Abstract
Boron (B) is essential for plant cell-wall structure and membrane functions. Compared with its role in cross-linking the pectic domain rhamnogalacturonan II (RG-II), little information is known about the biological role of B in membranes. Here, we investigated the involvement of glycosylinositol phosphorylceramides (GIPCs), major components of lipid rafts, in the membrane requirement for B. Using thin-layer chromatography and mass spectrometry, we first characterized GIPCs from Rosa cell culture. The major GIPC has one hexose residue, one hexuronic acid residue, inositol phosphate, and a ceramide moiety with a C18 trihydroxylated mono-unsaturated long-chain base and a C24 monohydroxylated saturated fatty acid. Disrupting B bridging (by B starvation in vivo or by treatment with cold dilute HCl or with excess borate in vitro) enhanced the GIPCs’ extractability. As RG-II is the main B-binding site in plants, we investigated whether it could form a B-centred complex with GIPCs. Using high-voltage paper electrophoresis, we showed that addition of GIPCs decreased the electrophoretic mobility of radiolabelled RG-II, suggesting formation of a GIPC–B–RG-II complex. Last, using polyacrylamide gel electrophoresis, we showed that added GIPCs facilitate RG-II dimerization in vitro. We conclude that B plays a structural role in the plasma membrane. The disruption of membrane components by high borate may account for the phytotoxicity of excess B. Moreover, the in-vitro formation of a GIPC–B–RG-II complex gives the first molecular explanation of the wall–membrane attachment sites observed in vivo. Finally, our results suggest a role for GIPCs in the RG-II dimerization process.
Keywords: boron, cross-linking, glycosylinositol phosphorylceramides, glycosylinositol phosphoceramides, lipid rafts, rhamnogalacturonan II, cell wall, plasma membrane, Rosa sp
Introduction
Normal plant growth and development require the element boron (B) (Warington, 1923; Blevins and Lukaszewski, 1998; Goldbach and Wimmer, 2007), although our current understanding of the biochemical basis of this B dependency lacks important detail and needs to be explored from several novel perspectives. B deprivation causes many anatomical, physiological and biochemical changes and, because of the rapidity and the wide variety of symptoms that follow B deficiency, determining the primary function of B in plants is one of the greatest challenges in plant nutrition. Why excess B is highly toxic to plants is also a mystery (Aquea et al., 2012). B deficiency leads to the development of brittleness of tissues (Loomis and Durst, 1992), abnormal cell-wall thickness, decreased root cell-wall elasticity (Findeklee and Goldbach, 1996) and pollen-tube growth deceleration within 2 min of B withdrawal. Collenchyma, which is particularly rich in pectin, is strongly compromised by B deficiency; conversely, the Poales, which are poor in pectin, have a low B requirement (Hu et al., 1996). Thus, a special significance of pectins in the function of B is widely accepted.
Pectins are complex acidic polysaccharides of the primary cell wall that contain at least three major domains: homogalacturonan, rhamnogalacturonan I (RG-I) and RG-II. Of these, RG-II is the most complex, being composed of a short α-(1→4)-linked homogalacturonan backbone substituted with five structurally different side chains (the oligosaccharides A and B, disaccharides C and D, and arabinose) and is described as the main, and maybe exclusive, cell-wall binding site for B (Matoh et al., 1996). Much of the RG-II in the cell wall is usually present as a dimer cross-linked by a borate diester via the cis-diol groups of two apiose (Api) residues of side-chain A (O’Neill et al., 1996). The structure of RG-II is highly conserved between species (Matsunaga et al., 2004; O’Neill et al., 2004), with relatively minor variation (Pabst et al., 2013), and it has been shown that modification of its structure affects its ability to dimerize, correlating with severe growth phenotypes and pollen-tube growth defects (O’Neill et al., 2001; Ahn et al., 2006; Delmas et al., 2008; Voxeur et al., 2011).
Much evidence suggests that B also plays a role in the structure and function of the plant plasma membrane (Loomis and Durst, 1992; Blevins and Lukaszewski, 1998). Within minutes, B deprivation inhibits Pi and Rb+ uptake (Pollard et al., 1977; Goldbach, 1985) and ferricyanide-induced H+ release (Goldbach et al., 1990), suggesting that B does not act only by maintaining wall integrity. Withholding B also causes visible abnormalities at the wall–membrane interface and is likely to be involved in the organisation of transvacuolar cytoplasmic strands and/or to participate in wall–membrane attachment (Hirsch and Torrey, 1980; Bassil et al., 2004). It has also been proposed that B could be involved in the structure of lipid rafts by forming cross-links with cis-diol groups present in raft components (Wimmer et al., 2009) such as the mannose residues in GPI-anchored proteins and the sugar residues of glycolipids, thereby dictating the membrane’s physical state (Brown et al., 2002). Together, these observations suggest that a plant-specific, B-dependent membrane component may exist, although it is yet to be isolated.
Glycosylinositol phosphorylceramides (GIPCs) are the major sphingolipids in the plant plasma membrane (Markham et al., 2006, 2013), especially in lipid rafts (Borner et al., 2005). They are encountered only in plants and fungi, including yeasts (Sperling et al., 2005). One characteristic feature of GIPCs is the high degree of hydroxylation of the fatty acid and the long-chain base that compose their ceramide moiety (Lester and Dickson, 1993; Lynch and Dunn, 2004). The fatty acids of GIPCs are very long-chain moieties (C22 to C26) that contain a C-2 hydroxyl group (Lynch and Dunn, 2004). The long-chain base typically contains 18 carbon atoms and three hydroxyl groups: the C-1 and the C-3 hydroxyl groups arise from serine and palmitoyl-CoA precursors, respectively, whereas the C-4 hydroxyl group is subsequently added by a sphingoid base hydroxylase. Unlike those of fungi, including yeasts, plant GIPCs usually contain an acidic sugar (α-GlcA) linked to the inositol–phosphate–ceramide core structure; additional sugar units may also be attached to the GlcA. (Kaul and Lester, 1978; Buré et al., 2013).
In the present study, we investigated the role of B in the plasma membrane structure and tried to provide a better understanding of the role of B in wall–membrane attachment. First, using a thin-layer chromatography (TLC) and mass spectrometry (MS) approach, we characterized GIPCs from rose cell cultures. We discovered that B influences the extractability of GIPCs and that disruption of borate ester linkages led to the solubilisation of a detergent-insoluble fraction that includes lipid rafts. Finally, we showed that GIPCs are able to bind RG-II, possibly via a B bridge, and that they can favour the B-dependent dimerization of RG-II.
