After drying, the surfaces were challenged with E.coli (ATCC number 72002) according to the ISO 22196:2011 protocol, with minor adaptations. The protocol allows the use of other strains of E. coli then those suggested, but this should be mentioned by the researchers. This is the case in our work. Then, 400 µL bacterial suspensions (ca 105 CFU/mL) were added to the test and control surfaces, after which the samples were covered with a 40×40 mm plastic film. The bacterial suspension was incubated for 24 hours at 35°C (90% humidity). Next, bacteria were recovered in 10 mL soybean-casein-digest-lecithin-polysorbate 80 (SCDLP) medium and serially diluted. From each dilution, 1 mL was poured into Luria agar (LA) agar plates (nutrient agar mentioned in ISO 22196:2011 protocol) and incubated for 48 hours at 90% humidity, at 35°C. Obtained triplicates (not shown) were averaged, revealing: Ut and Ua (corresponding to control surface and coated surface, respectively, after incubation on LB agar plate). Ut and Ua were expressed as log values of the CFUs counted after bacterial challenge of the coated or noncoated substrates. The values were applied in the following algorithms:
Figure 1.
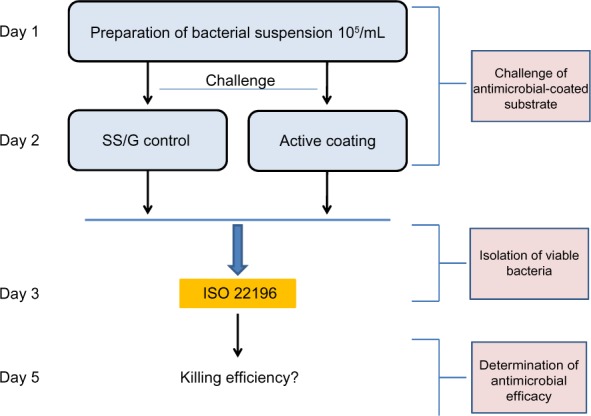
Study strategy to determine efficacy of different antimicrobial coatings.
Notes: Antimicrobial activity was determined by the golden standard, the ISO 22196:2011 protocol, which comprises three major phases. In the first phase we challenged the antimicrobial coatings (AMC) and control surfaces with a suspension of Escherichia coli. Bacteria were recovered after 24 hours of incubation at 35°C. After 2 days of additional incubation using the pour plate method, the number of CFUs in the obtained suspensions was determined. The killing efficiency of the applied coatings was calculated as described in “Materials and methods”.
Abbreviations: AMC, antimicrobial coating; CFU, colony-forming units; G, glass; ISO, International Organization for Standardization; SS, stainless steel.
| (1) |
where R is the log reduction in bacterial load due to the presence of coating, and
| (2) |
where % is the percent inhibition of bacterial growth.
Statistical analysis
When comparing the performance of silver-based coatings to others, the Mann–Whitney U test was performed, since the populations did not fit a normal distribution (assessed by Kolmogorov–Smirnov test).
Results
Different coatings available in the European market were obtained for testing, by allowing suppliers (eleven in total) to deposit their antimicrobial products onto materials that were provided by us (stainless steel and/or glass). We then investigated antimicrobial activity, using the standard ISO assay. This allowed an unprecedented comparison of, in total, 23 antimicrobial products (32 samples in total, on stainless steel and/or glass) whose mechanisms of action differ from each other. The received coatings were based on nanomaterials (n=13) or on non-nanomaterial-based active ingredients (n=10). Within the nanocoating group, the majority (12/13) contained nanosilver in some form, whereas one coating contained TiO2. The characterization of the tested coatings included within our survey seems to imply that nanocoatings dominate the total market (57% of all tested coatings). There was a large variety in the type of active ingredients within the non-nanomaterial-based group, ranging from natural wood extracts all the way to classical biocides embedded in polymer matrix (Table 1).
