Abstract
Context
Celastrol, a natural compound derived from the herb Tripterygium wilfordii, is known to have anticancer activity, but is not soluble in water.
Objective
Formation of celastrol liposomes, to avoid the use of toxic solubilizing agents.
Materials and methods
Two different formulations of pegylated celastrol liposomes were fabricated. Liposomal characteristics and serum stability were determined using dynamic light scattering. Drug entrapment efficacy and drug release were measured spectrophotometrically. Cellular internalization and anticancer activity was measured in prostate cancer cells.
Results
Liposomal celastrol displayed efficient serum stability, cellular internalization and anticancer activity, comparable to that of the free drug reconstituted in dimethyl sulfoxide.
Discussion and conclusion
Liposomal celastrol can decrease the viability of prostate cancer cells, while eliminating the need for toxic solubilizing agents.
Keywords: celastrol, liposomes, prostate cancer
Introduction
Small molecule drugs have several disadvantages and limitations, such as rapid renal clearance and low tumor accumulation. Therefore, there exists a need to develop delivery systems that could improve the pharmacodynamic properties of a therapeutic agent. One potential small molecule drug candidate for cancer therapy is celastrol (tripterine), which is derived from the classic Chinese medicinal herb, Tripterygium wilfordii (Song et al., 2011). Celastrol has generally been publicized for its anti-inflammatory effects, but it was recently shown to posses anticancer activity in various cancer cell lines (Liu et al., 2011). Furthermore, celastrol can augment the antitumor activity of standard chemotherapeutics, such as doxorubicin and paclitaxel (Sethi et al., 2007). In preclinical studies, celastrol has been mixed with toxic solvents and then administered intraperitoneally, because of the highly hydrophobic nature of the drug (Yang et al., 2006, Dai et al., 2009). Hence, the potential of celastrol could be realized by formulating a delivery system that mitigates the drug’s low aqueous solubility, while demonstrating stability and low toxicity in physiological conditions.
Liposomal encapsulation of hydrophobic drugs can circumvent the problem of low water solubility. Liposomes are generally formulated from cholesterol and biologically inert lipids, but may also be conjugated with polymers. The bilayer structure of liposomes enables the loading of both hydrophobic and hydrophilic agents. Hydrophobic drugs can be embedded within the bilayer and hydrophilic drugs can be loaded into the aqueous core. Currently, six liposomal formulations of anticancer drugs have received clinical approval (Chang and Yeh, 2012, Koudelka and Turanek, 2012). There is also a substantial amount of literature demonstrating the beneficial properties of liposomal chemotherapeutics in the preclinical settings. For instance, liposomal formulations of anthracycline chemotherapeutics and gemcitabine have led to a marked improvement in tumoricidal activity (Cosco et al., 2009a, Paolino et al., 2010, Pulini et al., 2009).
Compositionally modifying liposomes based upon the target and the enclosed agent can provide an efficient means of physiological transport. Variations in lipid make-up and liposomal size have amounted to differential uptake by the non-specific immune system, allowing for a more controlled deposition into organs such as the lungs and liver (Schroit and Fidler, 1982), which typically accumulate nanoparticles. The phospholipid composition can be tailored to increase the blood circulation time and the accumulation of liposomes in the tumor. Liposomes and other vesicular carriers are often coated with polyethylene glycol (PEG), which mimics the glycoproteins on the surface of cells, thereby reducing uptake by the reticuloendothelial system (RES) that recognizes foreign particles (Pasut and Veronese, 2012, Celia et al., 2013, Molinaro et al., 2013, Cosco et al., 2009b, Carafa et al., 2002, Carafa et al., 2004). Liposomes coated with this polymer are dubbed ‘stealth liposomes’ due to their ability to evade the immune system and to provide more favorable circulatory characteristics than their standard counterparts (Immordino et al., 2006). However, it should be noted that antibodies against pegylated particles might arise after repeated administration. This effect, termed the accelerated blood clearance (ABC) phenomenon, involves the rapid immunological recognition and uptake of particles (Abu Lila et al., 2013a, Abu Lila et al., 2013b, Ichihara et al., 2013).
To date, two studies have shown that liposomal formulations with celastrol exhibit antitumor efficacy in a glioma and a lung carcinoma model (Huang et al., 2012, Song et al., 2011). Here, we encapsulated celastrol in two types of pegylated (PEG2000) liposomes: i) 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC)/cholesterol and ii) 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC)/cholesterol. The formulation that demonstrated higher stability and encapsulation efficacy was chosen for in vitro experiments. This paper represents the first study evaluating the anticancer efficacy of celastrol liposomes in prostate cancer. The goal was to assess whether liposomal celastrol could have similar anticancer efficacy as the free drug, which requires toxic solubilizing agents, such as dimethyl sulfoxide (DMSO). DMSO has been demonstrated to have harmful effects in primates (Vogin et al., 1970) and be toxic when applied topically at high concentrations (Swanson, 1985), thus making it an unsuitable agent for clinical applications. The exchange of harmful solvents for biocompatible phospholipids provides a means for reducing adverse side effects, while maintaining therapeutic efficacy.
Materials & Methods
Materials
Celastrol (3-hydroxy-9β,13α-dimethyl-2-oxo-24,25,26-trinoroleana-1(10),3,5,7-tetraen-29-oic acid) and cholesterol were purchased from Sigma Aldrich, while DOPC, DSPC and 1,2-Distearoyl-phosphatidylethanolamine-methyl-polyethyleneglycol conjugate-2000 (DSPEmPEG2000) were obtained from Avanti Polar Lipids. Nuclear Track-Etch membranes for extrusion were acquired from Whatman.