Results
GIPC characterization of rose cells
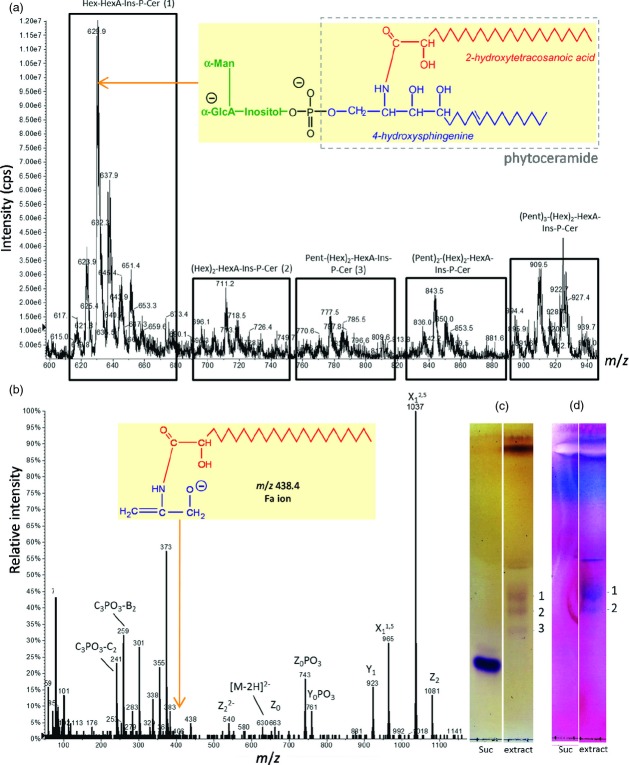
In order to characterize GIPCs from a rose cell culture, we extracted and analysed them according to a protocol described previously (Buré et al., 2011). Mass spectrometric analyses of GIPCs revealed five clusters of compounds (Figure1a). The most intense peak was found in the first cluster at m/z 629.9 ([M-2H]2− ion) corresponding to a GIPC with one hexuronic acid residue, one hexose residue and a t18:1 h24:0 ceramide moiety (where t18:1 indicates a trihydroxylated long-chain base with 18 C atoms and one C=C bond, and h24:0 indicates a monohydroxylated fatty acid with 24 C atoms and no C=C bonds) and containing one 13C atom (out of the 60). The other peaks of this cluster were mainly attributed to mono-hexosylated GIPCs composed of long-chain base t18:0 and t18:1 and fatty acid chains h22:0 to h28:0. Ions of doubly charged species corresponding to the other clusters were assigned to dihexosylated GIPC (m/z = 711.2), and the same containing up to three pentoses and thus having mainly a t18:1 h24:0 ceramide moiety (m/z = 777.5, 843.5, 909.5). These structures were further confirmed by MS fragmentation analysis (Figure1b). The hexose and hexuronic acid present in the major GIPC were not identified; however, they are most likely to be α-d-mannose and α-d-glucuronic acid respectively because the same Rosa culture releases into its culture medium a GIPC-derived fragment which has been characterized as α-d-mannopyranosyl-(1→4)-α-d-glucuronopyranosyl-(1→2)-myo-inositol (Smith and Fry, 1999; Smith et al., 1999).
Figure 1.
Mass spectrometric and thin-layer chromatographic analysis of Rosa glycosylinositol phosphorylceramides (GIPCs). (a) ESI-MS analysis of GIPC extract from Rosa cell culture. The spectrum was acquired in the negative ion mode. Abbreviations: Hex, hexose residue (probably α-mannose); HexA, hexuronic acid residue (probably α-glucuronic acid); Pent, pentose residue; Ins, myo-inositol; P, phosphate; Cer, phytoceramide. Inset: proposed structure of the predominant GIPC species; phytoceramide moiety in grey box. (b) ESI-MS/MS (collision-induced dissociation spectrum) analysis of the predominant Hex-HexA-Ins-P-Cer peak seen in (a) as the [M-2H]2− ion at m/z 630. Nitrogen was used as collision gas in a Q-TRAP instrument, with the collision energy set to −40 eV. The standard nomenclature for glycolipid fragmentation has been applied (Costello and Vath, 1990; Levery et al., 2001). Inset: proposed identity of the ion at m/z = 438.4, indicating an h24:0 ceramide moiety. (c, d) Thin-layer chromatography (TLC) of GIPC extract. Lipids were chromatographed in CHCl3/CH3OH/4 m NH4OH (9:7:2, by vol.) with 0.2 m ammonium acetate (Kaul and Lester, 1978) and located by orcinol reagent (c) or periodic acid–Schiff staining (d). Lipid bands are labelled: 1, Hex-HexA-Ins-P-Cer; 2, (Hex)2-HexA-Ins-P-Cer; 3, Pent-(Hex)2-HexA-Ins-P-Cer.
The separation of the lipid extract by TLC followed by orcinol staining to visualise the glycolipids revealed three low-mobility bands corresponding to GIPCs (Figure1c). Periodic acid–Schiff staining allowed the detection of the two most intense bands (bands 1 and 2; Figure1d) and of authentic commercial phytoceramide (not shown) but did not stain sucrose suggesting that the staining was specific to the cis-diol of the lipid moiety. According to the intensity of the bands obtained with the different stains and their relative mobility, the fastest-migrating band(band 1) was assigned to mono-hexosylated GIPCs, band 2 to dihexosylated GIPC and band 3 to GIPC containing one pentose. The average mono-hexosylated GIPC:dihexosylated GIPC ratio obtained from image analysis of TLCs stained by the periodic acid–Schiff method was around 4:1.
Impact of boron on GIPC extraction
To explore the putative existence of borate-bridged GIPC in vivo, we took advantage of aqueous solubility of GIPCs (Markham et al., 2006) and investigated the effect of boric acid (H3BO3) and HCl treatment on GIPC extractability. We extracted GIPCs from rose cell cultures grown with (B+) or without (B−) the routine concentration (3.3 μm) of H3BO3. Also, as cold 0.1 m HCl is able to hydrolyse the borate diester linkage in RG-II, we used acidic aqueous ethanol (H+) or non-acidic aqueous ethanol (H−). When B+ cultures were investigated, the use of H− as extractant resulted in the appearance of a prominent cloudy layer during the subsequent butan-1-ol/water phase-partitioning, interpreted as B-bridged lipid-rich material (Figure2a,d). This layer was less abundant in B−H−than in B+H−, and nearly absent in B−H+ and B+H+ (data not shown). Adding 0.1 m HCl to the B+H−sample during the butanol/water phase-partition step made this cloudy layer disappear (Figure2a-iii,vii). In the B−H+ and B+H+ samples (lacking a cloudy layer, as mentioned) no cloudiness subsequently formed after removal of the acidity by neutral acetone washes, suggesting that the disappearance of the cloudy layer was not simply pH dependent. As a consequence, a chemical modification such as the disruption of borate bridging must be responsible for the non-formation or the disappearance of the cloudy layer. Likewise, excess borate buffer, which could potentially disrupt B bridges (figure 7 of Bassil et al., 2004), solubilised all the cloudy layer, whereas ammonium buffer at the same concentration and pH did not (Figure2a-v,vi). Finally, 10 mm methyl β-cyclodextrin (βMCD), a cholesterol- and phytosterol-complexing agent that is capable of disrupting detergent-insoluble glycolipid-enriched complexes (potentially lipid rafts; Roche et al., 2008), solubilised most of, but not all, the cloudy layer (Figure2a-ii). The cloudy layer left was solubilised by addition of 0.1 m HCl.
Figure 2.