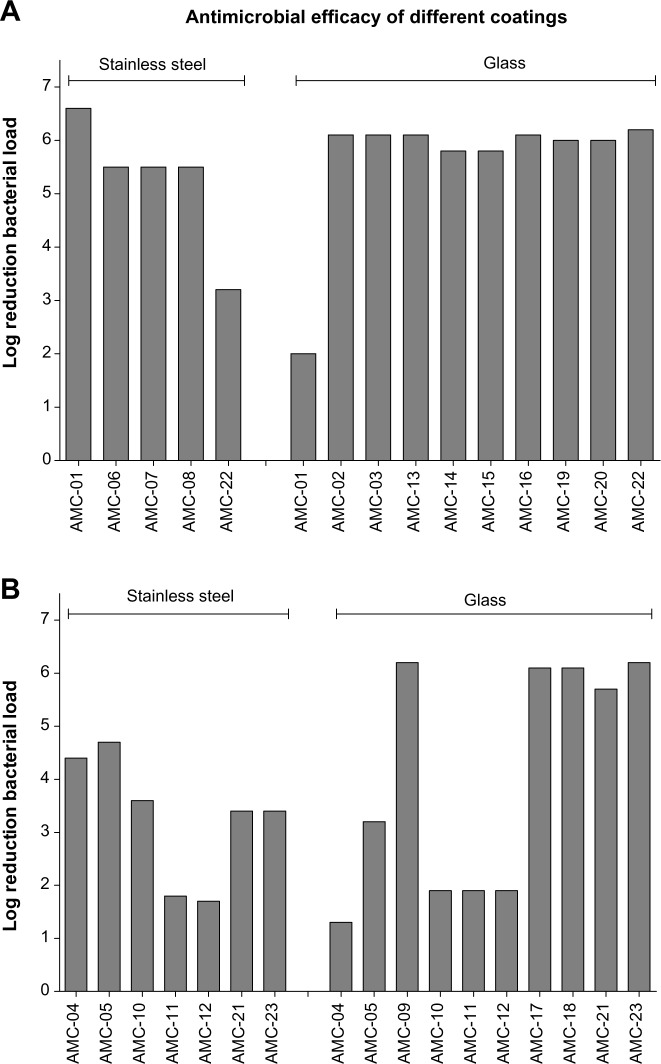
As expected, the activity, as determined by the ISO protocol, differed considerably among coatings, where the log reduction (R) values attained varied between 1.3 and 6.1 as a maximum (Table 1 and Figure 2). The interpretation of the variation in R-values was performed by focusing on the nanosized coatings (Figure 2A) or on the non-nanosized coatings (Figure 2B) based on nanosized active ingredients and those based on classical biocide ingredients.
Figure 2.
Logarithmic reduction of bacterial load after incubation on provided coatings.
Notes: Bacterial suspensions (400 µL, of approximately 105 CFU/mL) were cultured on substrates (stainless steel or glass, as indicated by hatches in top of graphs) coated with antimicrobial agents. Logarithmic reduction in bacterial load after 24 hour incubation, compared with the control substrate (stainless steel or glass alone) is depicted. Bars represent values of pooled triplicate samples obtained from (A) nanomaterial-based coatings and (B) coatings based on other antimicrobial agents.
Abbreviation: AMC, antimicrobial coating.
Within the nanocoatings group (R-value range 2.0–6.1) (Figure 2A), minimal R-value differences can be observed (median [interquartile range {IQR}] =6 [5.5–6.1]), and bacterial killing performance seems to be independent of the substrate onto which the nanocoating was applied (Figure 2A). Only antimicrobial coating (AMC)-01 (TiO2) and AMC-22 (silver) deviated in their killing performance (R) in comparison with other nanocoatings within the subset of tested coatings based on nanosized active ingredients. AMC-01 has a lower log reduction on glass (R=2.0), and AMC-22 (silver) has a lower log reduction on stainless steel (R =3.2). When considering the conventionally based coatings (range =1.3–6.6) (Figure 2B), our analysis shows that the variation in log reduction values is much larger among the coatings within this subset (median [IQR] =3.4 [1.9–5.9]). This observation is in contrast with the overall performance of the nanomaterial-based coatings.
Interestingly, when making a distinction between the silver (nanomaterial) and the nonsilver (non-nanomaterial and TiO2) coatings, the silver-based coatings appear to have far more potent bacterial killing effects. For nanosilver-based coatings, log reduction values range from 3.2 to 6.2 (median [IQR] =6.0 [5.5–6.1]) (Figure 3A). However, for the nonsilver-based coatings, significantly lower log reduction values are observed; the range of the R-values is 1.3 to 6.6, and the median (IQR) corresponds to 3.4 (1.9–6.1) (P=0.0324, by Mann–Whitney U test) (Figure 3A).
Figure 3.