Liposome preparation
The two compositions of the celastrol liposomes were as follows: i) DOPC, cholesterol, and DSPEmPEG2000 (7:4:3 molar ratio) and ii) DSPC, cholesterol, and DSPEmPEG2000 (6:3:1 molar ratio). Celastrol was added at a concentration of 1 mg/40 mg of phospholipids. The components were subsequently placed in a round bottom flask and dissolved using a mixture of methanol and chloroform (1:2 v/v). The lipid film was made with a Rotavapor® (55 °C, 50 rpm).
The formulations were hydrated with phosphate buffered saline (PBS, 1 ml/20 mg phospholipids) and subsequently heated in a 60 °C water bath for 3 min and mixed with a vortex mixer for 3 min. The hydration process was repeated three times. The formulations were then extruded through a series of polycarbonate membranes to increase homogeneity (pore size: 400 nm, 200 nm and 100 nm) using an extrusion device from Lipex Biomembranes (Northern Lipids Inc., Vancouver, BC, Canada).
Liposomal characterization
The average size (Zavg) and polydispersity index (PDI) of celastrol-loaded liposomes in Milli-Q water were determined by dynamic light scattering using a Zetasizer Nano ZS (ZEN 3600, Malvern Instruments). Zeta potential determination was done with the same instrument by evaluating the electrophoretic mobility and applying the Smoluchowski constant. For this analysis, the samples were diluted using phosphate buffer and placed within Zetasizer-Nano Folded Capillary Cells. The measurements were taken in duplicates (10 runs for each measurement). For analysis of stability, the liposomes were stored at 4 °C for 14 days, after which the size, PDI, and zeta potential were measured.
Drug entrapment efficiency
Celastrol liposomes were purified using ultracentrifugation (45,000 rpm, 4 °C, 1 h) to separate the vesicles from the supernatant containing the unentrapped drug. The remaining pellet was then solubilized with methanol to release the drug from the liposome, allowing the drug concentration to be determined by creating a standard curve in methanol. The absorbance was read with a spectrophotometer at a wavelength of 424 nm.
Serum stability
The stability of celastrol liposomes in a serum solution, consisting of 70 % fetal bovine serum (FBS) and 30 % phosphate buffered saline (PBS), was determined by adding 250 μl of the liposome formulation to 1 ml of serum solution. The resulting mixture was incubated at 37 °C and stirred with an electronic stirrer (700 rpm), to mimic the conditions present in the blood circulation. Samples were taken at set time points and diluted in MilliQ water (1:50 v/v) for dynamic light scattering analysis of size and poldispersity, using a Zetasizer Nano ZS (ZEN 3600, Malvern Instruments).
Drug release
Celastrol liposomes (1 ml) were placed in a pre-wetted dialysis tube with a cellulose ester membrane that had a molecular weight cutoff of 100 kD (Float-A-Lyzer, Spectrum Laboratories, Inc.). This membrane was used in order to allow serum proteins to interact with the liposomes (e.g. albumin, 66 kDa), thus mimicking an in vivo environment. The tube was immersed in 200 ml of either a water/ethanol mixture (4:1 v/v) or a PBS/fetal bovine serum mixture (7:3 v/v). The solution was stirred with a magnetic stirrer at 600 rpm. At set time points, 1 ml of solution was taken from the surrounding liquid for analysis, and replaced with fresh solution. Subsequently, the quantification of the drug amount was done by creating a standard curve with the above-mentioned solutions, followed by spectrophotometric reading of the absorbance at a wavelength of 424 nm.
Cell viability
The in vitro anticancer efficacy of celastrol formulations was measured using a Cell Counting Kit-8 (CCK8, Dojindo Molecular Technologies). Vertebral-cancer of the prostate (VCaP) cells were seeded in 96-well plates (1*105 cells/ml). Cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) with 10 % fetal bovine serum (FBS) at 37 °C and 5 % CO2. The cells were incubated with five different concentrations of celastrol dissolved in dimethyl sulfoxide (DMSO, 0.1 % v/v) or liposomal celastrol (0, 0.1, 0.5, 2.5, 5.0 mM). The CCK8 assay was performed according to the manufacturer’s instructions.
Cellular internalization
Tests for intracellular accumulation were done as previously described in the literature (Celia et al., 2008). VCaP human prostate cancer cells were seeded in 24-well plates (1.365×107 cells/ml) and incubated for different time spans (0.5, 1, 2, 4, 6, 24, 48 h). Liposomal and free celastrol were added to the cells at a concentration of 0.5 μM. Following incubation, 100 μl of lysis buffer (10 ml RIPA, 100 μl 100x protease inhibitor, 100 μl triton x-100) were added to each well and the drug concentration was measured spectrophotometrically as described previously.
Statistical analysis
The data is presented as the mean±SD. The statistical significance between two samples was assessed by the paired Student’s t-test.