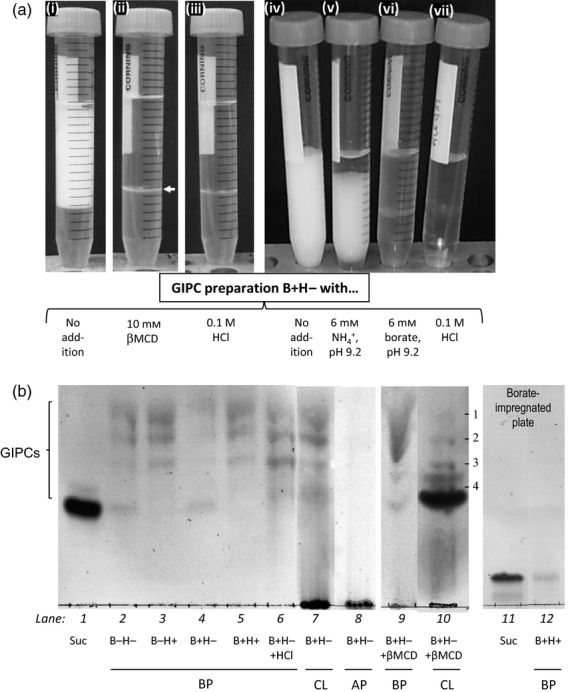
Influence of boron on glycosylinositol phosphorylceramide (GIPC) extraction. (a) A cloudy layer was observed during butanol/water phase-partitioning of a GIPC-enriched lipid sample extracted with neutral ethanol from Rosa cell cultures that had been grown in the usual B concentration (i, iv). This cloudy layer disappeared in the presence of 0.1 m HCl (iii, vii), 10 mm βMCD (ii), or 6 mm borate buffer, pH 9.2 (vi). The horizontal arrow indicates the slight cloudy layer left in the presence of βMCD (butanol above). In contrast, 6 mm ammonium buffer, pH 9.2 (v), only led to a partial disappearance. (b) TLC of the different phases after butanol/water phase-partitioning of a GIPC-rich lipid extract from Rosa cell cultures grown in media with (B+) or without boron (B−). The lipids had been extracted in 70% ethanol that contained 0.1 m HCl (H+) or lacking acid (H−). BP, butanol phase; CL, cloudy layer; AP, aqueous phase; Suc, sucrose (marker). In lanes 9 and 10, 10 mm βMCD was present during the partitioning step. Lipids labelled on lane 10: bands 1–3, as in Figure1; band 4, (Pent)2-(Hex)2-HexA-Ins-P-Cer.
As judged by TLC, more GIPC was present in the butanol phase (BP) obtained from B-deficient cell cultures with non-acidified ethanol (B−H−; Figure2b, lane 2) than in that obtained from control cell cultures (B+H−; Figure2b, lane 4). Using acidified ethanol clearly increased the GIPC amount present in the BP from the B+ extract (B+H+; Figure2b, lane 5) but not in that from B– preparation (B−H+, Figure2b, lane 3), suggesting that acid treatment interfered with the tethering of GIPC molecules within a lipid raft by disrupting potential borate ester linkages. Later addition of 0.1 m HCl to a previously neutral (B+H−) preparation during the phase-partition step also promoted the recovery of soluble GIPC in the BP (B+H−+HCl, Figure2b, lane 6). TLC of the compounds present in the cloudy layer of a never-acidified B+H−sample gave the same lipid profile as in the BP of a B+H+ sample (Figure2b, lane 7) but, in addition, high-molecular-weight (chromatographically immobile) carbohydrate-containing compounds were present. After acidification, these high molecular compounds were released into the aqueous phase (AP) (Figure2b, lane 8). Adding βMCD led to the partial recovery of GIPC in the BP (Figure2b, lane 9), and GIPCs associated with high-molecular-weight material were also found in the fraction of the cloudy layer (CL) that was non-solubilised by βMCD (Figure2b, lane 10). Interestingly, relatively more (Pent)1 and 2-(Hex)2-HexA-Ins-P-Cer (Figure2b, lane 10, bands 3 and 4) were found in the CL non-solubilised by βMCD compared with the native cloudy layer. The band below band 4 in lane 10 is unidentified, and probably arose from the βMCD.
Interestingly, the separation of the GIPC-enriched extracts on borate-impregnated silica gel plates showed a unique band of very low mobility instead of the four bands expected. The material in this band, assumed to be borate-cross-linked GIPCs, could not be eluted in butanol, suggesting that borate-bridged GIPCs were not soluble in butanol. They were, however, eluted in ethanol and when re-run on a borate-free TLC plate migrated with the same very low mobility, suggesting that the borate-bridged GIPC was stable.
RG-II–GIPC binding assays
In order to test for possible RG-II–GIPC interactions, we tried to obtain GIPC as pure as possible by adapting the method of Buré et al. (2011). The main difference from the original protocol was the addition of a phase-partition step with methanol/chloroform/formic acid/water (Figure3a). As judged by TLC of the two phases obtained, the less polar lipids were found in the organic phase, the contaminating sugars in the AP and the GIPC at the interface (Figure3b). This interface material was then submitted to an acidic butanol/water phase partition, which removed polymers. The purified GIPC-containing BP was collected.
Figure 3.
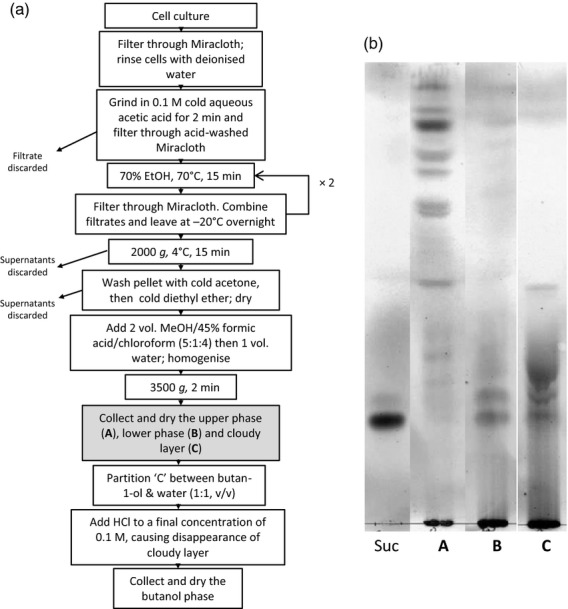
Purification of glycosylinositol phosphorylceramide (GIPC) from Rosa cell culture. (a) GIPC purification scheme adapted from Buré et al. (2011). (b) Thin-layer chromatography (TLC) of the phase A, B and C obtained during the step shaded grey in (a). Suc, sucrose marker.
