Abstract
Lay Abstract
Restricted interests and repetitive behaviors in autism can lead to an ‘insistence on sameness’ for routines and decision-making. The ability to adapt choice patterns when external contingencies change is commonly referred to as cognitive flexibility. To date, there are limited options for treating cognitive inflexibility in autism. Risperidone, an atypical antipsychotic, is approved to treat irritability in autism, but less is known of whether it is effective in treating cognitive inflexibility. Risperidone acts at multiple receptors although only actions at a subset of these receptors may be beneficial for cognitive flexibility. 5HT2A receptor blockade represents one pharmacological action of risperidone. Rodent studies have shown that 5HT2A receptor antagonists improve attention and cognitive flexibility. The present studies investigated whether risperidone and/or M100907, a 5HT2A receptor antagonist, improved cognitive flexibility in the BTBR mouse model of autism. The BTBR mouse compared to C57BL/6J (B6) mice exhibit a deficit in reversing learned choice patterns comparable to that in individuals with autism. The present experiments used a two-choice probabilistic reversal learning test in which the ‘correct’ choice was reinforced on 80% of trials and the ‘incorrect’ choice reinforced on 20% of trials. After initial acquisition, the contingencies were reversed. Both risperidone and M100907 improved probabilistic reversal learning performance in BTBR mice. The same treatments did not improve reversal learning in B6 mice. Because risperidone can often lead to unwanted side effects, treatment with a 5HT2A receptor antagonist may offer an alternative for improving cognitive flexibility in individuals with autism.
Scientific Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impaired social interactions with restricted interests and repetitive behaviors (RRBs). RRBs can severely limit daily living and be particularly stressful to family members. To date, there are limited options for treating this feature in ASD. Risperidone, an atypical antipsychotic, is approved to treat irritability in ASD, but less is known about whether it is effective in treating ‘higher-order’ RRBs, e.g. cognitive inflexibility. Risperidone also has multiple receptor targets in which only a subset may be procognitive and others induce cognitive impairment. 5HT2A receptor blockade represents one promising and more targeted approach as various preclinical studies have shown that 5HT2A receptor antagonists improve cognition. The present studies investigated whether risperidone and/or M100907, a 5HT2A receptor antagonist improved probabilistic reversal learning performance in the BTBR T+ tf/J (BTBR) mouse model of autism. The effects of these treatments were also investigated in C57BL/6J (B6) mice as a comparison strain. Using a spatial reversal learning test with 80/20 probabilistic feedback, similar to one in which ASD individuals exhibit impairments, both risperidone (0.125 mg) and M100907 (0.01 and 0.1 mg) improved reversal learning in BTBR mice. Risperidone (0.125 mg) impaired reversal learning in B6 mice. Improvement in probabilistic reversal learning performance resulted from treatments enhancing the maintenance of the newly correct choice pattern. Because risperidone can lead to unwanted side effects, treatment with a specific 5HT2A receptor antagonist may improve cognitive flexibility in individuals with ASD while also minimizing unwanted side effects.
Keywords: autism, cognitive flexibility, BTBR, reversal learning, serotonin, risperidone
Introduction
Autism spectrum disorder (ASD) is a persistent neurodevelopmental disorder characterized by both deficits in social communication and the presence of repetitive and restricted behaviors (RRBs) [Bodfish et al., 2000; Fombonne, 2009; Rapin & Tuchman, 2008]. RRBs include both repetitive manipulation of objects, stereotyped movements, and an “insistence on sameness” or rigid adherence to a rule or routine [Goldman et al., 2009; Hughes et al., 1994; Lam & Aman, 2007; Szatmari et al., 2006]. The ability to switch actions or thought patterns based on situational demands commonly defines cognitive flexibility. Thus, an insistence on sameness can be characterized as a reduction in cognitive flexibility [Geurts et al., 2009]. Currently, there are no FDA approved treatments for cognitive flexibility deficits in ASD or other disorders. This is particularly problematic as RRBs in ASD can be the most distressing feature to family members [Bishop et al., 2007, Turner et al., 1999].
Several studies have used the Wisconsin Card Sorting Task (WCST) to determine whether ASD individuals exhibit cognitive flexibility impairments [Memari, et al., 2013; Ozonoff & Jensen, 1999; Pooragha, et. al, 2013; Robinson et al., 2009; Williams & Jarrold, 2013]. In general, these investigations found that children or adults with ASD display impaired performance [Ozonoff et al., 1991; Ozonoff & Jensen, 1999; Pooragha, et. al, 2013; Williams & Jarrold, 2013]. Although many studies attribute WCST impairments specifically to cognitive inflexibility, successful task performance requires several processes including feedback incorporation, appropriate strategy detection, response inhibition, and maintenance of newly appropriate response patterns following switches that occur throughout testing [Geurts et al., 2009]. While the WCST allows detection of cognitive flexibility deficits, other tests that examine specific cognitive processes related to cognitive flexibility allow identification of particular operations altered in ASD which underlie cognitive inflexibility.
The findings from recent studies suggest that individuals with ASD have difficulties in learning when reward feedback is probabilistic or uncertain. Solomon et al., [2011] found that ASD and healthy individuals show comparable learning for a two-choice discrimination when a ‘correct’ choice is positively reinforced on 70 or 80% of trials. However, ASD subjects are initially slower at learning, showing a decreased sensitivity to positive feedback. More recently, D’Cruz et al., [2013] reported that ASD individuals perform similar to controls during an initial two-choice spatial discrimination using an 80/20 reinforcement schedule, but exhibit a specific reversal learning impairment. In particular, ASD participants could initially inhibit the previously correct choice (stop perseveration) as quickly as controls, but were more likely to regress back to the previously correct choice after the initial switch. This occurred because of an increased sensitivity to negative reinforcement when choosing the new correct choice on a trial that was followed by negative reinforcement [D’Cruz et al., 2013]. Thus, ASD subjects could initially shift choice patterns, but were impaired in maintaining the new correct choice pattern.
There has been increasing interest in developing animal models of autism that display comparable behavioral features observed in ASD, such as cognitive inflexibility. The BTBR T+ tf/J (BTBR) mouse is an inbred mouse strain that has garnered attention related to ASD because the mouse exhibits social deficits, as well as repetitive behaviors, e.g. increased self-grooming and marble burying [Amodeo et al., 2012; McFarlane et al., 2008; Moy et al., 2008; Pearson et al., 2011; Pobbe et al., 2010; Yang et al., 2007]. Recently, BTBR and C57BL/6J (B6) mice were tested in a spatial reversal learning test in which feedback was provided in a probabilistic manner (80/20) [Amodeo et al., 2012]. BTBR mice acquired the test as quickly as B6 mice, but were impaired during the reversal learning phase. Moreover, the reversal learning deficit in BTBR mice resulted from a significant increase in regressive errors. These results are strikingly similar to the findings observed in ASD individuals using a similar probabilistic reversal learning paradigm [D’Cruz et al., 2013]. Thus, the BTBR mouse model may be beneficial for understanding the neural basis of cognitive inflexibility in ASD, as well as for testing potential pharmacotherapies to ameliorate these impairments.