Results
Liposome characterization
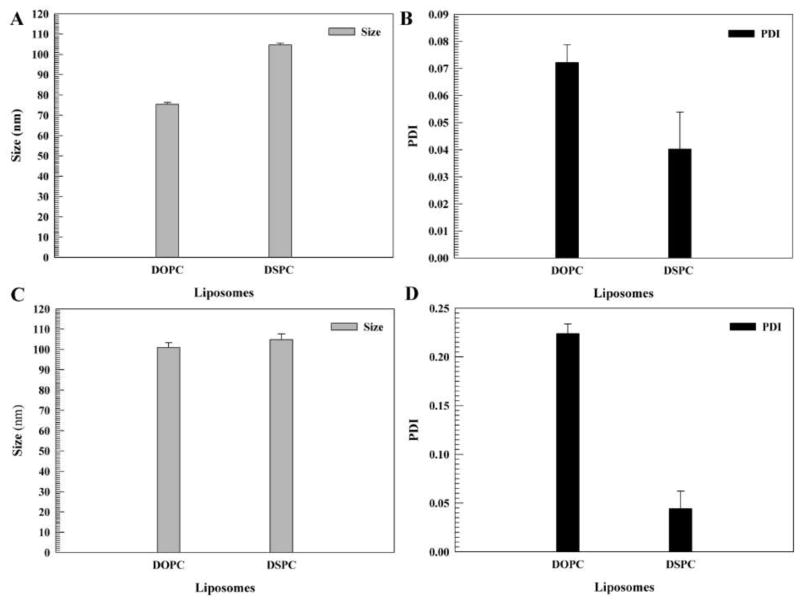
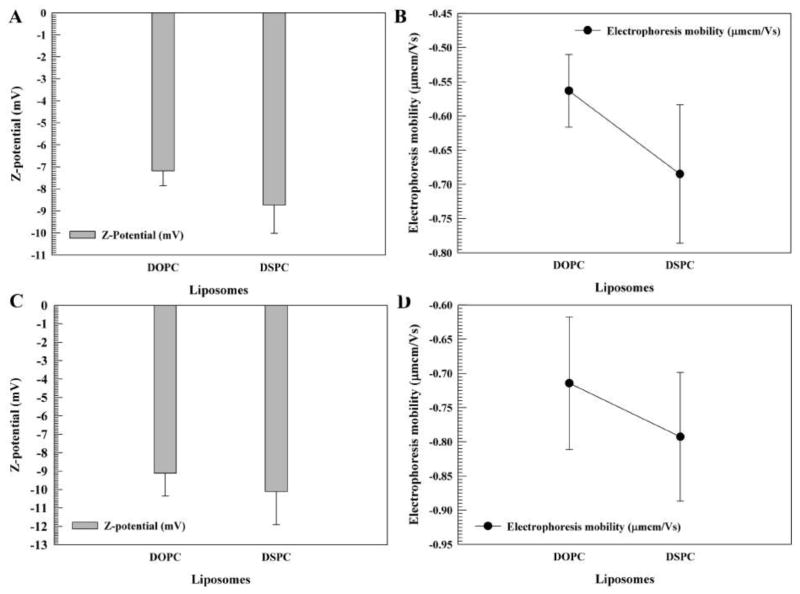
The DOPC and DSPC formulations of liposomal celastrol were semi-transparent orange solutions. Both the DOPC and DSPC formulations exhibited homogeneity with a polydispersity index (PDI) of less than 0.2 (Fig. 1B). The DSPC liposomes were larger in size, approximately 104 nm, while the DOPC liposomes were around 75 nm (Fig. 1A). The zeta potential and electrophoretic mobility values for the DOPC liposomes was approximately −7 mV and −0.6 μmcm/Vs, while the DSPC liposomes had a zeta potential and electrophoretic mobility of approximately −10 mV and −0.7 μmcm/Vs (Fig. 2A–B). After 14 days, the DSPC liposomes maintained their size and PDI, while the DOPC liposomes increased in size by almost 20 nm and had a PDI of more than 0.2 (Fig. 1C–D), suggesting a lack of stability. After a two-week period, the zeta potential and the electrophoretic mobility of the DSPC and DOPC liposomes remained relatively similar (Fig. 2C–D).
Figure 1. Size and polydispersity index (PDI) of liposomes.
Liposomes consisted of pegylated 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC)/cholesterol/celastrol and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC)/cholesterol/celastrol. The size (A, C) and PDI (B, D) of liposomes after 0 days (A, B) and 14 days (C, D). The measurements were taken in duplicate (10 runs for each measurement). The data is presented as the mean±SD. Error bars, if not shown, are located within the symbols.
Figure 2. Zeta potential values and electrophoresis mobility of liposomes.
Liposomes consisted of pegylated 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC)/cholesterol/celastrol and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC)/cholesterol/celastrol liposomes. The zeta potential (A, C) and electrophoretic mobility (B, D) of liposomes after 0 days (A, B) and 14 days (C, D). The measurements were taken in duplicate (10 runs for each measurement). The data is presented as the mean±SD.
Drug entrapment efficiency
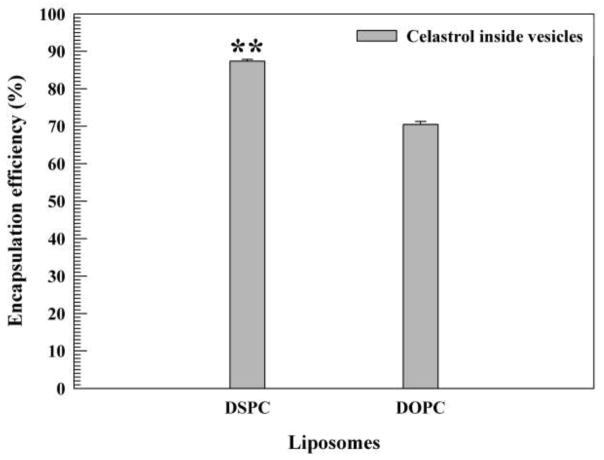
The drug entrapment efficiency of the DSPC and DOPC liposomal celastrol formulations was measured and quantified with a spectrophotometer. The liposomes prepared using DSPC exhibited a greater vesicular drug entrapment than those prepared with DOPC (87.37% and 70.45%, respectively) (Fig. 3). The difference in drug loading capacity between the formulations was statistically significant (P < 0.01).