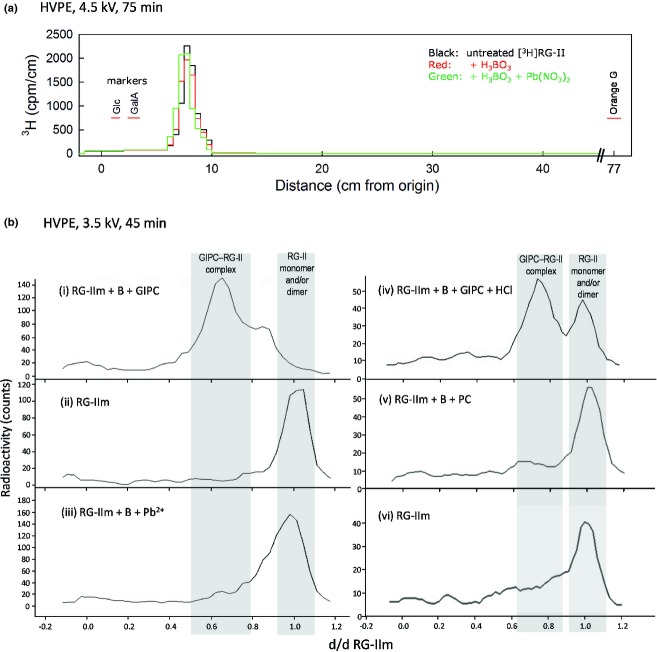
Using this extract, we investigated the ability of GIPC to bind RG-II by testing the effect of GIPC on the mobility of radiolabelled RG-II on paper electrophoresis. We incubated tracer quantities of [3H]RG-II with an excess of the purified GIPC under conditions suitable for RG-II dimerization (Chormova et al., 2013). On paper electrophoresis at pH 2.0, monomeric [3H]RG-II and the same preparation partially or fully dimerized by H3BO3, without or with 0.5 mm Pb2+ respectively, all migrated approximately 8 cm towards the anode (Figure4a). Thus dimeric RG-II had approximately 1.6× the charge of monomeric RG-II [estimated by application of Offord’s law, which states that mobility on paper electrophoresis is proportional to the Q:Mr2/3 ratio, where Q is the molecule’s net charge and where the molecular weight to the power of 2/3 is an indication of the molecule’s surface area (Offord, 1966; Fry, 2011)]. Co-migration of monomeric and dimeric RG-II is confirmed in Figure4b-ii,iii).
Figure 4.
High-voltage paper electrophoresis of radiolabelled RG-II and the effect of boric acid, Pb2+ and glycosylinositol phosphorylceramides (GIPCs). (a) [3H]RG-II was loaded on to the paper after 9 h of pre-treatment of monomeric [3H]RG-II in 250 mm pyridine buffer, pH 4.7, with or without 1 mm H3BO3 or 1 mm H3BO3 plus 0.5 mm Pb(NO3)2. After electrophoresis, the paper was cut into strips, which were assayed for tritium by scintillation-counting. The positions of non-radioactive markers (glucose, galacturonic acid and Orange G) are also shown. (b) Samples were loaded on the paper after 4 h of pre-treatment of monomeric [3H]RG-II (RGIIm) in 50 mm ammonium acetate, pH 4.8, with or without various combinations of 1.2 mm boric acid (B), 0.5 mm Pb(NO3)2 (Pb2+), GIPCs (purified as described in Figure3), commercial phytoceramide (PC), and 0.1 m hydrochloric acid (HCl). After electrophoresis, papers were read on a LabLogic AR2000 radio-TLC Imaging Scanner. The distance migrated (d) is given relative to dRGIIm (the distance migrated by monomeric RG-II, that is to say approximately 6 cm). Grey bands indicate the positions of monomeric RG-II (right) and the GIPC–RG-II complex (left).
Incubation of monomeric [3H]RG-II with GIPC caused a decrease in the electrophoretic mobility of the radioactive moiety. It gave a major peak with a shoulder, suggesting the existence of two species resulting from the interaction of RG-II with GIPC (Figure4b-i). Similar results were obtained when B was not deliberately added to the reaction mixture (data not shown), suggesting that enough B for RG-II–GIPC cross-linking was already present in the samples; but pre-treatment of the mixture with 0.1 m HCl for 1 h led to the partial re-formation of free [3H]RG-II (Figure4b-iv), compatible with the cleavage of borate bridges. To test the specificity of the GIPC, the same experiment was performed either with a Rosa lipid extract that did not contain GIPC (data not shown) or with authentic commercial phytoceramide (Figure4b-v). None of these lipids induced any change in the electrophoretic mobility of the [3H]RG-II, implying a special affinity of GIPC for RG-II.
Impact of GIPC on RG-II dimerization
In order to test whether GIPC plays a role in RG-II dimerization, we incubated GIPC with purified RG-II plus H3BO3 under conditions devised for demonstrating Pb2+-induced dimerization. After 4 h, the reaction mixture was analysed by polyacrylamide gel electrophoresis (PAGE) (Chormova et al., 2013). Some experiments showed that GIPC was able to enhance dimer formation as effectively as Pb2+ in vitro (Figure5a). Other repeat experiments demonstrated a smaller effect of GIPC (Figure5b), although the dimerization rate was always higher with GIPC + H3BO3 than with H3BO3 alone.
Figure 5.
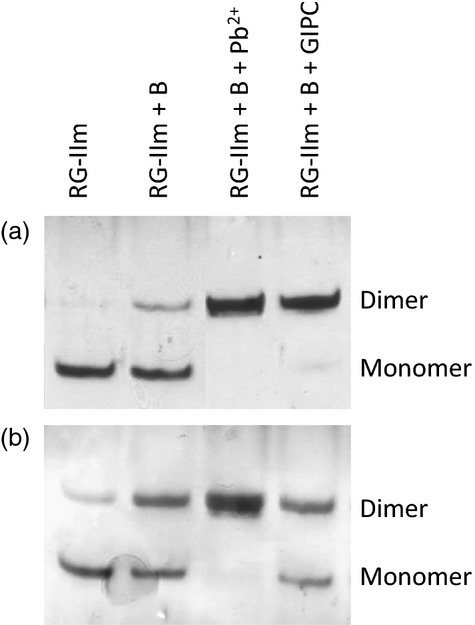
Glycosylinositol phosphorylceramide (GIPC)-induced dimerization of monomeric RG-II. After 4 h of pre-treatment of monomeric RG-II without or with 1.2 mm H3BO3 ± GIPC or with 1.2 mm H3BO3 and 0.5 mm Pb(NO3)2, samples were analysed by polyacrylamide gel electrophoresis and silver stained. Abbreviations as in Figure4. Parts (a) and (b) show the results of two independent experiments.
Discussion
Characterization of GIPC from rose cell cultures
To characterize biochemically GIPCs from rose cell culture, we analysed the GIPC-enriched extract by MS. Five clusters of species were identified corresponding to GIPCs that contained one hexuronic acid residue (HexA; most likely α-d-glucuronic acid), one or two hexose residues (Hex; at least one of which is probably α-d-mannose), and from one to three pentose residues (Pent), agreeing with the polar head variability recently reported by Cacas et al. (2013). As relative abundance of different GIPC species could not be assessed on the basis of full-scan mass spectra (Buré et al., 2011), and as the relative abundance of GIPCs could not be accurately assessed on TLC by orcinol staining because of the variable number and composition of sugar units, we developed a TLC staining method based on periodic acid–Schiff staining. This method allowed the instantaneous staining of lipids containing a vicinal diol whereas no band appeared for the sucrose suggesting that, under the conditions used, this stain was specific for the cis-diol of the lipid moiety. The average mono-hexosylated GIPC/dihexosylated GIPC ratio estimated by this method was roughly 4:1, which is consistent with previous results in Arabidopsis (Mortimer et al., 2013).