Currently, the FDA has only approved the use of the atypical antipsychotics risperidone and aripiprazole for the treatment of irritability in ASD patients [Canitano & Scandurra, 2011; Politte & McDougle, 2013; Rosenbaum et al. 2005; see McPheeters et al., 2011 for a review]. Risperidone has also been shown to improve clinical ratings of RRBs in ASD while being ineffective at reducing social impairments [McDougle et al. 2005]. Less is known about whether risperidone may be effective in reducing cognitive inflexibility in ASD. Comparable to clinical reports in individuals with ASD, acute risperidone treatment in BTBR mice decreases repetitive behaviors, such as grooming and marble burying [Silverman et al., 2010; Gould et al., 2011]. Although, risperidone reduced grooming only at a dose that also led to sedation [Silverman et al., 2010]. Animal models are often an important initial step in evaluating new treatment approaches, but even in BTBR mice, it is unknown whether risperidone treatment alleviates cognitive flexibility deficits.
An additional issue regarding risperidone treatment is that the drug has multiple receptor actions that include blockade of serotonin [5-HT] 2A, 2C and 7 receptors, dopamine 2 (D2) receptor blockade, α1 and α2-adrenergic receptor antagonism and inverse agonist actions at H1-histaminergic receptors [Schotte et al., 1996; Scott & Dhillon, 2007]. The complex pharmacology of a drug like risperidone, while potentially having procognitive effects, may also lead to severe and unwanted side effects, e.g. weight gain and sedation [Aman et al., 2002; Findling et al., 2000; Rummel-Kluge et al; 2010;McPheeters et al., 2011; Dove et al., 2012]. Alternatively, a drug with a more specific receptor target and at least similar behavioral benefit may prove more effective for treating cognitive inflexibility in ASD while limiting unwanted side effects.
Recent findings from studying the relationship of hyperserotonemia and ASD suggest that 5HT2A receptor antagonists represent one promising treatment approach [Veenstra-VanderWeele et al., 2012]. Elevated whole-blood 5HT or hyperserotonemia is a consistent finding in approximately 30% of ASD individuals [Cook & Leventhal, 1996; Mulder et al., 2004]. Furthermore, 5HT transporter gene (SLC6A4) variants are associated with increased risk of hyperserotonemia in ASD individuals [Coutinho et al., 2004]. The 5HT transporter gene Ala56 variant confers risk in ASD and has been associated with compulsive behaviors [Sutcliffe et al., 2005]. Veenstra-VanderWeele and colleagues [2012] developed a mouse that expresses the Ala56 variant which leads to hyperserotonemia, increased brain 5HT clearance, as well as increased 5HT2A receptor sensitivity. Moreover, treatment with a 5HT2A receptor antagonist in rats facilitates cognitive flexibility by improving the maintenance of a new, correct response pattern [Baker et al., 2011]. In combination, these findings suggest that 5HT2A receptor blockade may be effective in treating certain symptoms in ASD.
The present experiments examined whether risperidone and/or a selective 5HT2A receptor antagonist, M100907 improved probabilistic reversal learning in BTBR and/or B6 mice. Because BTBR mice only exhibit a reversal learning impairment and not an initial learning deficit, treatment effects were only investigated in the reversal learning phase.
Materials and Methods
Subjects
Forty-one male B6 and 66 male BTBR mice from the Jackson Laboratory [Bar Harbor, ME] were tested. Mice were singly housed in plastic cages [28 cm wide × 17 cm long × 12 cm high] in a humidity [30%] and temperature [22 °C] controlled room with a 12 hour light/dark cycle [lights on at 07:00 AM]. Fourteen days after introduction to the vivarium, behavioral testing procedures began. Both B6 and BTBR mice began training at 8 weeks of age. Animal care and use was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and was approved by the Institutional Laboratory Animal Care and Use Committee at the University of Illinois at Chicago.
Apparatus
Spatial discrimination training and testing was conducted in a black acrylic rectangle maze [76 cm long × 50 cm wide × 30 cm high] as used in Amodeo et al., [2012]. The maze was divided into a start and choice area by a plastic guillotine door [52 cm high × 49 cm wide] that extended the maze width. To assure that mice started each trial from the same central location without bias toward any particular side, a small plastic door [10 cm high × 5 cm wide] opened up at the bottom-center of the large guillotine door. In the choice area, an acrylic piece [30 cm long × 16 cm high] extended out from the back wall which divided the choice area into two equally-sized and visually distinct spatial locations. Both choice locations were adorned with distinct spatial cues attached to the back and side walls. In each choice location, a single food well was centered and located 3 cm away from the back wall.
Spatial Discrimination Training
One week prior to training mice were stabilized at 85% of ab libitum body weight. Mice were trained for 2–4 days before receiving acquisition testing. At the beginning of each training session, mice were placed into the start area. The start door was opened 1 min following placement into the holding chamber, allowing the mouse to freely navigate in the choice area and consume a ½ piece of Fruity Pebbles cereal [Post Foods, St. Louis, MO] from each food well. After cereal pieces were consumed from both choice locations, the guillotine door was raised to allow a mouse to enter the start area. After a mouse had returned to the start area, the guillotine door was closed and the food wells were re-baited. The start door was subsequently re-opened to begin a new trial. This procedure was repeated until 15 minutes had elapsed. Mice where considered trained once they successfully completed two consecutive days in which 4–6 trials were completed in the 15 min session.
Spatial Discrimination: Acquisition and Reversal Learning
A similar procedure was used as described in Amodeo et al. [2012]. At the beginning of testing a mouse was placed in the start area. The start door was opened and a mouse could choose to enter one of two spatial locations. Only one of the two food wells was baited with a ½ piece of cereal in each trial. Prior to testing, one choice location was designated as the “correct” spatial location and contained a ½ piece of cereal on 80% of trials. On the other 20% of trials, the ‘incorrect’ location was baited with a ½ piece of cereal. The first two trials of each test phase always contained a food reinforcement in the “correct” arm. In any given trial, only one choice location was baited. Learning criterion was achieved when a mouse chose the “correct” location on 6 consecutive trials. This is the same learning criterion used in other reversal learning tests [Tait et al., 2007; Tait & Brown, 2008; Amodeo et al., 2012]. If a mouse chose the “correct” location, it was allowed to eat the cereal piece; then the guillotine door was raised and subsequently lowered after a mouse returned to the start area. If a mouse chose the incorrect spatial location, it was allowed to navigate to the unbaited food well in that spatial location. Following navigation to the unbaited food well, the guillotine door was raised allowing a mouse to return to the start area. After incorrect choices, the baited food well was temporarily removed to prevent a mouse from quickly navigating over to the correct spatial location and obtain a cereal reinforcer after making an incorrect choice. Between trials, the choice area was cleaned with 2% ammonium chloride solution to minimize use of odor cues.
Reversal learning was conducted the day after acquisition testing. Just prior to the reversal learning test, each mouse received a retention test. The retention test was conducted to have mice began at a similar level of the originally learned discrimination just prior to reversal learning. In the retention test, a mouse was reinforced on 80% of trials for choosing the spatial location that was correct in acquisition trials. Criterion was achieved when a mouse successfully chose the “correct” spatial location (as in acquisition) on 5 out of 6 trials. Immediately after achieving retention criterion, the reversal learning session began. All aspects of the reversal learning test were identical to those in the acquisition phase, except that the opposite spatial location was reinforced as it was in acquisition. Successful reversal learning criterion was met when a mouse made 6 consecutive correct choices.