Figure 3. Drug entrapment efficiency of liposomes.
Liposomes consisted of pegylated 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC)/cholesterol/celastrol and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC)/cholesterol/celastrol liposomes. The data is presented as the mean±SD. **P ≤ 0.01.
Liposome stability in serum
DSPC liposomes were incubated in 70% FBS and the PDI and size were monitored. The liposomes exhibited a slight reduction in size and a small increase in the PDI over time (Fig. 4), which is consistent with previously reported results (Wolfram et al., 2013). These changes were more prominent during the first few hours in serum. Furthermore, the liposomes did not aggregate or break down in serum, as was evident from the relatively consistent Zavg and PDI values and the presence of a single intensity peak in the dynamic light scattering measurements.
Figure 4. Liposomal stability in 70% fetal bovine serum (FBS).
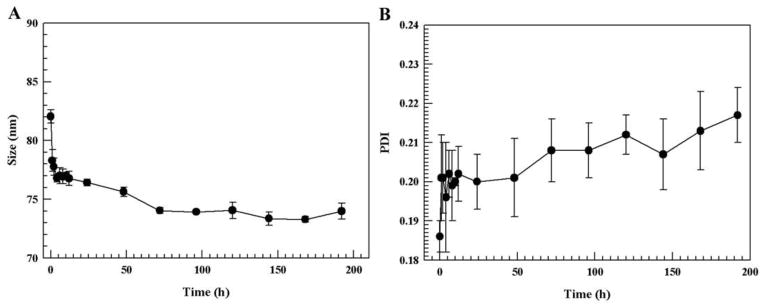
The size (A) and polydispersity index (PDI) (B) where measured at different time points.
Drug release
The release behavior of DOPC and DSPC formulations loaded with celastrol was studied. The drug release kinetic was evaluated in a water/ethanol solution (4:1 v/v) (Fig. 5A) and an FBS/PBS solution (7:3 v/v) (Fig 5B). An FBS/PBS mixture was used to mimic an in vivo environment, while a water/ethanol mixture was used as a receptor medium to maintain a sink condition for celastrol. Both formulations exhibited a zero-order release kinetic. However, in serum a lag phase of drug release occurred for approximately 8 h, after which the release rate increased almost hundredfold, in comparison to that of the water/ethanol mixture. After 240 h the majority of the drug had been released from the liposomes incubated in serum, while only 10% of the drug had been released after 900 h in the water solution. The drug release rate for DOPC liposomes in serum was greater than that of DSPC liposomes (Fig. 5). For instance, at 240 h, 48% of the drug had been released from the DOPC liposomes, while only 28% had been released from the DSPC liposomes (*P ≤ 0.05).
Figure 5. Cumulative drug release of liposomes.
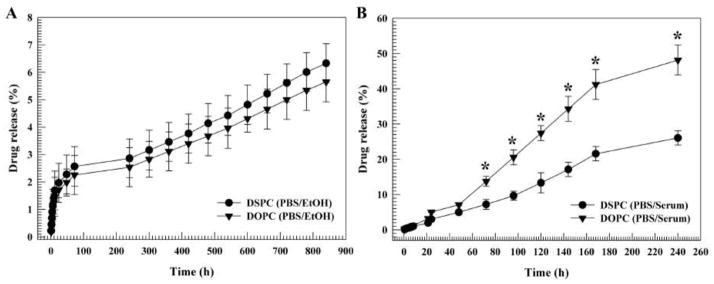
Liposomes consisted of pegylated 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC)/cholesterol/celastrol and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC)/cholesterol/celastrol. The drug release was measured in a water/ethanol solution (4:1 v/v) (A) and a serum/PBS (7:3 v/v) solution (B). Measurements were done in triplicate. The data is presented as the mean±SD. Error bars, if not shown, are located within the symbols. *P ≤ 0.05.
Reduction of cell viability
To evaluate the differential effects of free and liposomal celastrol on cell viability, a CCK-8 assay for viability was utilized. Free celastrol and liposomal celastrol caused a similar reduction in VCaP cell viability (Fig. 6). A dramatic reduction of cell viability was observable at concentrations exceeding 1 μM.
Figure 6. Cell viability of VCaP prostate cancer cells.
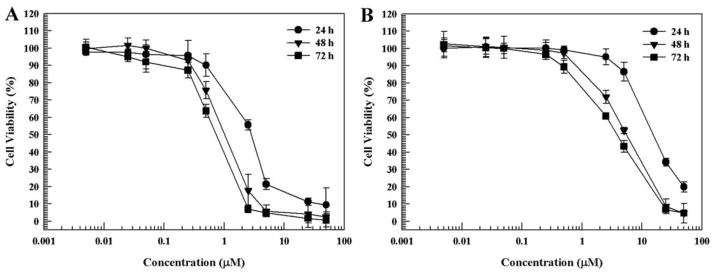
(A) Free celastrol. (B) Liposomal celastrol. Measurements were done in triplicate. The data is presented as the mean±SD. Error bars, if not shown, are located within the symbols.