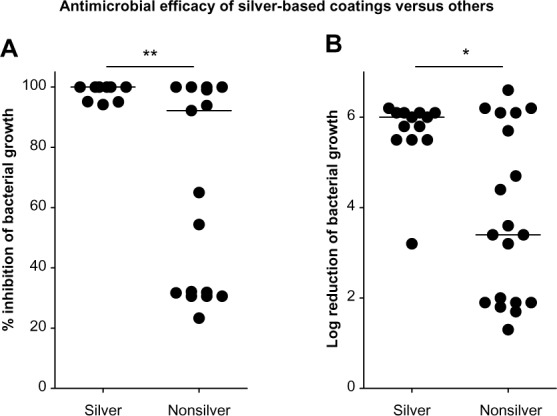
Relative inhibition of bacterial load by nanosilver versus other coatings.
Notes: Bacterial suspensions (400 µL of approximately 105 CFU/mL) were cultured on substrates (stainless steel or glass). Data obtained using stainless steel- and glass-based coatings were pooled for this analysis (A) Log reduction and (B) Percent reduction in bacterial load after 24 hour incubation, relative to the appropriate control substrate (stainless steel or glass) is depicted. Dots represent values of pooled triplicate samples. Bold lines represent median values. *P<0.05, **P<0.01 (Mann–Whitney U test).
Next, we determined the percentage of total killing as defined by the number of bacteria killed by the tested coating in relation to the total number of bacteria present on control surfaces. The difference in antibacterial performance between the nanosilver-based and nonsilver-based coatings, became even more evident (Figure 3B). For the silver coatings, the percentage killing values range from 94% to 100%, with a corresponding median (IQR) of 100% (98%–100%). For the nonsilver coatings, a significantly less potent killing is observed; the range included 23% to 100%, with a median (IQR) of 92% (32%–100%) (P=0.0077 by Mann–Whitney U test). However, these observations need to be considered carefully since measurements were generated under optimal conditions, and no measurements were done after aging or wearing of the tested coatings.
Discussion
After comparative analysis of the antibacterial activity of the provided coatings, the following results were obtained. Very high log reduction values (R), of 6 or more, were found for coatings based on silver (n=7), TiO2 (n=1), triclosan (n=1), quaternary ammonium (n=2), and zinc pyrithione (n=1). These R-values corresponded with 100% effective killing of the bacteria. Of note, in some other cases with 100% killing of E. coli, the log reduction values were underestimated (Table 1). This discrepancy can be due to a low starting number of bacteria directly after inoculation, which can result from a reduced adherence to the surface (in the case of AMC-04 and AMC-05)14,15 or to a technical limitation of the study. Also, the substrate specificity of TiO2 was unexpected and cannot be explained. Unfortunately, we had no additional substrates to revalidate these samples.
Based on our panel of coatings and submission by suppliers, nanosilver-based coatings appear to dominate the market of antimicrobial coatings. However, we need to be aware that this study is no way a randomized or representative selection of all coatings on the market. Nevertheless, it is remarkable that almost all of the nanocoatings were based on active silver, although exact details and embedding have not been disclosed to us. We observed a solid high performance of silver coatings, as determined by the ISO 22196:2011. However, the precise mechanism has remained elusive.16 Quite recently, Morones-Ramirez et al17 demonstrated that silver actively disrupts multiple cellular processes required for bacterial survival. Considering the current threat of resistance to antibiotics, these researchers also demonstrated that silver can synergize with antibiotic agents both in vitro and in vivo. More importantly, in a mouse model when researchers induced biofilms on catheters, they were able to restore the susceptibility of bacteria resistant to systemic gentamicin treatment by coadministration of silver. Given the added benefit of silver through restoring susceptibility to antibiotics, our data partly clarify its apparent domination as an active ingredient in antimicrobial products. This is shown by our finding of consistent performance of high antimicrobial activity by silver coatings.