Experiment 1: The Effect of Acute Risperidone Treatment on Probabilistic Reversal Learning
In Experiment 1, all mice were injected with saline 30 minutes prior to acquisition. This was done to be consistent in mice receiving an injection prior to each test phase. The following day, BTBR mice were injected with either saline, 0.0125, or 0.125 mg/kg risperidone. B6 mice were injected with either saline or 0.125 mg/kg risperidone [Sigma Aldrich, St Louis, MO]. In reversal learning, each mouse only received a single treatment. The highest dose of risperidone was only tested in B6 mice because this was the only dose that had a behavioral effect in BTBR mice. Risperidone was dissolved in 0.9% physiological saline by vortex to thoroughly mix the solution. IP injections occurred in 10 ml/kg volume. Thirty minutes following an injection, a mouse began retention and reversal learning testing. These doses were selected because a pilot study demonstrated that doses used in the present study did not significantly affect locomotor activity. The acquisition test session ranged from 14 to 102 min. The reversal learning session ranged from 35 to 134 min. All mice in this experiment reached acquisition and reversal learning criterion. Thus, no mice were excluded from the behavioral analyses.
Experiment 2: Acute M100907 Treatment on Probabilistic Reversal Learning
In Experiment 2, all mice received an injection of 10% DMSO sterile water prior to acquisition. Mice received an IP injection twenty four hours after acquisition testing of either 10% DMSO sterile water, 0.01, or 0.1 mg/kg of M100907 [Sigma Aldrich, St Louis, MO]. M100907 was mixed in 10% DMSO sterile water. Thirty minutes after an injection, mice began retention trials followed immediately by the reversal learning test. The acquisition test session ranged from 20 to 91 min. The reversal learning session ranged from 33 to 110 min. All mice in this experiment reached acquisition and reversal learning criterion. Thus, no mice were excluded from the behavioral analyses.
Error Analysis
As in previous experiments, [Amodeo et al., 2012; Brown et al., 2012; Floresco et al., 2006; McCool et al., 2008], an error analysis of reversal learning was conducted to determine whether a treatment affected the ability to initially inhibit the previously relevant choice pattern [perseverative errors] and/or the ability to maintain the new choice pattern after being initially selected and reinforced [regressive errors]. The first trial of reversal learning was not counted as a perseverative error, but served as initial negative feedback. On subsequent trials, if a mouse chose the previously correct spatial location these were recorded as perseverative errors until a mouse first chose the new correct spatial location. After selecting the correct spatial location for the first time, all subsequent entries into the previously reinforced spatial location were scored as regressive errors. For example, a mouse could have initially learned to choose spatial location A and then during reversal learning had to choose spatial location B instead. The following represents an example of the spatial location chosen on consecutive trials during reversal learning: A,A,A,B,A,B,A,A,B,B,B,B,B,B. Therefore, a mouse would have two perseverative errors (bold) and three regressive errors (italics).
Statistical Analysis
For Experiments 1 and 2, a t-test was conducted to determine whether there was a difference between strains in trials to criterion for acquisition. For retention and reversal learning, a two-way analysis of variance (ANOVA) with strain and treatment as the factors was conducted to determine whether there was a significant difference in trials to criterion. A separate analysis was conducted to determine significance differences for perseverative and regressive errors. Post-hoc Newman-Keuls tests were used to determine significant differences between specific groups.
Results
Experiment 1: The effect of risperidone on probabilistic reversal learning
All mice received a vehicle injection 30 minutes prior to the acquisition session. B6 and BTBR mice required approximately 70 trials to reach criterion in the acquisition phase (see Figure 2A). Analysis of the acquisition results revealed that there was not a significant difference between B6 and BTBR mice in acquiring the spatial discrimination (t(45) = 1.42, P > 0.05). On the second test day, all treatment groups required approximately 9 trials to reach criterion in the retention test (data not shown). Similar to acquisition, a two-way ANOVA indicated that there was not a significant effect of strain (F(1,42) = 0.13, P > 0.05) or treatment (F(1,42) = 0.51, P > 0.05) for retention. The strain x treatment interaction for retention also was not significant (F(2,42) = 0.03, P > 0.05). Thus mice did not differ in their ability to remember the originally learned discrimination before reversal learning.
Figure 2.
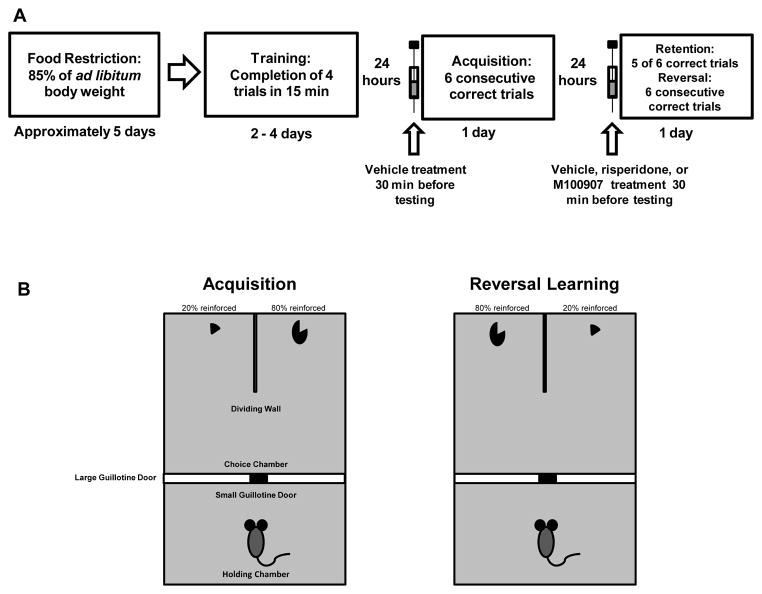
Risperidone treatment improves probabilistic reversal learning in BTBR mice. Each mouse was tested across two consecutive days in a spatial discrimination test. Each mouse received an i.p. injection of vehicle 30 minutes prior to an acquisition session. In reversal learning, mice received either vehicle or risperidone 30 minutes prior to testing. The treatments on the x-axis represent the treatment received prior to acquisition [top] and prior to reversal learning [bottom]. [A] Mean [±SEM] trials to criterion on acquisition. All mice received vehicle treatment in acquisition. B6: vehicle-vehicle [n = 9], vehicle-0.0125 [n = 8]; BTBR: vehicle-vehicle [n = 10], vehicle-0.0125 [n = 10], vehicle-0.125 [n = 10]. [B] Mean [±SEM] trials to criterion on reversal learning. B6 mice treated with vehicle required significantly less trials to criterion than BTBR vehicle-treated mice and BTBR mice receiving the low dose of risperidone. Risperidone at 0.125 mg/kg treatment in BTBR mice significantly improved reversal learning performance compared to that of BTBR mice treated with vehicle and low dose of risperidone.* = p < .05 vs. B6-vehicle and # = p < .05 vs BTBR-vehicle and BTBR-0.0125.
Immediately following the retention test, mice received a reversal learning test. B6 controls required approximately 70 trials to reach criterion while BTBR controls required approximately 120 trials (see Figure 2B). A two-way ANOVA revealed that there was a there was a significant effect for strain (F(1,42) = 7.21, P < 0.05) and treatment (F(2,42) = 5.14, P < 0.05). The analysis also showed that there was a significant strain x treatment interaction (F(2,42) = 3.92, P < 0.05). Post-hoc Newman-Keuls tests indicated that B6 mice treated with vehicle required significantly less trials to criterion than BTBR vehicle-treated mice (P < 0.05) and BTBR mice receiving the low dose of risperidone (P < 0.05). The high dose of risperidone treatment in BTBR mice significantly improved reversal learning performance compared to that of BTBR mice treated with vehicle or the low dose of risperidone (P’s < 0.05). No other comparisons were significant.