Cellular uptake of liposomes
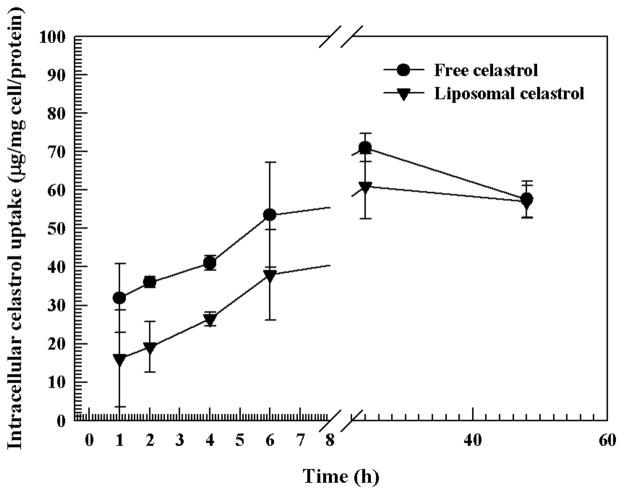
The amount of intracellular uptake of celastrol was evaluated to discern whether differential uptake patterns between the liposomes and the free drug (DMSO solubilized) could be observed. The assay demonstrated similar uptake patterns for both the free celastrol and liposomal celastrol. In general, the uptake of celastrol liposomes was slightly lower, however this difference was not statistically significant at all time points (Fig. 7). After 48 h equal levels of drug had been internalized in both groups.
Figure 7. Intracellular accumulation of free celastrol and liposomal celastrol in VCaP prostate cancer cells.
Measurements were done in triplicate. The data is presented as the mean±SD.
Discussion
Several studies have reported that celastrol exerts an antiproliferative effect on prostate cancer cells, through modification of nuclear factor-kappaB (NF-kB) signaling and inhibition of proteasome function (Shao et al., 2013, Dai et al., 2010, Yang et al., 2006). In particular, prostate cancer cells are sensitive to celastrol, since the majority of them express a fusion gene that stimulates NF-kB signaling (Shao et al., 2013). Nevertheless, the use of celastrol as an anticancer agent has been limited by the hydrophobic nature of the drug (Song et al., 2011). However, the encapsulation of this therapeutic agent within liposomes eliminates the need to use solubilizing agents, hence mitigating non-specific toxicity. Moreover, liposomal entrapment can protect the drug compound from enzymatic degradation, renal clearance and uptake by the immune system, resulting in prolonged blood circulation (Gentile et al., 2014). Consequently, the drug accumulation in the tumor can be increased, further incentivizing the use of liposomes. In addition, liposomes have the potential to overcome drug resistance due to higher drug deposition in the tumor and different mechanisms of cellular internalization and trafficking (Gentile et al., 2014).
In this study, the liposomal formulation containing DSPC showed more favorable properties than the DOPC formulation. Namely, the DSPC liposomes showed greater storage stability over a two-week period, had higher drug encapsulation efficiency and displayed a slower rate of drug release in FBS than that of the DOPC liposomes. These features make the DSPC formulation more suitable for clinical applications, as the liposomes are likely to remain intact and in circulation for longer periods of time. It is not surprising that the DSPC liposomes are more stable, since the lipids have saturated hydrocarbon chains that can be tightly packed in the bilayer. In contrast, DOPC lipids have two double bonds that produce kinks in the bilayer, causing increased liposomal flexibility. This difference in bilayer structure is also apparent in the transition temperature (Tm, from gel to liquid phase), where DSPC and DOPC have a Tm of 55 °C and −20 °C, respectively (information provided by Avanti Polar Lipids). These factors may explain why the DSPC liposomes displayed more favorable properties. However, there was no apparent difference in drug release between the formulations when incubated in PBS. This observation suggests that the DOPC liposomes can remain stable for shorter periods of time in the absence of serum proteins. Indeed, the presence of cholesterol may have aided in increasing liposomal stability, as this molecule has been previously been reported to enhance the rigidity of lipid membranes (Liang et al., 2004).
Furthermore, the DSPC liposomes remained stable when incubated with serum, despite a slight reduction in size and a small increase in the PDI, which may be explained by osmotic forces. Serum contains many proteins that are impermeable to the liposomal membrane, possibly causing water to escape from the aqueous core and subsequent shrinking of the liposomes. Indeed, previous studies have shown that liposomes may decrease in size due to osmotic forces caused by the presence of multivalent ions in the solution, thereby supporting the aforementioned explanation (Sabin et al., 2006, Hupfeld et al., 2010, Pencer et al., 2001). In addition, one study showed that both pegylated and non-pegylated liposomes undergo a reduction in size when exposed to a serum solution (Wolfram et al., 2013). The authors found a correlation between the serum concentration and the degree of shrinkage, speculating that the proteins in the serum triggered osmosis.