Structural role of boron in the plasma membrane
Boron deficiency has been shown to disrupt the membrane transport of Pi and Rb+ and the activity of membrane-localised proteins such as ATPase (Robertson and Loughman, 1974; Pollard et al., 1977; Smyth and Dugger, 1980; Goldbach, 1985). Interestingly, amongst proteins specifically detected in the detergent-insoluble (lipid-raft-associated) fractions of tobacco BY-2 cells, isoforms of H+-ATPase or subunits of vacuolar H+-ATPases and three isoforms of Pi transporter have been identified (Morel et al., 2006). We therefore suggest that an alteration of membrane structure and perhaps of lipid raft structure, caused by a B deficiency, could be responsible for the observed effects. As the role of B in membrane structure had never been directly demonstrated and as GIPCs have interesting cis-diol groups that are able to form borate ester bridges, we investigated the effect of boron on GIPC extractability.
We showed that GIPCs were more extractable from cells grown without B than from cells grown under normal conditions and that stripping of B with HCl improved GIPC recovery, suggesting that B influences the GIPC extractability. Moreover, the use of non-B-stripping extractants (neutral 70% ethanol) led to the appearance of a GIPC-containing CL during butanol/water phase-partitioning. This phenomenon, which was more pronounced in cells grown with B, is characteristic of micelle aggregation. The addition of βMCD [which has been reported to disrupt lipid rafts (Roche et al., 2008)] led to the almost total solubilisation of the CL, suggesting that this layer was mainly composed of lipid raft-containing micelles. Interestingly, the disruption of potential borate cross-links by excess borate or 0.1 m HCl also led to the total solubilisation of the CL. Together, these data indicate a potential structural role for B in the plasma membrane, possibly in lipid rafts. Furthermore, more (Pent)n-(Hex)2-HexA-Ins-P-Cer than Hex-HexA-Ins-P-Cer was found in the small quantity of the CL that was not solubilised by βMCD than in the native cloudy layer. This observation suggests that the major GIPCs that bind B in vivo are the highly glycosylated species.
As we showed that excess borate can solubilise GIPCs from the detergent-insoluble CL, we suggest a possible basis for the phytotoxicity of soils that have a high concentration of soluble B, which are prevalent in over-irrigated semi-arid areas (Al-Mustafa et al., 1993). Although a small amount of B appears to be required for the structural integrity of the membrane in plants, serving to cross-link GIPCs to other membrane components (possibly glycoproteins), excess B may interfere with such cross-linking. High B may independently bond to the two organic partners such that a single B can no longer cross-link them. We suggest that, at high B concentrations, B–GIPC and B–glycoprotein bonds form separately, precluding the formation of GIPC–B–glycoprotein bridges and thus interfering in the assembly of the membrane and perhaps of lipid rafts.
GIPC–RG-II interactions
Previous work by Bassil et al. (2004) showed that B is involved in cell-wall–membrane attachment. Furthermore, fumonisin B1 (FB1), which specifically inhibits the incorporation of very-long-chain fatty acids into sphingolipids, caused a detachment of the plasma membrane (Markham et al., 2011), suggesting a role of GIPCs in the wall–membrane attachment. As RG-II is the only strong B-binding site in the cell wall, we investigated whether an RG-II–GIPC complex could be formed and potentially involved in the wall–membrane attachment. Using radioactive RG-II and high-voltage paper electrophoresis, we demonstrated that GIPCs, unlike other lipids tested, were able to form complexes with RG-II. HCl (0.1 m) disrupted the complex, as it does with RG-II–B–RG-II complexes, again supporting the existence of GIPC–B–RG-II complexes; however, it remains to be shown directly whether B was involved in the GIPC–RG-II complex. The RG-II–GIPC complex appears to be reasonably stable under acidic conditions, as it remained intact during about 1 h of electrophoresis at pH 2.0. The quantity of [3H]RG-II used in this study was very small, so the traces of soluble B present in ordinary laboratory buffers may have been sufficient for GIPC–RG-II bonding, explaining why exogenous H3BO3 did not modify the results obtained.
Analysis of wall-localised RG-II consistently shows the presence of 10–15% in the monomeric form, even in plants with an adequate boron supply (Matsunaga and Ishii, 2006). It is possible that this percentage represents RG-II molecules which, in vivo, had been present as a GIPC–RG-II complex but released in free form during the alkali treatment that is routinely applied before endopolygalacturonase digestion. Although that idea is speculative, some clues pointing to the existence of a GIPC–RG-II complex can be found in the literature. It has been discovered, by use of anti-RG-II antiserum in Pisum sativum symbiotic root nodules developed in presence of B, that RG-II interacted with a 175-kDa arabinogalactan-protein–extensin (AGPE) hybrid glycoprotein (Reguera et al., 2010). Some AGPs, owing to their glycosylphosphatidylinositol (GPI) lipid anchor, are associated with detergent-resistant membrane (Seifert and Roberts, 2007). Thus, it is likely that some lipids such as GIPCs, tightly associated with the GPI anchor, could be present in the 175-kDa nodule complex containing RG-II antigen. Moreover, immunocytochemical studies using RG-II-specific antibodies demonstrated a denser label proximal to the plasma membrane (Matoh et al., 1998), suggesting that RG-II could be bound to the membrane. These previous results strengthen the possible presence of a GIPC–B–RG-II complex in vivo.
The dimerization of RG-II through B bridges occurs only very slowly when RG-II and H3BO3 are mixed in vitro, but the process can be greatly expedited by addition of certain metal ions, especially Pb2+ or Sr2+ (O’Neill et al., 1996; Ishii et al., 1999). Boron-mediated cross-linking of newly synthesised RG-II occurs very rapidly in vivo (Chormova et al., 2013). The factors that promote the cross-linking in vivo are unknown, but it is clear that they do not normally include Pb2+ or Sr2+, which are not essential elements. What biological agent ‘mimics’ Pb2+ in vivo? One attractive possibility, suggested by the present work, is that a GIPC serves as a B ligand (L) promoting boryl-transfer to RG-II, in reactions of the type:
| Reaction 1 |
| Reaction 2 |
| Reaction 3 |
In reaction 1, boric acid binds to the ligand (GIPC); in reaction 2, the GIPC forms a B-centred complex with RG-II; then in reaction 3, the GIPC is displaced by an incoming second RG-II molecule, resulting in a firmly cross-linked RG-II–B–RG-II dimer, as seen in Figure5.
Furthermore, results from Mortimer et al. (2013) on gonst1 (Golgi localised nucleotide sugar transporter) mutants altered in the mannosylation of mono- and dihexosylated GIPCs showed no decrease in RG-II dimerization rate. However, no difference in the Ara/GlcA ratio of the GIPCs of the mutants was observed, suggesting that GIPCs with more than three sugar residues were not affected by the mutation. Taken together with our evidence that the GIPCs with the strongest ability to bind B in vivo are highly glycosylated, it is likely that GIPCs potentially involved in RG-II dimerization are the (Pent)n-(Hex)2-HexA-Ins-P-Cer species.
