Abstract
We report a reaction of direct electrophilic fluorination of phenolsulfonphthalein at mild conditions. This reaction affords the synthesis of novel positron-emitting 18F-labeled pH indicators. These compounds are useful for non-invasive in vivo pH measurement in biological objects.
Keywords: Phenol Red, Fluorine, Electrophilic fluorination, pH measurement
Introduction
Electrophilic fluorination under mild conditions allows the introduction of the positron emitting isotope fluorine-18 into molecules. However, this synthetic method is rarely used due to the nature of fluorine gas. Unlike other halogens, fluorine usually reacts with complex organic molecules in a non-specific way, completely destroying the target precursor. As a result, only few 18F-labeled compounds are currently synthesized by this method [1].
18F is well known as a tracer for detection of the labeled molecule by γ-radiation, which is produced due to annihilation of the emitted positron with an electron (positron emission tomography, PET). Addition of this isotope label can significantly increase the possibilities of application of molecular probes. For example, 18F-labeled pH indicators could be used for non-invasive in vivo pH measurement in water solutions by combination of radioactive (for probe quantification) and optical signals. Another potential application of this type of compounds is for the measurement of blood volume [2].
In the current work we report the direct fluorination of phenolsulfonphthalein (also known as and Phenol Red, IUPAC name 3,3-bis(4-hydroxyphenyl)-3H-2,1λ6-benzoxathiol-1,1-dione) and characterization of the fluorine-containing products of this reaction. This reaction allows synthesis of novel fluorine-18 labeled pH indicators for non-invasive in vivo pH measurement in biological objects.
Materials and methods
All the reagents were purchased from Sigma-Aldrich and used as received.
Fluorination of Phenol Red (PR) was performed in conditions, similar to direct fluorination with [18F]-F2 gas under acidic conditions [3–5]. Diluted fluorine gas (0.1% F2 in Ne or Ar) was bubbled through a freshly prepared solution of PR sodium salt in glacial acetic acid (2 mg/mL) for 5 minutes. The total amount of fluorine in the added gas mixture was determined independently after its absorption by KI solution and titration of released iodine with sodium thiosulfate. In a typical experiment, 35 μmol of F2 were added to different volumes of PR solution with the ratio of [F2]0:[PR]0 varied from 1:1 to 2:1. The reaction mixture was then evaporated under vacuum at 120 °C, redissolved in 2 mL water, injected onto a semi-preparative HPLC column (Phenomenex, Synergi 4 μm Hydro-RP 80 Å 10×250 mm), and eluted with 25 % ethanol-water at 2 mL/min rate detecting absorption at 430 nm. Under these conditions elution times were about 15 min for PR, and from 18 to 30 min for fluorinated products. Separation of individual UV signals from semi-preparative HPLC was not possible due to the very high extinction coefficients of the indicators. In order to analyze the product mixture successive fractions were collected and analyzed by HPLC using an analytical (4.6×250 mm) column of the same type with a water-acetonitrile gradient. Spectroscopic measurements of the products were performed using a Beckman-Coulter DU 720 spectrophotometer using acetate, phosphate, TRIZMA, and borate buffers for different values of pH.
Independent syntheses of the reaction products was performed by a Friedel-Crafts reaction between 2-sulfobenzoic acid cyclic anhydride and fluorophenol (for the difluorinated product) or a mixture of phenol and fluorophenol (1:4 for the monofluorinated product) in the presence of zinc chloride (10 %) catalyst at 170 °C. These reaction mixtures were separated by semi-preparative HPLC using the same methods as the fluorination products and compared by analytical HPLC and spectroscopic measurements.
Results
HPLC analysis of the products of direct fluorination of Phenol Red allowed us to identify the presence of the non-reacted precursor and three main fluorinated compounds. Two products of the reaction were found to be identical with synthetic monofluorophenol red (MFPR; 3-(3-[18F]fluoro-4-hydroxyphenyl)-3-(4-hydroxyphenyl)-1,1-dioxo-2,1λ6-benzoxathiol-7-yl) and difluorophenol red (DFPR; 3,3-bis(3-[18F]fluoro-4-hydroxyphenyl)-1,1-dioxo-3H-2,1λ6-benzoxathiol-7-yl), the third product is presumed to be trifluorophenol red (TFPR; 6-[18F]fluoro-3,3-bis(3-[18F]fluoro-4-hydroxyphenyl)-1,1-dioxo-3H-2,1λ6-benzoxathiol-7-yl). The overall fluorination reaction is described in Figure 1. MFPR and DFPR were characterized by comparison of their HPLC signals (Figure 2) and absorption spectra at different pH (Figure 3) with the same compounds synthesized by the condensation reaction. The behavior of the products was identical in both cases, indicating identical structures of these compounds.