The rate of drug release in 70 % serum was increased by almost a hundredfold in comparison to the release rate in a water/ethanol solution. This phenomenon suggests that the binding of serum proteins to the liposomal surface greatly accelerates drug release, despite the presence of PEG. Indeed, it has previously been suggested that the surface of conventional stealth liposomes is not fully covered by PEG-chains, thus leaving the liposomal membrane vulnerable to protein interactions (Li and Huang, 2010, Kim et al., 2007). In particular, the density and conformation of PEG chains dictates the extent of protein binding (Bartucci et al., 2002, Kaufmann et al., 2011). Namely, sparsely spaced PEG chains display a mushroom conformation, which allows for proteins to interact with the liposomal surface. On the contrary, densely spaced PEG chains exhibit a brush confirmation, thereby obstructing protein binding. Furthermore, it has been shown that in an in vivo environment PEG-chains may rapidly detach from the liposomal surface, allowing various proteins to establish contact with the lipid surface (Parr et al., 1994). Consequently, such interactions at the nano-bio interface may influence the rate of drug release. In essence, incomplete surface coverage and detachment of PEG chains may explain the accelerated rate of drug release observed in serum. Notably, as proteins bind to the lipid surface they may interact with celastrol, causing it to be released from the bilayer.
Conclusion
Pegylated DSPC celastrol liposomes were shown to maintain stability under storage conditions and when incubated with serum. The liposomes displayed efficient cellular uptake and anticancer efficacy in prostate cancer cells, comparable to that of the free drug reconstituted in DMSO. The advantage of encapsulating celastrol in liposomes is the elimination of the need for solubilizing agents, such as DMSO, which exhibit non-specific toxicity that has hindered their clinical use (Ruiz-Delgado et al., 2009, Rubin, 1975, Swanson, 1985, Montaguti et al., 1994, Vogin et al., 1970). Furthermore, celastrol and other small molecule drugs have short blood circulation times, due to rapid excretion by the kidneys. By embedding celastrol in a liposomal bilayer, the drug can be released over a prolonged period of time, increasing the bioavailability of the agent and reducing the frequency of dosing. The results of this study warrant further investigation of the in vivo use of celastrol liposomes as a therapeutic agent for prostate cancer.
Acknowledgments
The research was supported by funds from the Methodist Hospital Research Institute. Partial funds were acquired from: the Ernest Cockrell Jr. Distinguished Endowed Chair (M.F.), the US Department of Defense (W81XWH-09-1-0212) (M.F.), the National Institute of Health (U54CA143837, U54CA151668) (M.F.), Nylands nation Finland (J.W.), Department of Defense grant W81XWH-12-1-0414 (M.F.) and the State of Texas CPRIT grant RP121071 (M.F. and H.S.).
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Contributor Information
Joy Wolfram, Email: jvwolfram@tmhs.org.
Krishna Suri, Email: krishnasuri11@gmail.com.
Yi Huang, Email: huangyi214@yahoo.com.cn.
Roberto Molinaro, Email: robertomolinaro@unicz.it.
Carlotta Borsoi, Email: cborsoi@tmhs.org.
Bronwyn Scott, Email: blscott@tmhs.org.
Kathryn Boom, Email: klboom@tmhs.org.
Donatella Paolino, Email: paolino@unicz.it.
Massimo Fresta, Email: fresta@unicz.it.
Jianghua Wang, Email: jwang1@bcm.edu.
Mauro Ferrari, Email: mferrari@tmhs.org.
Christian Celia, Email: c.celia@unich.it.
Haifa Shen, Email: hshen@tmhs.org.
References
- ABU LILA AS, ICHIHARA M, SHIMIZU T, ISHIDA T, KIWADA H. Ex-Vivo/in-Vitro Anti-polyethylene Glycol (PEG) Immunoglobulin M Production from Murine Splenic B Cells Stimulated by PEGylated Liposome. Biol Pharm Bull. 2013a;36:1842–8. doi: 10.1248/bpb.b13-00562. [DOI] [PubMed] [Google Scholar]
- ABU LILA AS, KIWADA H, ISHIDA T. The accelerated blood clearance (ABC) phenomenon: Clinical challenge and approaches to manage. J Control Release. 2013b;172:38–47. doi: 10.1016/j.jconrel.2013.07.026. [DOI] [PubMed] [Google Scholar]
- BARTUCCI R, PANTUSA M, MARSH D, SPORTELLI L. Interaction of human serum albumin with membranes containing polymer-grafted lipids: spin-label ESR studies in the mushroom and brush regimes. Biochim Biophys Acta. 2002;1564:237–42. doi: 10.1016/s0005-2736(02)00458-3. [DOI] [PubMed] [Google Scholar]
- CARAFA M, MARIANECCI C, LUCANIA G, MARCHEI E, SANTUCCI E. New vesicular ampicillin-loaded delivery systems for topical application: characterization, in vitro permeation experiments and antimicrobial activity. J Control Release. 2004;95:67–74. doi: 10.1016/j.jconrel.2003.10.022. [DOI] [PubMed] [Google Scholar]