Co-expression analysis
Results retrieved (Figure6) from Atted-II database (Obayashi et al., 2014) revealed that genes involved in GIPC biosynthesis, such as LAG ONE HOMOLOGUE (LOH) encoding a ceramide synthase (Ternes et al., 2011), were co-expressed in several species with genes involved in the biosynthesis of RG-II such as ketodeoxyoctulosonic acid [involved in biosynthesis of 3-deoxy-d-manno-2-octulosonic acid (KDO)] and RGXT (RG-II xylosyltransferase; involved in side-chain A biosynthesis). Likewise, LOH genes are co-expressed with genes involved in the biosynthesis of RG-II’s homogalacturonan backbone (GAUT1, GAUT7 and GAUT8, encoding three galacturonosyltransferases). Moreover, both RG-II and GIPC biosynthesis occur in the Golgi apparatus (Mohnen, 2008); therefore, if the B-dependent dimerization of RG-II does require the intermediacy of a GIPC–B–RG-II complex, all necessary participants in the process would be present together in the endo-membrane/exocytosis system – which is the major location of RG-II dimerization in vivo (Chormova et al., 2013).
Figure 6.
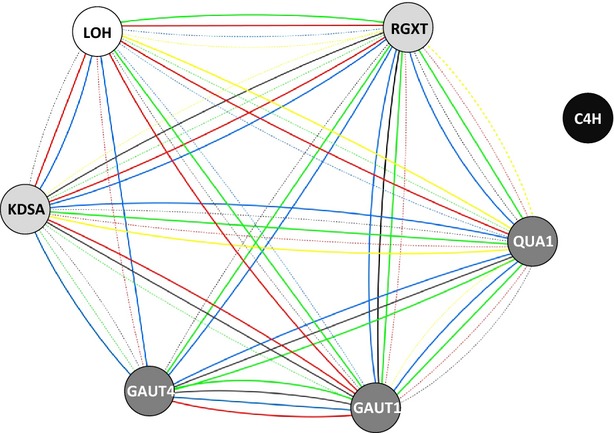
Co-expression network between genes involved in RG-II and glycosylinositol phosphorylceramide (GIPC) biosynthesis in various species using an edge-weighted force-directed approach, based on data retrieved from ATTED-II and visualised in Cytoscape 2.8 (http://www.cytoscape.org). Circles: white circle, LOH (encoding a ceramide synthase); pale grey, encoding enzymes involved in synthesis of RG-II side-chains; dark grey, encoding enzymes involved in synthesis of the homogalacturonan backbone; black, C4H (encoding cinnamate-4-hydroxylase, involved in lignin synthesis; included as a control). Lines: green lines, Arabidopsis thaliana; blue, Populus trichocarpa; red, Oryza sativa; black, Glycine max; yellow, Zea mays. Solid line, strong co-expression [mutual rank (MR) < 1000]; dotted line, weak co-expression (5000 > MR > 1000); no line, no transcriptomic data available or no co-expression (MR > 5000).
Conclusion
Our results indicate that the essential element B, well known to contribute to cell-wall assembly by cross-linking pectic RG-II domains, also plays a structural role in plant lipid rafts. The plant-specific membrane components with which B interacts are proposed to be GIPCs on the evidence that their extractability is affected by in-vivo B supply, and by in-vitro treatment with cold dilute HCl (known to cleave borate diester bonds) or with borate buffer. We argue that the ability of high borate concentrations to disrupt lipid rafts may account for the phytotoxicity of excess soluble B which is encountered in certain soils especially in semi-arid areas. Furthermore, the electrophoretically observed in-vitro formation of a GIPC–RG-II complex, proposed to be established via a diol–B(−)–diol diester bridge, gives the first molecular explanation of the wall–membrane attachment sites that have been reported in vivo. Finally, our results suggest a synergistic role for GIPCs in the dimerization of pectic RG-II domains.
Experimental Procedures
Rosa cell-suspension cultures
Cell-suspension cultures of ‘Paul’s Scarlet’ rose [a complex hybrid; genus Rosa, initiated by Nickell and Tulecke (1959)], were grown as described by Chormova et al. (2013) in a medium that contained 3.3 μm H3BO3.
Extraction of GIPCs
GIPCs were extracted according to a method adapted from Buré et al., 2011, yielding almost pure GIPCs. For this procedure, 1 g of rose cells was ground with a pestle and mortar in 4 ml ice-cold 0.1 m aqueous acetic acid. The slurry was filtered through acid-washed Miracloth (Calbiochem, http://www.merckmillipore.co.uk/life-science-research/calbiochem/), on a funnel and the residue washed with cold 0.1 m acetic acid. The filtrate was discarded and the residue was then incubated in 70% ethanol at 70°C for 15 min with or without 0.1 m HCl. The slurry was filtered hot through Miracloth and washed with 70% ethanol (±0.1 m HCl). The residue was re-extracted twice more in the same manner. The combined ethanolic filtrates were chilled immediately and left at −20°C overnight. The GIPC-containing precipitate was pelleted by centrifugation at 2000 g at 4°C for 15 min. The pellet was washed with ice-cold acetone until washes were non-acidic, and finally with cold diethyl ether, yielding a whitish precipitate, which was dried in vacuo. For studying the impact of B on GIPC extraction, the dried precipitate was directly phase-partitioned between butan-1-ol and water (±0.1 m HCl or 10 mm βMCD or 6 mm borate buffer pH 9.2 or ammonium buffer pH 9.2). For purifying GIPCs, the dried precipitate was suspended in 10 ml methanol/45% (aqueous) formic acid/chloroform (5:1:4 by volume) followed by 5 ml of water and homogenised. Whitish GIPC-containing material that collected at the aqueous/organic interface was dried and then phase-partitioned between butan-1-ol and aqueous 0.1 m HCl (1:1, v/v). Finally, the upper (butanol-rich) phase was dried and the residue was dissolved in 70% ethanol.
ESI–MS and ESI–MS/MS
GIPC extracts were diluted 16-fold in 65:35 (v/v) isopropanol/water that contained 0.03% (w/v) ammonium acetate. Analyses were performed on a Q-TRAP mass spectrometer (Applied Biosystems, http://www.lifetechnologies.com) and samples infused at a flow rate of 7 μl min−1. ESI–MS/MS experiments were performed in accordance with Buré et al. (2011).
Separation and purification of GIPCs by TLC
Lipids were chromatographed on Merck silica gel ‘60’ TLC plates in CHCl3:CH3OH:4 N NH4OH (9:7:2, v/v) with 0.2 m ammonium acetate, a system described by Kaul and Lester (1975). In some cases the plate was immersed in 1% (w/v) di-sodium tetraborate decahydrate in MeOH for 15 sec and air-dried overnight before the samples were loaded. The chromatograms were sprayed with rhodamine 6G, which located the lipid spots, and with 1% sodium meta-periodate followed after a few minutes by 0.01% Schiff’s reagent (Sigma), which stains compounds with vicinal diol groups. After rhodamine spraying, glycolipid spots were specifically visualised by subsequent spraying with orcinol reagent (Skipski and Barclay, 1969). Relative band intensities were determined by use of ImageJ software (http://imagej.nih.gov/ij/).
High-voltage paper electrophoresis
High-voltage paper electrophoresis (PE) of samples that contained [3H]RG-II was performed on Whatman No. 20 paper in pH 2 buffer [water/formic acid/acetic acid (45:1:4, by vol.)] (Fry, 2011) at 4.5 kV for 75 min or at 3.5 kV for 45 min. The papers were read with an AR-2000 radio-TLC Imaging Scanner (LabLogic, http://www.lablogic.com/).