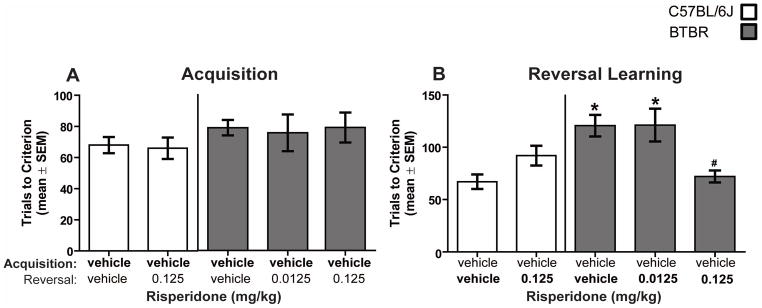
As observed previously, B6 and BTBR mice exhibited comparable levels of perseveration during reversal learning (see Figure 3A). Further, risperidone treatment did not affect perseveration. A two-way ANOVA indicated that there was not a significant effect of strain (F(1,42) = 2.23, P > 0.05) or treatment (F(2,42) = 0.87, P > 0.05). There was also no significant strain x treatment interaction (F(2,42) = 1.23, P > 0.05). Unlike perseverative errors, an effect of regressive errors was observed. B6 controls committed approximately 30 regressive errors, while BTBR controls committed approximately 70 regressive errors (see Figure 3B). Risperidone treatment reduced the number of regressive errors committed in BTBR mice. A two-way ANOVA revealed that there was a significant strain effect (F(1,42) = 10.0, P < .01), as well as a significant treatment effect (F(2,42) = 3.36, P < 0.05). The strain x treatment interaction was significant (F(2,42) = 3.92, P < 0.05). BTBR mice treated with vehicle or the low dose of risperidone had significantly more regressive errors than B6 mice receiving vehicle (P’s < 0.05). The high dose of risperidone in BTBR mice significantly decreased regressive errors compared to that of BTBR mice treated with vehicle (P < 0.05).
Figure 3.
Risperidone attenuates regressive errors committed by BTBR mice. Analysis of the errors committed in reversal learning was conducted to determine whether a treatment affected the ability to initially inhibit the previously relevant choice pattern [perseverative errors] and/or the ability to maintain the new choice pattern after initially being selected [regressive errors]. [A] Mean [±SEM] perseverative errors committed during reversal learning. Risperidone treatment did not affect perseverative errors committed. [B] Mean [±SEM] regressive errors committed during reversal learning. B6 mice did not differ on the number of regressive errors committed during reversal learning. BTBR mice treated with vehicle or the low dose of risperidone had significantly more regressive errors than B6 mice receiving vehicle. The high dose of risperidone in BTBR mice significantly decreased regressive errors compared to that of BTBR mice treated with vehicle. * = p < .05 vs. B6-vehicle; # = p < .05 vs. BTBR-vehicle.
Experiment 2: Effects of M100907 on probabilistic spatial reversal learning
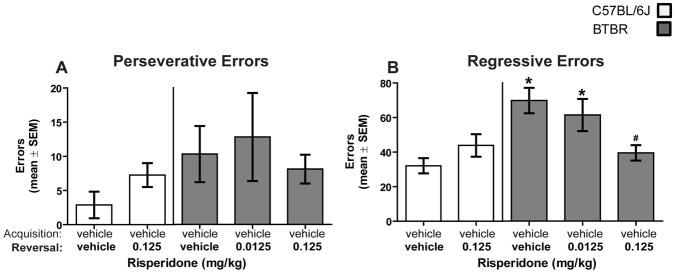
Figure 4A illustrates the findings for the spatial acquisition test in B6 and BTBR mice. All mice received vehicle treatment prior to acquisition testing and required approximately 70 trials to reach criterion. The analysis on trials to criterion for acquisition showed that there was not a significant difference in learning between the strains (t(58) = 0.68, P > 0.05. In the retention test, treatment groups ranged from 6–10 trials. The analysis on retention trials indicated that there was not a significant main effect for strain (F(1,54) = 1.57, P > 0.05), but there was a significant treatment effect (F(2,54) = 4.37, P < 0.05). This effect reflects that M100907 treatment reduced retention trials compared to that of vehicle treatment. There was not a significant strain x treatment interaction (F(2,54) = 1.57, P > 0.05). In the reversal learning test, B6 controls required approximately 80 trials to reach criterion while BTBR controls required approximately 110 trials to achieve criterion (see Figure 4B). The analysis of trials to criterion in reversal learning indicated that there was not a significant effect for strain (F(1,54) = 2.82, P > 0.05, but there was a significant treatment effect (F(2,54) = 4.13, P < 0.05). There was also a significant strain x treatment interaction (F(2,54) = 5.86, P < 0.01). Post-hoc analyses revealed that vehicle-treated BTBR mice required significantly more reversal learning trials compared to that of B6 vehicle-treated mice and BTBR mice receiving the high dose of M100907 (P’s < 0.01), as well as the BTBR mice receiving the low dose of M100907 (P < 0.05).
Figure 4.
M100907 treatment improves probabilistic reversal learning in BTBR mice. Each mouse received an i.p. injection of vehicle 30 minutes prior to an acquisition session. In reversal learning, mice received either vehicle or M100907 30 minutes prior to testing. The treatments on the x-axis represent the treatment received prior to acquisition [top] and prior to reversal learning [bottom]. [A] Mean [±SEM] trials to criterion on acquisition. All mice received vehicle treatment in acquisition. B6: vehicle-vehicle [n = 8], vehicle-0.01 [n = 8], vehicle-0.1 [n=8]; BTBR: vehicle-vehicle [n = 13], vehicle-0.01 [n = 12], vehicle-0.1 [n = 11]. [B] Mean [±SEM] trials to criterion on reversal learning. M100907 treatment did not affect reversal performance in B6 mice. Vehicle-treated BTBR mice required significantly more reversal learning trials compared to that of B6 vehicle-treated mice and BTBR mice receiving the high dose of M100907, as well as the BTBR mice receiving the low dose of M100907.* = p < .01 vs. B6-vehicle; and # p < 0.05 vs. BTBR-vehicle.
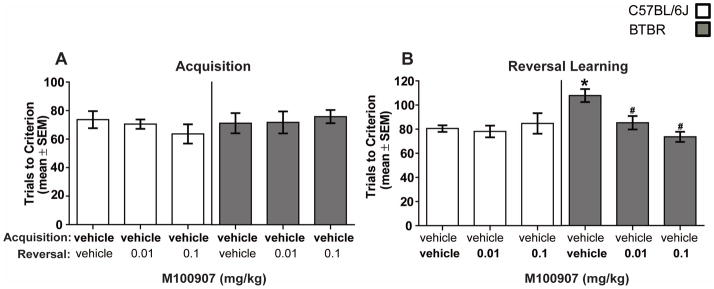
The perseverative errors in reversal learning are shown in Figure 5A. B6 mice in all treatment conditions committed approximately 3–5 perseverative errors. In BTBR mice, there was greater variability in the number of perseverative errors committed across the treatment groups with vehicle treatment leading to approximately 7 errors, the low dose of M100907 resulting less than 5 perseverative errors and the high dose of M100907 leading to near 15 perseverative errors. A two-way ANOVA revealed that there was a significant effect of strain (F(1,54) = 8.15, P < 0.01), reflecting the greater number of perseverative errors committed by BTBR mice. There was also a significant treatment effect (F(2,54) = 4.80, P < 0.05) reflecting that the high dose of M100907 increased perseverative errors. However, there was not a significant strain x treatment interaction (F(2,54) = 1.49, P > 0.05). For regressive errors, B6 controls committed approximately 35 regressive errors while BTBR controls committed approximately 55 regressive errors. M100907 treatment in BTBR mice reduced regressive errors with the largest effect observed at the high dose (Figure 5B). A two-way ANOVA indicated that there was not a significant effect of strain (F(1,54) = 3.24, P > 0.05) or treatment (F(2,54) = 2.23, P > 0.05), but there was a significant strain x treatment interaction (F(2,54) = 3.66, P < 0.05). Post-hoc analyses revealed that in the vehicle treated groups, BTBR mice committed significantly more regressive errors than B6 mice (P < 0.05). The high dose of M100907 in BTBR mice significantly reduced regressive errors compared to that of vehicle-treated BTBR mice (P < 0.05).