Figure 1.
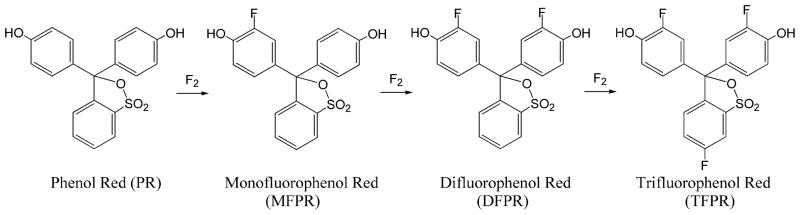
Reaction of direct fluorination of phenolsulfophthalein in acetic acid by 0.1% F2 – Ne gas mixture..
Figure 2.
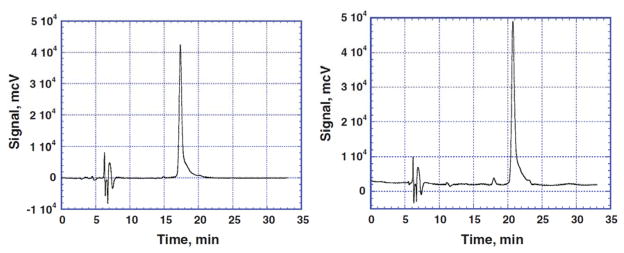
Analytical HPLC signals of the products of PR fluorination separated by HPLC. The left panel shows a diagram of the fraction containing monofluorophenol red (MFPR), the sample on the right panel is represented mostly by difluorophenol red (DFPR). Signals from the products prepared by Friedel-Crafts reaction were identical.
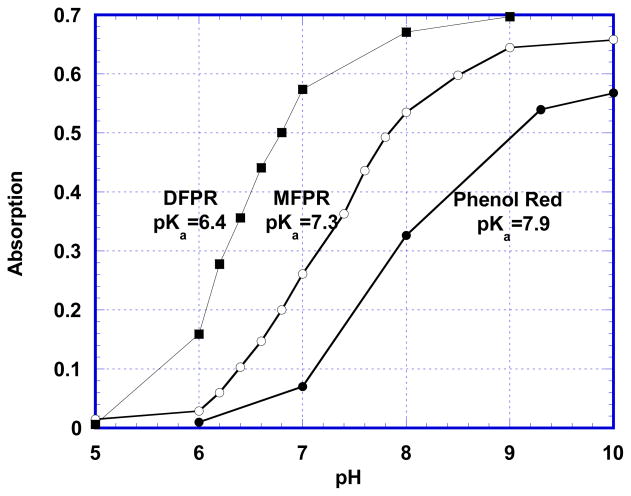
Figure 3.
pH dependence of A560 for Phenol Red, A564 for Monofluorophenol Red (MFPR), and A570 for Difluorophenol Red (DFPR) demonstrating shift of pKa due to introduction of fluorine atom into molecule.
Quantitative analysis of the reaction products at a stoichiometric ratio of fluorine and PR shows that the mixture contains 6% of non-reacted precursor, 8% of MFPR, 11% of DFPR, and 4% of TFPR. The elution times for these compounds on reverse phase HPLC column increased consecutively, which correlates with increased hydrophobic character of the molecule after addition of the fluorine atom [6].
Increasing the relative amount of fluorine resulted in a simultaneous decreasing of the PR signal and the yield of reaction products to only a few percent. A double excess of fluorine (ratio [F2]:[PR] 2:1) resulted in discoloring of the reaction mixture and presence of only trace amounts of the identifiable products.
One of the features indicating that the aromatic system of PR survives the fluorination is the retention of its indicator properties. PR is a widely used acid-base indicator with wavelength change from 433 nm to 558 nm at pKa=7.9. All the above-mentioned products of fluorination also act as pH indicators with increased wavelength and intensity of absorption upon transfer from acidic to basic form. Figure 3 illustrates the dependence of intensity of absorption maxima on the pH of the solution for the basic forms of phenol red and the products of its fluorination. MFPR acts like an indicator with absorption maximum at 436 nm for acidic and 564 nm for basic form. The transition between these two forms occurs at pKa = 7.3. The basic form of DFPR has an absorption maxima at 428 nm and converts into basic form with a 570 nm absorption maxima at pKa = 6.4. The trifluorinated derivative also acted as an indicator with absorption maximum for basic form at 572 nm and pKa = 5.9.