- CARAFA M, SANTUCCI E, LUCANIA G. Lidocaine-loaded non-ionic surfactant vesicles: characterization and in vitro permeation studies. Int J Pharm. 2002;231:21–32. doi: 10.1016/s0378-5173(01)00828-6. [DOI] [PubMed] [Google Scholar]
- CELIA C, CALVAGNO MG, PAOLINO D, BULOTTA S, VENTURA CA, RUSSO D, FRESTA M. Improved in vitro anti-tumoral activity, intracellular uptake and apoptotic induction of gemcitabine-loaded pegylated unilamellar liposomes. J Nanosci Nanotechnol. 2008;8:2102–13. doi: 10.1166/jnn.2008.065. [DOI] [PubMed] [Google Scholar]
- CELIA C, TRAPASSO E, LOCATELLI M, NAVARRA M, VENTURA CA, WOLFRAM J, CARAFA M, MORITTU VM, BRITTI D, DI MARZIOL, PAOLINO D. Anticancer activity of liposomal bergamot essential oil (BEO) on human neuroblastoma cells. Colloids Surf B Biointerfaces. 2013 doi: 10.1016/j.colsurfb.2013.09.017. [DOI] [PubMed] [Google Scholar]
- CHANG HI, YEH MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomedicine. 2012;7:49–60. doi: 10.2147/IJN.S26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSCO D, BULOTTA A, VENTURA M, CELIA C, CALIMERI T, PERRI G, PAOLINO D, COSTA N, NERI P, TAGLIAFERRI P, TASSONE P, FRESTA M. In vivo activity of gemcitabine-loaded PEGylated small unilamellar liposomes against pancreatic cancer. Cancer Chemother Pharmacol. 2009a;64:1009–20. doi: 10.1007/s00280-009-0957-1. [DOI] [PubMed] [Google Scholar]
- COSCO D, PAOLINO D, MUZZALUPO R, CELIA C, CITRARO R, CAPONIO D, PICCI N, FRESTA M. Novel PEG-coated niosomes based on bola-surfactant as drug carriers for 5-fluorouracil. Biomed Microdevices. 2009b;11:1115–25. doi: 10.1007/s10544-009-9328-2. [DOI] [PubMed] [Google Scholar]
- DAI Y, DESANO J, TANG W, MENG X, MENG Y, BURSTEIN E, LAWRENCE TS, XU L. Natural proteasome inhibitor celastrol suppresses androgen-independent prostate cancer progression by modulating apoptotic proteins and NF-kappaB. PLoS One. 2010;5:e14153. doi: 10.1371/journal.pone.0014153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAI Y, DESANO JT, MENG Y, JI Q, LJUNGMAN M, LAWRENCE TS, XU L. Celastrol potentiates radiotherapy by impairment of DNA damage processing in human prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:1217–25. doi: 10.1016/j.ijrobp.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENTILE E, CILURZO F, DI MARZIO L, CARAFA M, VENTURA CA, WOLFRAM J, PAOLINO D, CELIA C. Liposomal chemotherapeutics. Future Oncology. 2014 doi: 10.2217/fon.13.146. [DOI] [PubMed] [Google Scholar]
- HUANG Y, ZHOU D, HANG T, WU Z, LIU J, XU Q, XIE X, ZUO J, WANG Z, ZHOU Y. Preparation, characterization, and assessment of the antiglioma effects of liposomal celastrol. Anticancer Drugs. 2012;23:515–24. doi: 10.1097/CAD.0b013e3283514b68. [DOI] [PubMed] [Google Scholar]
- HUPFELD S, MOEN HH, AUSBACHER D, HAAS H, BRANDL M. Liposome fractionation and size analysis by asymmetrical flow field-flow fractionation/multi-angle light scattering: influence of ionic strength and osmotic pressure of the carrier liquid. Chem Phys Lipids. 2010;163:141–7. doi: 10.1016/j.chemphyslip.2009.10.009. [DOI] [PubMed] [Google Scholar]
- ICHIHARA M, MORIYOSHI N, LILA AS, ISHIDA T, KIWADA H. Anti-PEG IgM production via a PEGylated nano-carrier system for nucleic acid delivery. Methods Mol Biol. 2013;948:35–47. doi: 10.1007/978-1-62703-140-0_4. [DOI] [PubMed] [Google Scholar]
- IMMORDINO ML, DOSIO F, CATTEL L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- KAUFMANN S, BORISOV O, TEXTOR M, REIMHULT E. Mechanical properties of mushroom and brush poly(ethylene glycol)-phospholipid membranes. Soft Matter. 2011;7:9267–9275. [Google Scholar]
- KIM HR, ANDRIEUX K, DELOMENIE C, CHACUN H, APPEL M, DESMAELE D, TARAN F, GEORGIN D, COUVREUR P, TAVERNA M. Analysis of plasma protein adsorption onto PEGylated nanoparticles by complementary methods: 2-DE, CE and Protein Lab-on-chip system. Electrophoresis. 2007;28:2252–61. doi: 10.1002/elps.200600694. [DOI] [PubMed] [Google Scholar]
- KOUDELKA S, TURANEK J. Liposomal paclitaxel formulations. J Control Release. 2012;163:322–34. doi: 10.1016/j.jconrel.2012.09.006. [DOI] [PubMed] [Google Scholar]
- LI SD, HUANG L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J Control Release. 2010;145:178–81. doi: 10.1016/j.jconrel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIANG X, MAO G, NG KY. Mechanical properties and stability measurement of cholesterol–containing liposome on mica by atomic force microscopy. J Colloid Interface Sci. 2004;278:53–62. doi: 10.1016/j.jcis.2004.05.042. [DOI] [PubMed] [Google Scholar]