RG-II purification and radio-labelling
RG-II was prepared from suspension-cultured Rosa cells and monomerised as described (Chormova et al., 2013) and was kindly given by Dr Dimitra Chormova. In some experiments, we used monomeric [3H]RG-II of specific activity 17 MBq/μmol RG-II, also prepared by the method of Chormova et al. (2013).
In-vitro RG-II dimerization and gel electrophoresis
In-vitro RG-II dimerization and gel electrophoresis were performed according to Chormova et al. (2013).
Acknowledgments
We thank Dr Dimitra Chormova for providing purified RG-II and for help with gel electrophoresis, and the BBSRC (UK) (grant reference BB/H000690/1) for financial support. We thank Dr Christophe Rihouey from the University of Rouen for help with the MS.
References
- Ahn J, Verma R, Kim M, Lee J, Kim Y, Bang J, Reiter W, Pai H. Depletion of UDP-d-apiose/UDP-d-xylose synthases results in rhamnogalacturonan-II deficiency, cell-wall thickening, and cell death in higher plants. J. Biol. Chem. 2006;281:13708–13716. doi: 10.1074/jbc.M512403200. [DOI] [PubMed] [Google Scholar]
- Al-Mustafa WA, Falatah AM, El-Shall AA. Effect of excess boron fertilization on status and availability of boron in calcareous soils. Fertilizer Res. 1993;36:71–78. [Google Scholar]
- Aquea F, Federici F, Moscoso C, Vega A, Jullian P, Haseloff JIM, Arce-Johnson P. A molecular framework for the inhibition of Arabidopsis root growth in response to boron toxicity. Plant, Cell Environ. 2012;35:719–734. doi: 10.1111/j.1365-3040.2011.02446.x. [DOI] [PubMed] [Google Scholar]
- Bassil E, Hu H, Brown PH. Use of phenylboronic acids to investigate boron function in plants. possible role of boron in transvacuolar cytoplasmic strands and cell-to-wall adhesion. Plant Physiol. 2004;136:3383–3395. doi: 10.1104/pp.104.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins DG, Lukaszewski KM. Boron in plant structure and function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:481–500. doi: 10.1146/annurev.arplant.49.1.481. [DOI] [PubMed] [Google Scholar]
- Borner GHH, Sherrier DJ, Weimar T, Michaelson LV, Hawkins ND, Macaskill A, Napier JA, Beale MH, Lilley KS, Dupree P. Analysis of detergent-resistant membranes in Arabidopsis. Evidence for plasma membrane lipid rafts. Plant Physiol. 2005;137:104–116. doi: 10.1104/pp.104.053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PH, Bellaloui N, Wimmer MA, Bassil ES, Ruiz J, Hu H, Pfeffer H, Dannel F, Römheld V. Boron in plant biology. Plant Biol. 2002;4:205–223. [Google Scholar]
- Buré C, Cacas JL, Wang F, Gaudin K, Domergue F, Mongrand S, Schmitter J. Fast screening of highly glycosylated plant sphingolipids by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2011;25:3131–3145. doi: 10.1002/rcm.5206. [DOI] [PubMed] [Google Scholar]
- Buré C, Cacas J, Mongrand S, Schmitter J. Characterization of glycosyl inositol phosphoryl ceramides from plants and fungi by mass spectrometry. Anal. Bioanal. Chem. 2013;1:16. doi: 10.1007/s00216-013-7130-8. [DOI] [PubMed] [Google Scholar]
- Cacas JL, Buré C, Furt F, Maalouf JP, Badoc A, Cluzet S, Schmitter JM, Antajan E, Mongrand S. Biochemical survey of the polar head of plant glycosylinositolphosphoceramides unravels broad diversity. Phytochemistry. 2013;96:191–200. doi: 10.1016/j.phytochem.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Chormova D, Messenger DJ, Fry SC. Boron bridging of rhamnogalacturonan-II, monitored by gel electrophoresis, occurs during polysaccharide synthesis and secretion, but not post-secretion. Plant J. 2013;77:534–546. doi: 10.1111/tpj.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello CE, Vath JE. Tandem mass spectrometry of glycolipids. Methods Enzymol. 1990;193:738–768. doi: 10.1016/0076-6879(90)93448-t. [DOI] [PubMed] [Google Scholar]
- Delmas F, Séveno M, Northey JGB, Hernould M, Lerouge P, McCourt P, Chevalier C. The synthesis of the rhamnogalacturonan II component 3-deoxy-dmanno-2-octulosonic acid (KDO) is required for pollen tube growth and elongation. J. Exp. Bot. 2008;59:2639–2647. doi: 10.1093/jxb/ern118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findeklee P, Goldbach HE. Rapid effects of boron deficiency on cell wall elasticity modulus in Cucurbita pepo roots. Bot. Acta. 1996;109:463–465. [Google Scholar]
- Fry SC. High-voltage paper electrophoresis (HVPE) of cell-wall building blocks and their metabolic precursors. Methods Mol. Biol. 2011;715:55–80. doi: 10.1007/978-1-61779-008-9_4. [DOI] [PubMed] [Google Scholar]
- Goldbach H. Influence of boron nutrition on net uptake and efflux of 32P and 14C-glucose in helianthus annuus roots and cell cultures of daucus carota. J. Plant Physiol. 1985;118:431–438. doi: 10.1016/S0176-1617(85)80203-0. [DOI] [PubMed] [Google Scholar]
- Goldbach HE, Wimmer MA. Boron in plants and animals: is there a role beyond cell-wall structure? J. Plant Nutr. Soil Sci. 2007;170:39–48. [Google Scholar]
- Goldbach HE, Hartmann D, Rötzer T. Boron is required for the stimulation of the ferricyanide-induced proton release by auxins in suspension-cultured cells of Daucus carota and Lycopersicon esculentum. Physiol. Plant. 1990;80:114–118. [Google Scholar]
- Hirsch AM, Torrey JG. Ultrastructural changes in sunflower root cells in relation to boron deficiency and added auxin. Can. J. Bot. 1980;58:856–866. [Google Scholar]
- Hu H, Brown PH, Labavitch JM. Species variability in boron requirement is correlated with cell wall pectin. J. Exp. Bot. 1996;47:227–232. [Google Scholar]
- Ishii T, Matsunaga T, Pellerin P, O’Neill MA, Darvill A, Albersheim P. The plant cell wall polysaccharide rhamnogalacturonan II self-assembles into a covalently cross-linked dimer. J. Biol. Chem. 1999;274:13098–13104. doi: 10.1074/jbc.274.19.13098. [DOI] [PubMed] [Google Scholar]
- Kaul K, Lester RL. Characterization of inositol-containing phosphosphingolipids from tobacco leaves: isolation and identification of two novel, major lipids: N-acetylglucosamidoglucuronidoinositol phosphorylceramide and glucosamidoglucuronidoinositol phosphorylceramide. Plant Physiol. 1975;55:120–129. doi: 10.1104/pp.55.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul K, Lester RL. Isolation of 6 novel phosphoinositol-containing sphingolipids from tobacco leaves. Biochemistry. 1978;17:3569–3575. doi: 10.1021/bi00610a023. [DOI] [PubMed] [Google Scholar]