Discussion
The electrophilic fluorination reaction with fluorine gas is not a straightforward process, significantly dissimilar to addition of other halogens to double bonds or substitution in aromatic cycles. Due to the very high negative enthalpy of the reaction, fluorine gas is able to cause a cleavage of carbon-carbon bonds, which results in nonspecific fluorination and subsequent destruction of the target molecule. This unwanted behavior can be prevented by performing the reaction with diluted fluorine gas at the temperature of dry ice [7]. However, these conditions lead to significant limitations resulting from the decreased solubility of target compounds at low temperature. Previously we found that strong acidic media (trifluoroacetic acid) can be considered as one factor, which favors nondestructive addition of fluorine to double bonds at room temperature [8]. This effect occurs for target molecules which are able to bind a proton in acidic media [5]. The protonated molecule acquires the positive charge with subsequent electron impoverishment and decreased energy of the fluorination reaction. As the result, the target molecule becomes more resistant to destructive electrophilic attack by fluorine. The same type of stabilization most likely occurs in the case of fluorination of nucleosides in acetic acid [3, 4], which becomes a successful process due to cytidine protonation (pKa=4.2 [9]) under these conditions.
The results of our current work represent another reaction of direct fluorination of a complex molecule in acidic conditions at room temperature. Phenol red exists in acidic media as a zwitterion with a structure shown in Figure 4 on the right [10]. Most likely a positive charge is not located solely on the oxygen atoms, but rather distributed through the aromatic system, as it is shown for other resonance forms in Figure 4. As result an entire aromatic system will have a delocalized positive charge, which favors its mild fluorination with dilute fluorine gas. A significance of this structural feature is evident from our unsuccessful attempts to perform the same fluorination reaction with a similar indicator phenolphthalein. Although the formal difference between these two compounds is only the presence of a carboxyl instead of a sulfonic group, the result of the reaction was quite opposite. We could detect only gradual destruction of phenolphthalein by fluorine gas without the appearance of any fluorinated indicator product. This distinction can be explained by different structure of acidic forms of these two indicators: phenolphthalein does not form a zwitterion and exists as an uncharged cyclic lactone [10], which subsequently can not survive an attack of fluorine due to lack of positive charge in the aromatic system.
Figure 4.

Resonance structures of Phenol Red in strongly acidic media.
The right structure is proposed in [10] as the main one; most likely the positive charge should be distributed through the aromatic system with 4 resonance structures shown in the middle and one on the left
The addition of one fluorine atom to the molecule does not inactivate it for further substitution, as often occurs for other halogens. Due to its high electronegativity, the fluorine atom will make an aromatic system even more electron-deficient and increases its ability to survive attack by another fluorine molecule. As result of this rather paradoxical effect we can see an immediate fluorine introduction into other rings with formation of a mixture of mono-, di-, and trifluorosubstituted products with higher yield of DFPR at equimolar ratio of the reagents. An increased amount of fluorine does not shift the reaction to higher overall yield of polyfluorinated products, but causes their destruction. This kind of subsequent substitution would not be appropriate for bulk preparation of the reaction products, but can be applied for processes on the micro scale level, for example the labeling of the molecule with 18F. Electrophilic fluorination with [18F]-F2 gas is performed on milligram quantities of reagents and usually produces a complex mixture of products with similar structure. Separation of this kind of mixture always requires application of semi-preparative HPLC, so all the products of interest can be isolated from the mixture. We were able to perform a proper separation of mono-, di-, and trifluorinated phenol red derivatives using a Phenomenex semi-preparative hydroxylated C-18 column.. As it is evident from Figure 2, the products of fluorination are essentially pure, which validates the separation method and allows their further application as pH indicators.
Introduction of a halogen atom into a molecule of phenol red causes an alteration of its indicator properties. PR is commonly used in cell culture media because its transition of absorption maximum from 433 nm to 558 nm with pKa = 7.9 [11] visually indicates an undesirable pH increase. Other widely used indicators of this type are Bromophenol Red (BR) and Chlorophenol Red (CR), which have one halogen atom in each OH-containing ring. These indicators have a transition from UV absorption to 574 nm at pKa=6.3 for BR and from UV to 573 nm at pKa = 6.0 for CR [11]. An overall effect of introduction of two halogen atoms into the molecule of PR is quite obvious: it causes a hypsochromic shift for the acidic form and a bathochromic for the basic form thus overall decreasing of pKa value. Our current results show a definite similarity of the effects of fluorine and other halogens. Introduction of one fluorine atom into a molecule of PR changes its absorption maxima to 436 nm and 564 nm, shifting the transition between these forms to pKa = 7.3 for MFPR. The presence of two fluorine atoms in the molecule of DFPR increases the shift of absorption maxima to 428 nm for acidic form, 570 nm for basic form, and the value of pKa to 6.4. The third product of fluorination has even higher shift of both wavelength (to 572 nm) and pKa (to 5.9), suggesting an addition of a third fluorine atom into the molecule, presumably into position 4 of sulfonated ring, as is shown in Figure 1.