Figure 5.
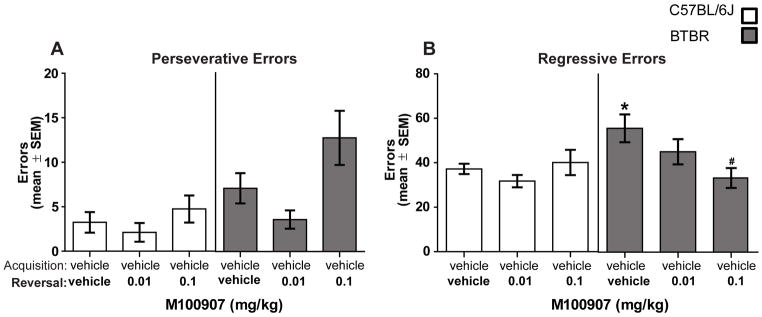
M100907 treatment effects on perseverative and regressive errors. [A] Mean [±SEM] perseverative errors committed during reversal learning. [B] Mean [±SEM] regressive errors committed during reversal learning. B6 mice did not differ on the number of perseverative errors committed during reversal learning. Vehicle-treated BTBR mice committed significantly more regressive errors than B6 mice. The high dose of M100907 in BTBR mice significantly reduced regressive errors compared to that of vehicle-treated BTBR mice. * = p < .05 vs. B6-vehicle; # = p < .05 vs. BTBR-vehicle.
Discussion
The present experiments investigated the effects of the atypical antipsychotic, risperidone, as well as the selective 5HT2A receptor antagonist, M100907 on probabilistic reversal learning in BTBR and B6 mice. In both studies, BTBR and B6 mice acquired a spatial discrimination at comparable rates. However, during reversal learning, BTBR mice required more trials to reach criterion compared to that of B6 mice. This replicates our previous findings with BTBR and B6 mice in a probabilistic reversal learning task [Amodeo et al., 2012]. As previously shown, BTBR mice exhibited a greater number of regressive errors compared to that of B6 mice, but generally showed similar perseverative error profiles. This pattern of findings suggests that BTBR mice can initially inhibit a previously learned choice pattern and shift to the newly correct choice pattern, but are impaired in maintaining a new choice pattern after it is initially selected. A similar selective increase in regressive errors during probabilistic reversal learning was recently reported in ASD individuals [D’Cruz et al, 2013], indicating that BTBR mice share similar behavioral deficits as patients with ASD, and thus may serve as a useful model for understanding behavioral inflexibility in this population. In addition, Kaland et al., [2007] demonstrated that individuals with high functioning autism showed a greater ‘failure to maintain set’ compared to healthy controls in the WCST. Thus, under various conditions that demand a flexible switch in choice patterns, ASD individuals may exhibit a select impairment in maintaining a new choice pattern after initially being selected.
In Experiment 1, acute risperidone treatment in BTBR mice improved probabilistic reversal learning performance in a dose-dependent manner. These findings in BTBR mice expand on past studies demonstrating that risperidone treatment decreased repetitive self-grooming [Silverman et al., 2010] and reduced marble burying [Gould et al., 2011; Thomas et al., 2009], both measures of repetitive behavior. The 0.125 mg/kg dose of risperidone that improved probabilistic reversal learning did so by significantly reducing regressive errors. Thus, in BTBR mice, risperidone improved probabilistic reversal learning by facilitating the maintenance of a new choice pattern after initially being selected. Although Silverman et al., [2010] reported that 0.125 mg/kg reduced locomotor activity in BTBR mice, this dose did not increase the trials completed per minute during reversal learning in the present study. In contrast to the study by Silverman et al. [2010], in the current experiments mice were food restricted and required to approach the food bowl to attain a cereal reinforcement for every trial. One possibility is that food restriction counteracts or reduces the sedating effects of risperidone at higher doses. The present results with risperidone are also comparable to those in which mice with a knockout of the CNTNAP2 gene, which is associated with ASD, exhibited behavioral flexibility impairments that were attenuated by acute risperidone treatment [Penagarikano et al., 2011]. Although risperidone has been shown to be effective in decreasing repetitive behaviors in different mouse models of autism, several studies indicate that risperidone treatment is not effective in decreasing social approach impairments in BTBR mice [Chadman, 2011; Gould et al., 2011; Silverman et al., 2010]. This parallels clinical observations, as McCracken et al., [2002] reported that risperidone treatment significantly attenuated stereotypy and hyperactivity in ASD individuals, but did not affect scores for social withdrawal and inappropriate speech. Thus, while risperidone treatment may be effective for reducing certain RRBs and possibly cognitive inflexibility, it may not be effective in treating social impairments in ASD.
One unexpected finding was that risperidone at the higher dose actually impaired performance in B6 mice. The risperidone-induced deficit appeared due to a modest increase in both perseverative and regressive errors, although neither increase achieved statistical significance. The risperidone effect in B6 mice did not result from an overall slowing of behavioral responding in the test, as vehicle and risperidone treated mice did not differ on trials completed per minute in a test session (data not shown). Relatively few studies have investigated the effects of risperidone in wild-type or control mouse strains on behavioral flexibility. However, one study did report that risperidone, at a similar dose that impaired reversal learning in B6 mice, reduced active avoidance performance in BALB/C mice [Aguilar et al., 1997]. This is a test that requires mice to repeatedly reverse positions in a chamber to avoid a footshock. Thus, risperidone may be effective in treating certain symptoms in autism or other disorders, but at the same doses may be impairing in a typical developing population.
As described above, risperidone has multiple receptor actions. One pharmacological action of risperidone is the blockade of dopamine D2 receptors [Schotte et al., 1996; Scott & Dhillon, 2007]. Past studies in both healthy humans and rodents revealed that administration of a selective dopamine D2 receptor antagonist impairs reversal learning and set-shifting performance [Desteno & Schmauss, 2009; Floresco et al., 2006; Mehta et al., 1999; Mehta et al., 2004]. One possibility is that in individuals who do not exhibit a cognitive flexibility deficit, risperidone treatment may compromise behavioral flexibility, at least in part by blocking dopamine D2 receptors. Again, these findings raise the possibility that risperidone treatment may improve cognitive flexibility when a deficit is present, but can be detrimental when there is not an existing impairment.