This does not immediately imply that the use of silver should be advocated before a thorough benefit–risk analysis has been done.18 It is questionable whether the widespread use of nanosilver in consumer products may lead to adverse effects in ecosystems as well as to humans. The extensive use of silver (as was observed within our study, the majority were silver-based) may actually contribute to the spread and/or development of silver-resistance mechanisms. The present prevalence of silver resistance is low.19 Although resistance rates among clinical isolates have remained low despite long-term use, silver-resistance determinants may be located on mobile genetic elements that confer resistance to multiple other antibiotics. In accordance, silver-resistant bacterial strains have been isolated from (hospital) sewage systems.20,21 Another concern is the unresolved environmental effect of the widespread application of silver. At the moment, the data set that could be used to arrive at a valid risk assessment for human exposure is still too limited.22 However, recent subchronic toxicity studies in rats have shown that repeated administration of small amounts of soluble silver can alter the immune system.23 Also, the Ecotox Database is increasing, and work has now been done in zebra fish embryos, Caenorhabditis elegans and Daphnia spp. For example, both nanoparticle silver and soluble silver can affect different Daphnia subspecies development,24 and nanosilver has the highest effect on the fifth generation of animals of Daphnia magna.24 Taken together, we support a thorough benefit–risk analysis before a greater adoption of silver for various applications. Also, we did not quantify the amount of silver ions released from different coatings or the durability of antimicrobial efficacy of the different coatings. This could lead to an understanding of the minimal amount of silver needed to evoke bactericidal action, with limitation of leakage into the environment. Although also of importance to justify the large scale use of antimicrobial coatings, these experiments are beyond the scope of the current report.
As mentioned briefly above, this study has several shortcomings. First of all, the study is not representative of the total field of coatings and applications. In addition, for reasons of materials transfer, we allowed producers to perform the coating process to their best technical skills. Although this may have led to the introduction of nonsystematic error, as we had no control of the quality of this process, optical inspection did not reveal inhomogeneous or incomplete coatings.
Another limitation of this study is that the ISO protocol reflects an artificial situation under best-case laboratory conditions. This limits extrapolation of the data to performance in a real practical application of the coatings. The parameters used in the test, like humidity, temperature, presentation of the inoculums, and duration of incubation, do not reflect real-life application conditions, which could have led to overestimation of the efficacy of an antibacterial coating.13 In this sense, the close reinforced contact between bacteria and the film at high-load conditions is the opposite to low, incidental deposition of bacteria on different shaped surfaces that may occur outside the research setting. Therefore, it should be recommended to use the ISO 22196 only as a measure for the intrinsic, maximal efficacy. To further gain insight into the antibacterial efficacy of coatings in real-life scenarios, other tests that more closely mimic conditions, such as field tests, preferably in health care environments, are warranted. Within such tests, the durability and the effect of wear, cleaning, temperature and humidity on coatings need to be assessed. It is recommended that antibacterial surfaces should be evaluated using one rational and unified approach, preferably less time-consuming than the ISO 22196:2011, leading to benchmarking of antimicrobial coatings for specific applications. Comparative trials offer an easy and reliable method for a complete benchmark of the efficacy of antibacterial coatings on specific surfaces. For this, we would like to refer to work we performed on a similar set of coatings using a polymerase chain reaction (PCR) based assay that allows rapid quantitative detection of bacterial load (Muijers–Chen et al, unpublished data, 2013). It is the authors’ current intention to perform similar testing in a real-life environment and to consult with potential users before application of specific antimicrobial products. A widespread application of nanocoatings in our battle against antimicrobial resistance deserves an approach in which all aspects of the microbe’s life cycle are considered.
Acknowledgments
We thank the Foundation Innovation Alliance (SIA - Stichting Innovatie Alliantie) for the Regional Attention and Action for Knowledge circulation (RAAK) between Universities of Applied Science and Small and Medium Enterprises (MKB - Midden en Klein Bedrijf) grant: RAAK-MKB-AntiMicrobials.