- LIU Z, MA L, ZHOU GB. The main anticancer bullets of the Chinese medicinal herb, thunder god vine. Molecules. 2011;16:5283–97. doi: 10.3390/molecules16065283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLINARO R, WOLFRAM J, FEDERICO C, CILURZO F, DI MARZIO L, VENTURA CA, CARAFA M, CELIA C, FRESTA M. Polyethylenimine and chitosan carriers for the delivery of RNA interference effectors. Expert Opin Drug Deliv. 2013 doi: 10.1517/17425247.2013.840286. [DOI] [PubMed] [Google Scholar]
- MONTAGUTI P, MELLONI E, CAVALLETTI E. Acute intravenous toxicity of dimethyl sulfoxide, polyethylene glycol 400, dimethylformamide, absolute ethanol, and benzyl alcohol in inbred mouse strains. Arzneimittelforschung. 1994;44:566–70. [PubMed] [Google Scholar]
- PAOLINO D, COSCO D, RACANICCHI L, TRAPASSO E, CELIA C, IANNONE M, PUXEDDU E, COSTANTE G, FILETTI S, RUSSO D, FRESTA M. Gemcitabine-loaded PEGylated unilamellar liposomes vs GEMZAR: biodistribution, pharmacokinetic features and in vivo antitumor activity. J Control Release. 2010;144:144–50. doi: 10.1016/j.jconrel.2010.02.021. [DOI] [PubMed] [Google Scholar]
- PARR MJ, ANSELL SM, CHOI LS, CULLIS PR. Factors influencing the retention and chemical stability of poly(ethylene glycol)-lipid conjugates incorporated into large unilamellar vesicles. Biochim Biophys Acta. 1994;1195:21–30. doi: 10.1016/0005-2736(94)90004-3. [DOI] [PubMed] [Google Scholar]
- PASUT G, VERONESE FM. State of the art in PEGylation: the great versatility achieved after forty years of research. J Control Release. 2012;161:461–72. doi: 10.1016/j.jconrel.2011.10.037. [DOI] [PubMed] [Google Scholar]
- PENCER J, WHITE GF, HALLETT FR. Osmotically induced shape changes of large unilamellar vesicles measured by dynamic light scattering. Biophys J. 2001;81:2716–28. doi: 10.1016/S0006-3495(01)75914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULINI S, RUPOLI S, GOTERI G, PIMPINELLI N, ALTERINI R, BETTACCHI A, MULATTIERI S, PICARDI P, TASSETTI A, SCORTECHINI AR, FIORITONI G, LEONI P. Efficacy and safety of pegylated liposomal doxorubicin in primary cutaneous B-cell lymphomas and comparison with the commonly used therapies. Eur J Haematol. 2009;82:184–93. doi: 10.1111/j.1600-0609.2008.01197.x. [DOI] [PubMed] [Google Scholar]
- RUBIN LF. Toxicity of dimethyl sulfoxide, alone and in combination. Annals of the New York Academy of Sciences. 1975;243:98–103. doi: 10.1111/j.1749-6632.1975.tb25348.x. [DOI] [PubMed] [Google Scholar]
- RUIZ-DELGADO GJ, MANCIAS-GUERRA C, TAMEZ-GOMEZ EL, RODRIGUEZ-ROMO LN, LOPEZ-OTERO A, HERNANDEZ-ARIZPE A, GOMEZ-ALMAGUER D, RUIZ-ARGUELLES GJ. Dimethyl sulfoxide-induced toxicity in cord blood stem cell transplantation: report of three cases and review of the literature. Acta Haematol. 2009;122:1–5. doi: 10.1159/000227267. [DOI] [PubMed] [Google Scholar]
- SABIN J, PRIETO G, RUSO JM, HIDALGO-ALVAREZ R, SARMIENTO F. Size and stability of liposomes: a possible role of hydration and osmotic forces. Eur Phys J E Soft Matter. 2006;20:401–8. doi: 10.1140/epje/i2006-10029-9. [DOI] [PubMed] [Google Scholar]
- SCHROIT AJ, FIDLER IJ. Effects of liposome structure and lipid composition on the activation of the tumoricidal properties of macrophages by liposomes containing muramyl dipeptide. Cancer Res. 1982;42:161–7. [PubMed] [Google Scholar]
- SETHI G, AHN KS, PANDEY MK, AGGARWAL BB. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB activation. Blood. 2007;109:2727–35. doi: 10.1182/blood-2006-10-050807. [DOI] [PubMed] [Google Scholar]
- SHAO L, ZHOU Z, CAI Y, CASTRO P, DAKHOV O, SHI P, BAI Y, JI H, SHEN W, WANG J. Celastrol suppresses tumor cell growth through targeting an AR-ERG-NF-kappaB pathway in TMPRSS2/ERG fusion gene expressing prostate cancer. PLoS One. 2013;8:e58391. doi: 10.1371/journal.pone.0058391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONG J, SHI F, ZHANG Z, ZHU F, XUE J, TAN X, ZHANG L, JIA X. Formulation and evaluation of celastrol-loaded liposomes. Molecules. 2011;16:7880–92. doi: 10.3390/molecules16097880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWANSON BN. Medical use of dimethyl sulfoxide (DMSO) Reviews in clinical & basic pharmacology. 1985;5:1–33. [PubMed] [Google Scholar]
- VOGIN EE, CARSON S, CANNON G, LINEGAR CR, RUBIN LF. Chronic toxicity of DMSO in primates. Toxicol Appl Pharmacol. 1970;16:606–12. doi: 10.1016/0041-008x(70)90065-7. [DOI] [PubMed] [Google Scholar]
- WOLFRAM J, SURI K, YANG Y, SHEN J, CELIA C, FRESTA M, ZHAO Y, SHEN H, FERRARI M. Shrinkage of pegylated and non-pegylated liposomes in serum. Colloids Surf B Biointerfaces. 2013;114C:294–300. doi: 10.1016/j.colsurfb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG H, CHEN D, CUI QC, YUAN X, DOU QP. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66:4758–65. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]