While risperidone improved reversal learning in BTBR mice, this drug may not be ideal as a long-term treatment because risperidone can lead to weight gain, sedation and extra pyramidal symptoms [Aman et al., 2002; Findling et al., 2000; Rummel-Kluge et al; 2010; Tandon, 2002]. Because risperidone is known to act at multiple receptor sites prescribing drugs with greater receptor specificity may be more useful clinically in reducing cognitive flexibility deficits while minimizing unwanted side effects. Risperidone is known to have a high affinity for the 5HT2A receptor where it acts as an antagonist [Schotte et al., 1996]. A recent study found that acute treatment with the 5HT2A receptor antagonist ketanserin enhanced cognitive flexibility in rats [Baker et al., 2011]. In the present study, similar to effects observed with risperidone, we found that acute treatment with the selective 5HT2A receptor antagonist M100907 ameliorated cognitive flexibility deficits in BTBR mice. Both 0.01 mg/kg and 0.1 mg/kg M100907 treatment improved reversal learning performance in BTBR mice. M100907 at 0.01 mg/kg, tended to decrease both perseverative and regressive errors in BTBR mice. However, M100907 at 0.1 mg/kg showed a trend toward increasing perseverative errors, but significantly decreased regressive errors. The pattern of reduced regressive errors with M100907 is consistent with ketanserin treatment showing a reduction in regressive errors in a set-shifting test [Baker et al., 2011]. As the present study investigated the effects of acute administration, it is unknown whether chronic administration of a 5HT2A receptor antagonist would be effective in rescuing a reversal learning deficit and what cognitive processes underlying reversal learning might be affected by chronic treatment.
The high dose of M100907 both increasing perseverative errors and decreasing regressive errors during reversal learning, may be due to the high dose of the drug producing actions in different brain areas and therefore differentially affecting these processes. More specifically, past studies have shown that orbitofrontal cortex is important for reducing perseveration of the originally learned choice pattern during reversal learning [Chudasama & Robbins, 2003; Dias et al., 1997; Kim & Ragozzino, 2005]. Furthermore, a recent study found that a direct injection of M100907 into the orbitofrontal cortex impairs reversal learning in rats [Furr et al., 2012]. Thus, the high dose of M100907 may affect orbitofrontal cortex activity that initially leads to increased perseverative responding. In contrast, several studies demonstrated that optimal function of the dorsomedial striatum supports a low level of regressive errors and the consistent maintenance of a new choice pattern [Ragozzino & Choi, 2004; Lindgren et al., 2013; Palencia & Ragozzino, 2006; McCool & Ragozzino, 2008; Van Golf Racht-Delatour & Massioui, 1999]. One possibility is that the high dose of M100907 also acts at the dorsomedial striatum to reduce regressive errors and facilitate the reliable execution of the new choice pattern. Future studies that directly infuse M100907 into the orbitofrontal cortex and dorsomedial striatum in BTBR mice can test these possibilities and provide a greater understanding of how 5HT2A receptor blockade affects different neural systems to influence cognitive flexibility.
As we have demonstrated, cognitive flexibility impairments in the BTBR mouse strain can be attenuated with risperidone or M100907 treatment. As noted above, risperidone can often lead to unwanted side effects, although the present results raise the possibility that at lower doses risperidone may be effective in attenuating cognitive inflexibility symptoms. The findings with M100907 suggest that treatment with a 5HT2A receptor antagonist may serve as an alternative to treating RRBs in ASD. There is some evidence that treatment with a 5HT2A receptor antagonist can reduce symptoms related to psychiatric disorders [Vollenweider et al., 1998]. Furthermore, daily M100907 treatment in schizophrenic patients that lasted three to four weeks was well tolerated and did not produce extrapyramidal symptoms [Talvik-Lotfi et al., 2000]. Although the sample was small and the patient population different, the results suggest that testing M100907 may be suitable in the ASD population for reducing cognitive inflexibility and other related RRBs.
Although there have been advances in understanding the developmental and behavioral features of ASD, the search for reliable biomarkers are ongoing. Investigations into specific biomarkers for ASD have consistently shown elevated blood 5HT levels or hyperserotonemia in approximately 30% of ASD individuals [Cook & Leventhal, 1996; Gabriele et al., 2014; Veenstra-VanderWeele & Blakely, 2012]. While an overall increase in whole blood 5HT may be consistent, it does not specifically account for alterations in central 5HT function. Further examinations have found a link between the 5HT2A receptor gene (HTR2A) and ASD. Functional evidence suggests that the HTR2A is a primary candidate gene in autism [Veenstra-VanderWeele et al. 2002; Cho et al. 2007]. Moreover, recent development of a mouse model that expresses a variant of the 5HT transporter gene associated with ASD results in hyperserotonemia, increased 5HT clearance from the brain and increased 5HT2A receptor sensitivity [Veenstra-VanderWeele et al., 2012]. These findings further suggest that a 5HT2A receptor antagonist represents a potential target for alleviating core symptoms in ASD.
Treatment with a 5HT2A receptor antagonist may alternatively be most effective combined with other pharmacological treatments. Some treatment studies of autism have shown that selective-serotonin reuptake inhibitors (SSRIs) can reduce repetitive behaviors [Hollander et al., 2005, 2012; McDougle et al., 2000; Owley et al., 2005]. In addition, SSRI treatment rescues behavioral deficits in BTBR mice [Chadman et al., 2011]. Moreover, SSRI treatment improves probabilistic reversal learning in rats by reducing regressive errors [Brown et al., 2012]. Preclinical studies have also shown that treatment with a 5HT2A receptor antagonist can augment the anti-depressive effects of SSRIs [Marek et al., 2005; Quesseveur et al., 2013]. Taken together, these findings raise the possibility that combined pharmacotherapy with a SSRI and a 5HT2A receptor antagonist may be effective in reducing cognitive flexibility impairments in ASD.
In summary, the present experiments demonstrated that acute treatment with the atypical antipsychotic risperidone facilitated reversal learning performance in the BTBR mouse model of ASD. These findings suggest that risperidone may be an effective treatment in addressing the cognitive inflexibility that has been reported in ASD individuals using a test we developed to closely parallel the paradigm we used in the present study [D’Cruz et al., 2013]. Moreover, the specific 5-HT2A receptor antagonist M100907 similarly facilitated reversal learning performance in these mice. Thus, treatment with 5HT2A receptor antagonists may improve cognitive flexibility in ASD and possibly other neuropsychiatric disorders in which cognitive flexibility impairments significantly impact daily functioning.
Figure 1.
Acquisition and reversal learning of a spatial discrimination. [A] Training and testing schedule for B6 and BTBR mice. [B] Mice were tested on acquisition and reversal learning of a spatial discrimination with a 80/20 probabilistic reinforcement schedule.