Footnotes
Author contributions
Jacques Seezink was responsible for execution and interpretation of the experiments. Birgit EJ Teunissen was responsible for quality control of the data. Johan W Molling was the main author of the manuscript and did the analysis of the data. Birgit EJ Teunissen and Paul JA Borm participated in the study planning, management, and communications with suppliers. Inhua Muijrers-Chen provided guidance during the experimental portion of the research, was involved in the discussion of the results, and in the critical review of the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lee CR, Cho IH, Jeong BC, Lee SH. Strategies to minimize antibiotic resistance. Int J Environ Res Public Health. 2013;10(9):4274–4305. doi: 10.3390/ijerph10094274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tacconelli E, Cataldo MA, Dancer SJ, et al. European Society of Clinical Microbiology ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014;20(Suppl 1):S1–S55. doi: 10.1111/1469-0691.12427. [DOI] [PubMed] [Google Scholar]
- 3.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhalla A, Pultz NJ, Gries DM, et al. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol. 2004;25(2):164–167. doi: 10.1086/502369. [DOI] [PubMed] [Google Scholar]
- 5.Scott E, Bloomfield SF. The survival and transfer of microbial contamination via cloths, hands and utensils. J Appl Bacteriol. 1990;68(3):271–278. doi: 10.1111/j.1365-2672.1990.tb02574.x. [DOI] [PubMed] [Google Scholar]
- 6.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35(4):322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11(7):1034–1043. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 8.Knetsch MLW, Koole LH. New strategies in the development of antimicrobial coatings: the example of increasing usage of silver and silver nanoparticles. Polymers. 2011;3(1):340–366. [Google Scholar]
- 9.Hasan J, Crawford RJ, Ivanova EP. Antibacterial surfaces: the quest for a new generation of biomaterials. Trends Biotechnol. 2013;31(5):295–304. doi: 10.1016/j.tibtech.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Weber DJ, Rutala WA. Self-disinfecting surfaces: review of current methodologies and future prospects. Am J Infect Control. 2013;41(Suppl 5):S31–S35. doi: 10.1016/j.ajic.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Tiller JC, Liao CJ, Lewis K, Klibanov AM. Designing surfaces that kill bacteria on contact. Proc Natl Acad Sci U S A. 2001;98(11):5981–5985. doi: 10.1073/pnas.111143098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antimicrobial Coatings Market by Type (Silver, Copper, and Others), Application (Indoor air/HVAC, Medical, Mold remediation, Building and Construction, Food and Beverages, Textiles, and Others) and Geography(North America, Europe, Asia-Pacific, and ROW) – Global Trends and Forecasts to 2018. markets and markets.com 13.
- 13.Ojeil M, Jermann C, Holah J, Denyer SP, Maillard JY. Evaluation of new in vitro efficacy test for antimicrobial surface activity reflecting UK hospital conditions. J Hosp Infect. 2013;85(4):274–281. doi: 10.1016/j.jhin.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen TD, Perrin FX, Nguyen DL. New hybrid materials based on poly(ethyleneoxide)-grafted polysilazane by hydrosilylation and their anti-fouling activities. Beilstein J Nanotechnol. 2013;4:671–677. doi: 10.3762/bjnano.4.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee I, Pangule RC, Kane RS. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv Mater. 2011;23(6):690–718. doi: 10.1002/adma.201001215. [DOI] [PubMed] [Google Scholar]
- 16.Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 17.Morones-Ramirez JR, Winkler JA, Spina CS, Collins JJ. Silver enhances antibiotic activity against gram-negative bacteria. Sci Transl Med. 2013;5(190):190ra81. doi: 10.1126/scitranslmed.3006276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seltenrich N. Nanosilver: weighing the risks and benefits. Environ Health Perspect. 2013;121(7):A220–A225. doi: 10.1289/ehp.121-a220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakobsen L, Andersen AS, Friis-Møller A, Jørgensen B, Krogfelt KA, Frimodt-Møller N. Silver resistance: an alarming public health concern? Int J Antimicrob Agents. 2011;38(5):454–455. doi: 10.1016/j.ijantimicag.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Lima de Silva AA, de Carvalho MA, de Souza SA, et al. Heavy metal tolerance (Cr, Ag AND Hg) in bacteria isolated from sewage. Braz J Microbiol. 2012;43(4):1620–1631. doi: 10.1590/S1517-838220120004000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eze E, Eze U, Eze C, Ugwu K. Association of metal tolerance with multidrug resistance among bacteria isolated from sewage. Journal of Rural and Tropical Public Health. 2009;8:25–29. [Google Scholar]
- 22.Schäfer B, Brocke JV, Epp A, et al. State of the art in human risk assessment of silver compounds in consumer products: a conference report on silver and nanosilver held at the BfR in 2012. Arch Toxicol. 2013;87(12):2249–2262. doi: 10.1007/s00204-013-1083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Jong WH, Van Der Ven LT, Sleijffers A, et al. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials. 2013;34(33):8333–8343. doi: 10.1016/j.biomaterials.2013.06.048. [DOI] [PubMed] [Google Scholar]
- 24.Völker C, Boedicker C, Daubenthaler J, Oetken M, Oehlmann J. Comparative toxicity assessment of nanosilver on three Daphnia species in acute, chronic and multi-generation experiments. PLoS One. 2013;8(10):e75026. doi: 10.1371/journal.pone.0075026. [DOI] [PMC free article] [PubMed] [Google Scholar]