Although the directions of the shifts for maxima and pKa are the same for fluorine and other halogens, one could expect stronger effects of more electronegative fluorine in comparison with bromine and chlorine. In reality it is actually smaller for both absorption wavelengths and pKa, as it is obvious from comparison of characteristics of DFPR with CR and BR. This effect is another illustration of the certain differences between fluorine and other halogens. Unlike chlorine and bromine, fluorine does not act as a donor of electron pair for benzene ring, which can explain its specific influence on indicator properties. For our purposes this difference appears to be quite beneficial, because monofluorinated derivative has its pKa exactly at normal physiological pH, while difluorinated product has the pKa at the maximal level of acidosis that can be tolerated by cells. Subsequently, both derivatives can serve as pH indicators at physiological conditions.
Conclusion
Direct fluorination of phenol red in acidic conditions results in production of its mono-, di- and trifluorinated derivatives at mild yields. Successful introduction of fluorine atoms into phenol red retains its indicator properties, gradually decreasing pKa and increasing wavelength of absorption at basic conditions. These novel fluorine-containing pH indicators can be labeled with radioactive 18F and used for non-invasive in vivo pH measurement in biological objects.
Acknowledgments
Grant Support: Supported by the Institute for Translational Medicine and Therapeutics (ITMAT) Transdisciplinary Awards Program in Translational Medicine and Therapeutics Translational Biomedical Imaging Core Grant (TAPITMAT-TBIC)
Literature
- 1.Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N Radiolabels for Positron Emission Tomography. Angew Chem Int Ed. 2008;47:8998–9033. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- 2.Heinrich TK, Gottumukkala V, Snay E, Dunning P, Fahey FH, Treves ST, Packard AB. Synthesis of Fluorine-18 Labeled Rhodamine B: A Potential PET Myocardial Perfusion Imaging Agent. Appl Radiat Isot. 68:96–100. doi: 10.1016/j.apradiso.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visser GWM, Boele S, v Halteren BW, Knops GHJN, Herscheid JDM, Brinkman GA, Hoekstra A. Mechanism and Stereochemistry of the Fluorination of Uracil and Cytosine Using Fluorine and Acetyl Hypofluorite. J Org Chem. 1986;51:1466–1471. [Google Scholar]
- 4.Visser GWM, Noordhuis P, Zwaagstra O, Herscheid JDM, Hoekstra A. A Simplified Synthesis of 18F-Labelled Cytosine- and UraciI-Nucleosides. Appl Radiat Isot. 1986;37:1074–1076. [Google Scholar]
- 5.Dolbier WR, Li A-R, Koch CJ, Shiue C-Y, Kachur AV. [18F]-EF5 - a Marker for PET Detection of Hypoxia: Synthesis of Precursor and a New Fluorination Procedure. Appl Radiat Isot. 2001;53:73–80. doi: 10.1016/s0969-8043(00)00102-0. [DOI] [PubMed] [Google Scholar]
- 6.Mann J. Modern Methods for the Introduction of Fluorine into Organic Molecules: An Approach to Compounds with Altered Chemical and Biological Activities. Chem Soc Rev. 1987;16:381–436. [Google Scholar]
- 7.Rozen S, Brand M. Direct Addition of Elemental Fluorine to Double Bonds. J Org Chem. 1986;51:3607–3611. [Google Scholar]
- 8.Kachur AV, Dolbier WR, Xu W, Koch CJ. Catalysis of Fluorine Addition to Double Bond: an Improvement of Method for Synthesis of 18F PET Agents. Appl Radiat Isot. 2010;68:293–296. doi: 10.1016/j.apradiso.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oyelere AK, Strobel SA. Biochemical Detection of Cytidine Protonation within RNA. J Am Chem Soc. 2000;122:10259–10267. [Google Scholar]
- 10.Tamura Z, Maeda M. Differences Between Phthaleins and Sulfonphthaleins. Yakugaku Zasshi. 1997;117:764–770. doi: 10.1248/yakushi1947.117.10-11_764. [DOI] [PubMed] [Google Scholar]
- 11.Dean JA. Lange’s Handbook of chemistry. 13. McGraw-Hill Book Company; NY: 1985. [Google Scholar]