Acknowledgments
Grant sponsor: NIH Grant number: P50 HD055751
References
- Aguilar MA, Rodriguez-Arias M, Marí-Sanmillán MI, Miñarro J. Effects of risperidone on conditioned avoidance responding in male mice. Behavioural Pharmacology. 1997;8(8):669–676. doi: 10.1097/00008877-199712000-00001. [DOI] [PubMed] [Google Scholar]
- Aman MG, De Smedt G, Derivan A, Lyons B, Findling RL. Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. American Journal of Psychiatry. 2002;159(8):1337–1346. doi: 10.1176/appi.ajp.159.8.1337. [DOI] [PubMed] [Google Scholar]
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behavior. Behavioural Brain Research. 2012;227(1):64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Thompson JL, Sweeney JA, Ragozzino ME. Differential effects of 5-HT[2A] and 5-HT[2C] receptor blockade on strategy-switching. Behavioural Brain Research. 2011;219(1):123–131. doi: 10.1016/j.bbr.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Richler J, Cain AC, Lord C. Predictors of perceived negative impact in mothers of children with autism spectrum disorder. American Journal on Mental Retardation. 2007;112(6):450–461. doi: 10.1352/0895-8017(2007)112[450:POPNII]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: Comparisons to mental retardation. Journal of Autism and Developmental Disorders. 2000;30(3):237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Brown HD, Amodeo DA, Sweeney JA, Ragozzino ME. The selective serotonin reuptake inhibitor, escitalopram, enhances inhibition of prepotent responding and spatial reversal learning. Journal of Psychopharmacology. 2012;26(11):1443–1455. doi: 10.1177/0269881111430749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canitano R, Scandurra V. Psychopharmacology in autism: An update. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2011;35(1):18–28. doi: 10.1016/j.pnpbp.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Chadman KK. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacology Biochemistry and Behavior. 2011;(3):586–594. doi: 10.1016/j.pbb.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Cho IH, Yoo HJ, Park M, Lee YS, Kim SA. Family-based association study of 5-HTTLPR and the 5-HT2A receptor gene polymorphisms with autism spectrum disorder in Korean trios. Brain Research. 2007;1139:34–41. doi: 10.1016/j.brainres.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. Journal of Neuroscience. 2003;23(25):8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook E, Leventhal B. The serotonin system in autism. Curr Opin Pediatr. 1996;8:348–354. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Coutinho AM, Oliveira G, Morgadinho T, Fesel C, Macedo TR, Bento C, Marques C, Ataíde A, Miguel T, Borges L, Vicente AM. Variants of the serotonin transporter gene (SLC6A4) significantly contribute to hyperserotonemia in autism. Molecular Psychiatry. 2004;9(3):264–271. doi: 10.1038/sj.mp.4001409. [DOI] [PubMed] [Google Scholar]
- D’Cruz AM, Ragozzino ME, Mosconi MW, Shrestha S, Cook EH, Sweeney JA. Reduced behavioral flexibility in autism spectrum disorders. Neuropsychology. 2013;27(2):152–160. doi: 10.1037/a0031721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSteno DA, Schmauss C. A role for dopamine D2 receptors in reversal learning. Neuroscience. 2009;162(1):118–127. doi: 10.1016/j.neuroscience.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from “on-line” processing. The Journal of Neuroscience. 1997;17(23):9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove D, Warren Z, McPheeters ML, Taylor JL, Sathe NA, Veenstra-VanderWeele J. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics. 2012;130(4):717–726. doi: 10.1542/peds.2012-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, McNamara NORA, Branicky LA, Schluchter MD, Lemon E, Blumer JL. A double-blind pilot study of risperidone in the treatment of conduct disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(4):509–516. doi: 10.1097/00004583-200004000-00021. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set shifting. The Journal of Neuroscience. 2006;31(2):297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiology of pervasive developmental disorders. Pediatric Research. 2009;65(6):591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- Furr A, Lapiz-Bluhm MD, Morilak DA. 5-HT2A receptors in the orbitofrontal cortex facilitate reversal learning and contribute to the beneficial cognitive effects of chronic citalopram treatment in rats. International Journal of Neuropsychopharmacology. 2012;15(9):1295–1305. doi: 10.1017/S1461145711001441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriele S, Sacco R, Persico AM. Blood serotonin levels in autism spectrum disorder: A systematic review and meta-analysis. European Neuropsychopharmacology. 2014 doi: 10.1016/j.euroneuro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Corbett B, Solomon M. The paradox of cognitive flexibility in autism. Trends in Cognitive Sciences. 2009;13(2):74–82. doi: 10.1016/j.tics.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S, Wang C, Salgado MW, Greene PE, Kim M, Rapin I. Motor stereotypies in children with autism and other developmental disorders. Developmental Medicine & Child Neurology. 2009;51(1):30–38. doi: 10.1111/j.1469-8749.2008.03178.x. [DOI] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin [5-HT] transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+ tf/J mouse social behavior. Journal ofNeurochemistry. 2011;116(2):291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Phillips A, Chaplin W, Zagursky K, Novotny S, Wasserman S, Iyengar RA. Placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30(3):582–589. doi: 10.1038/sj.npp.1300627. [DOI] [PubMed] [Google Scholar]
- Hollander E, Soorya L, Chaplin W, Anagnostou E, Taylor BP, Ferretti CJ, Wasserman S, Swanson E, Settipani C. A double-blind placebo-controlled trial of fluoxetine for repetitive behaviors and global severity in adult autism spectrum disorders. American Journal of Psychiatry. 2012;169(3):292–299. doi: 10.1176/appi.ajp.2011.10050764. [DOI] [PubMed] [Google Scholar]
- Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Neuropsychologia. 1994;32(4):477–492. doi: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Kaland N, Mortensen EL, Smith L. Disembedding performance in children and adolescents with Asperger syndrome or high-functioning autism. Autism. 2007;11(1):81–92. doi: 10.1177/1362361307070988. [DOI] [PubMed] [Google Scholar]
- Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiology Learning and Memory. 2005;83(2):125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KS, Aman MG. The Repetitive Behavior Scale-Revised: independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(5):855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Lindgren HS, Wickens R, Tait DS, Brown VJ, Dunnett SB. Lesions of the dorsomedial striatum impair formation of attentional set in rats. Neuropharmacology. 2013;71:148–153. doi: 10.1016/j.neuropharm.2013.03.034. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Martin-Ruiz R, Abo A, Artigas F. The selective 5-HT2A receptor antagonist M100907 enhances antidepressant-like behavioral effects of the SSRI fluoxetine. Neuropsychopharmacology. 2005;30(12):2205–2215. doi: 10.1038/sj.npp.1300762. [DOI] [PubMed] [Google Scholar]
- McCool MF, Patel S, Talati R, Ragozzino ME. Differential involvement of M1-type and M4-type muscarinic cholinergic receptors in the dorsomedial striatum in task switching. Neurobiology of Learning & Memory. 2008;89:114–124. doi: 10.1016/j.nlm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, Arnold LE, Lindsay R, Nash P, Hollway J, McDougle CJ, Posey D, Swiezy N, Kohn A, Scahill L, Martin A, Koenig K, Volkmar F, Carroll D, Lancor A, Tierney E, Ghuman J, Gonzalez NM, Grados M, Vitiello B, Ritz L, Davies M, Robinson J, McMahon D. Risperidone in children with autism and serious behavioral problems. New England Journal of Medicine. 2002;347(5):314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Holmes JP, Carlson DC, Pelton GH, Cohen DJ, Price LH. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Archives of General Psychiatry. 1998;55(7):633–641. doi: 10.1001/archpsyc.55.7.633. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Kresch LE, Posey DJ. Repetitive thoughts and behavior in pervasive developmental disorders: treatment with serotonin reuptake inhibitors. Journal of Autism and Developmental Disorders. 2000;30(5):427–435. doi: 10.1023/a:1005551523657. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Scahill L, Aman MG, McCracken JT, Tierney E, Davies M, Arnold LE, Posey DJ, Martin A, Ghuman JK, Shah B, Chuang SZ, Swiezy NB, Gonzalez NM, Hollway J, Koenig K, McGough JJ, Ritz L, Vitiello B. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. American Journal of Psychiatry. 2005;162(6):1142–1148. doi: 10.1176/appi.ajp.162.6.1142. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+ tf/J mice. Genes, Brain and Behavior. 2008;7(2):152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McPheeters ML, Warren Z, Sathe N, Bruzek JL, Krishnaswami S, Jerome RN, Veenstra-Vanderweele J. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics. 2011;127(5):1312–1321. doi: 10.1542/peds.2011-0427. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Sahakian BJ, McKenna PJ, Robbins TW. Systemic sulpiride in young adult volunteers simulates the profile of cognitive deficits in Parkinson’s disease. Psychopharmacology. 1999;146(2):162–174. doi: 10.1007/s002130051102. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Manes FF, Magnolfi G, Sahakian BJ, Robbins TW. Impaired set-shifting and dissociable effects on tests of spatial working memory following the dopamine D2 receptor antagonist sulpiride in human volunteers. Psychopharmacology. 2004;176(4):331–342. doi: 10.1007/s00213-004-1899-2. [DOI] [PubMed] [Google Scholar]
- Memari AH, Ziaee V, Shayestehfar M, Ghanouni P, Mansournia MA, Moshayedi P. Cognitive flexibility impairments in children with autism spectrum disorders: Links to age, gender and child outcomes. Research in Developmental Disabilities. 2013;34(10):3218–3225. doi: 10.1016/j.ridd.2013.06.033. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman NJ, Segall SK, Andrade GM. Social approach and repetitive behavior in eleven inbred mouse strains. Behavioural Brain Research. 2008;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA, Minderaa RB. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. Journal of the American Academy of Child Adolescent Psychiatry. 2004;43:491–499. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Jensen J. Brief report: Specific executive function profiles in three neurodevelopmental disorders. Journal of Autism and Developmental Disorders. 1999;29(2):171–177. doi: 10.1023/a:1023052913110. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Pennington BF, Rogers SJ. Executive function deficits in high-functioning autistic individuals: Relationship to theory of mind. Journal of Child Psychology and Psychiatry. 1991;32(7):1081–1105. doi: 10.1111/j.1469-7610.1991.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Owley T, Walton L, Salt J, Guter SJ, Jr, Winnega M, Leventhal BL, Cook EH., Jr An open-label trial of escitalopram in pervasive developmental disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44(4):343–348. doi: 10.1097/01.chi.0000153229.80215.a0. [DOI] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The effect of N-methyl-d-aspartate receptor blockade on acetylcholine efflux in the dorsomedial striatum during response reversal learning. Neuroscience. 2006;143(3):671–678. doi: 10.1016/j.neuroscience.2006.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson BL, Pobbe RL, Defensor EB, Oasay L, Bolivar VJ, Blanchard DC, Blanchard RJ. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes, Brain and Behavior. 2011;216(1):228–235. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, Golshani P, Trachtenberg JT, Peles E, Geschwind DH. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147(1):235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behavioural Brain Research. 2010;214(2):443–449. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politte LC, McDougle CJ. Atypical antipsychotics in the treatment of children and adolescents with pervasive developmental disorders. Psychopharmacology. 2013 doi: 10.1007/s00213-013-3068-y. in press. [DOI] [PubMed] [Google Scholar]
- Pooragha F, Kafi SM, Sotodeh SO. Comparing response inhibition and flexibility for two components of executive functioning in children with autism spectrum disorder and normal children. Iranian Journal of Pediatrics. 2013;23(3):309–314. [PMC free article] [PubMed] [Google Scholar]
- Quesseveur G, Repérant C, David DJ, Gardier AM, Sanchez C, Guiard BP. 5-HT2A receptor inactivation potentiates the acute antidepressant-like activity of escitalopram: involvement of the noradrenergic system. Experimental Brain Research. 2013;226(2):285–295. doi: 10.1007/s00221-013-3434-3. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Annals of the New York Academy of Sciences. 2007;1121(1):355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Choi D. Dynamic changes in acetylcholine output in the medial striatum during place reversal learning. Learning & Memory. 2004;11(1):70–77. doi: 10.1101/lm.65404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behavioral Neuroscience. 2002;116(1):105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapin I, Tuchman RF. Autism: Definition, neurobiology, screening, diagnosis. Pediatric Clinics of North America. 2008;55(5):1129–1146. doi: 10.1016/j.pcl.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Robinson S, Goddard L, Dritschel B, Wisley M, Howlin P. Executive functions in children with autism spectrum disorders. Brain and Cognition. 2009;71(3):362–368. doi: 10.1016/j.bandc.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JFAG, Hyman SE, Labbate LA, Fava M. Handbook of psychiatric drug therapy. 5. Lippincott Williams and Wilkins; Philadelphia, PA: 2005. [Google Scholar]
- Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Lobos CA, Kisslinga W, Davisc JM, Leuchta S. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophrenia Research. 2010;123(2):225–233. doi: 10.1016/j.schres.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotte A, Janssen PFM, Gommeren WHML, Luyten WHML, Van Gompel P, Lesage AS, De Loore K, Leysen JE. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology. 1996;124(2):57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Dhillon S. Risperidone. Pediatric Drugs. 2007;9(5):343–354. doi: 10.2165/00148581-200709050-00006. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Babineau BA, Oliver CF, Karras MN, Crawley JN. Influence of Stimulant-induced hyperactivity on social approach in the BTBR mouse model of autism. Neuropharmacology. 2012;68:210–222. doi: 10.1016/j.neuropharm.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35(4):976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010;171(4):1197–1208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Smith AC, Frank MJ, Ly S, Carter CS. Probabilistic reinforcement learning in adults with autism spectrum disorders. Autism Research. 2011;4(2):109–120. doi: 10.1002/aur.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. The American Journal of Human Genetics. 2005;77(2):265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Georgiades S, Bryson S, Zwaigenbaum L, Roberts W, Mahoney W, Goldberg J, Tuff L. Investigating the structure of the restricted, repetitive behaviours and interests domain of autism. Journal of Child Psychology and Psychiatry. 2006;47(6):582–590. doi: 10.1111/j.1469-7610.2005.01537.x. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ. Lesions of the basal forebrain impair reversal learning but not shifting of attentional set in rats. Behavioural Brain Research. 2008;187(1):100–108. doi: 10.1016/j.bbr.2007.08.035. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. European Journal of Neuroscience. 2007;25(12):3719–3724. doi: 10.1111/j.1460-9568.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- Talvik-Lotfi M, Nyberg S, Nordström AL, Ito H, Halldin C, Brunner F, Farde L. High 5HT2A receptor occupancy in M100907-treated schizophrenic patients. Psychopharmacology. 2000;148(4):400–403. doi: 10.1007/s002130050069. [DOI] [PubMed] [Google Scholar]
- Tandon R. Safety and tolerability: how do newer generation “atypical” antipsychotics compare? Psychiatric Quarterly. 2002;73(4):297–311. doi: 10.1023/a:1020464017021. [DOI] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 2009;204(2):361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. Annotation: Repetitive behaviour in autism: A review of psychological research. Journal of Child Psychology and Psychiatry. 1999;40(6):839–849. [PubMed] [Google Scholar]
- Van Golf Racht-Delatour B, El Massioui N. Rule-based learning impairment in rats with lesions to the dorsal striatum. Neurobiology of Learning and Memory. 1999;72(1):47–61. doi: 10.1006/nlme.1998.3905. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Kim SJ, Lord C, Courchesne R, Akshoomoff N, Leventhal BL, Courchesne E, Cook EH. Transmission disequilibrium studies of the serotonin 5-HT2A receptor gene (HTR2A) in autism. American journal of medical genetics. 2002;114(3):277–283. doi: 10.1002/ajmg.10192. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(14):5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9(17):3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Williams D, Jarrold C. Assessing planning and set-shifting abilities in Autism: Are experimenter-administered and computerised versions of tasks equivalent? Autism Research. 2013 doi: 10.1002/aur.1311. in press. [DOI] [PubMed] [Google Scholar]
- Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Frontiers in Behavioral Neuroscience. 2007;2;1:1. doi: 10.3389/neuro.08.001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]