- Lester RL, Dickson RC. Sphingolipids with inositolphosphate-containing head groups. Adv. Lipid Res. 1993;26:253–274. [PubMed] [Google Scholar]
- Levery SB, Toledo MS, Straus AH, Takahashi HK. Comparative analysis of glycosylinositol phosphorylceramides from fungi by electrospray tandem mass spectrometry with low-energy collision-induced dissociation of Li+ adduct ions. Rapid Commun. Mass Spectrom. 2001;15:2240–2258. doi: 10.1002/rcm.505. [DOI] [PubMed] [Google Scholar]
- Loomis WD, Durst RW. Chemistry and biology of boron. BioFactors. 1992;3:229–239. [PubMed] [Google Scholar]
- Lynch DV, Dunn TM. An introduction to plant sphingolipids and a review of recent advances in understanding their metabolism and function. New Phytol. 2004;161:677–702. doi: 10.1111/j.1469-8137.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- Markham JE, Li J, Cahoon EB, Jaworski JG. Separation and identification of major plant sphingolipid classes from leaves. J. Biol. Chem. 2006;281:22684–22694. doi: 10.1074/jbc.M604050200. [DOI] [PubMed] [Google Scholar]
- Markham JE, Molino D, Gissot L, Bellec Y, Hématy K, Marion J, Belcram K, Palauqui J, Satiat-Jeunemaître B, Faure J. Sphingolipids containing very-long-chain fatty acids define a secretory pathway for specific polar plasma membrane protein targeting in Arabidopsis. Plant Cell. 2011;23:2362–2378. doi: 10.1105/tpc.110.080473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JE, Lynch DV, Napier JA, Dunn TM, Cahoon EB. Plant sphingolipids: function follows form. Curr. Opin. Plant Biol. 2013;16:350–357. doi: 10.1016/j.pbi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Matoh T, Kawaguchi S, Kobayashi M. Ubiquity of a borate–rhamnogalacturonan II complex in the cell walls of higher plants. Plant Cell Physiol. 1996;37:636–640. [Google Scholar]
- Matoh T, Takasaki M, Takabe K, Kobayashi M. Immunocytochemistry of rhamnogalacturonan ii in cell walls of higher plants. Plant Cell Physiol. 1998;39:483–491. [Google Scholar]
- Matsunaga T, Ishii T. Borate cross-linked/total rhamnogalacturonan II ratio in cell walls for the biochemical diagnosis of boron deficiency in hydroponically grown pumpkin. Anal. Sci. 2006;2:1125–1127. doi: 10.2116/analsci.22.1125. [DOI] [PubMed] [Google Scholar]
- Matsunaga T, Ishii T, Matsumoto S, Higuchi M, Darvill A, Albersheim P, O’Neill MA. Occurrence of the primary cell wall polysaccharide rhamnogalacturonan II in pteridophytes, lycophytes, and bryophytes. Implications for the evolution of vascular plants. Plant Physiol. 2004;134:339–351. doi: 10.1104/pp.103.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohnen D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008;11:266–277. doi: 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Morel J, Claverol S, Mongrand S, Furt F, Fromentin J, Bessoule J, Blein J, Simon-Plas F. Proteomics of plant detergent-resistant membranes. Mol. Cell. Proteomics. 2006;5:1396–1411. doi: 10.1074/mcp.M600044-MCP200. [DOI] [PubMed] [Google Scholar]
- Mortimer JC, Yu X, Albrecht S, et al. Abnormal glycosphingolipid mannosylation triggers salicylic acid-mediated responses in Arabidopsis. Plant Cell. 2013;25:1881–1894. doi: 10.1105/tpc.113.111500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell LG, Tulecke W. Responses of plant tissue cultures to gibberellin. Bot. Gaz. 1959;24:5–250. [Google Scholar]
- Obayashi T, Okamura Y, Ito S, Tadaka S, Aoki Y, Shirota M, Kinoshita K. Atted-II in 2014: evaluation of gene coexpression in agriculturally important plants. Plant Cell Physiol. 2014;55:e6. doi: 10.1093/pcp/pct178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord RE. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966;211:591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- O’Neill MA, Warrenfeltz D, Kates K, Pellerin P, Doco T, Darvill AG, Albersheim P. Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester. In vitro conditions for the formation and hydrolysis of the dimer. J. Biol. Chem. 1996;271:22923–22930. doi: 10.1074/jbc.271.37.22923. [DOI] [PubMed] [Google Scholar]
- O’Neill MA, Eberhard S, Albersheim P, Darvill AG. Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science. 2001;294:846–849. doi: 10.1126/science.1062319. [DOI] [PubMed] [Google Scholar]
- O’Neill MA, Ishii T, Albersheim P, Darvill AG. Rhamnogalacturonan II: Structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu. Rev. Plant Biol. 2004;55:109–139. doi: 10.1146/annurev.arplant.55.031903.141750. [DOI] [PubMed] [Google Scholar]
- Pabst M, Fischl RM, Brecker L, Morelle W, Fauland A, Köfeler H, Altmann F, Léonard R. Rhamnogalacturonan II structure shows variation in the side chains monosaccharide composition and methylation status within and across different plant species. Plant J. 2013;76:61–72. doi: 10.1111/tpj.12271. [DOI] [PubMed] [Google Scholar]
- Pollard AS, Parr AJ, Loughman BC. Boron in relation to membrane function in higher plants. J. Exp. Bot. 1977;28:831–841. [Google Scholar]
- Reguera M, Abreu I, Brewin NJ, Bonilla I, Bolaños L. Borate promotes the formation of a complex between legume AGP-extensin and rhamnogalacturonan II and enhances production of Rhizobium capsular polysaccharide during infection thread development in Pisum sativum symbiotic root nodules. Plant, Cell Environ. 2010;33:2112–2120. doi: 10.1111/j.1365-3040.2010.02209.x. [DOI] [PubMed] [Google Scholar]
- Robertson GA, Loughman BC. Reversible effects of boron on the absorption and incorporation of phosphate in Vicia faba L. New Phytol. 1974;73:291–298. [Google Scholar]
- Roche Y, Gerbeau-Pissot P, Buhot B, Thomas D, Bonneau L, Gresti J, Mongrand S, Perrier-Cornet JM, Simon-Plas F. Depletion of phytosterols from the plant plasma membrane provides evidence for disruption of lipid rafts. FASEB J. 2008;22:3980–3991. doi: 10.1096/fj.08-111070. [DOI] [PubMed] [Google Scholar]
- Seifert GJ, Roberts K. The biology of arabinogalactan proteins. Annu. Rev. Plant Biol. 2007;58:137–161. doi: 10.1146/annurev.arplant.58.032806.103801. [DOI] [PubMed] [Google Scholar]
- Skipski VP, Barclay M. Thin-layer chromatography of lipids. Methods Enzymol. 1969;14:530–598. [Google Scholar]
- Smith CK, Fry SC. Biosynthetic origin and longevity in vivo of α-d-mannopyranosyl-(1→4)-α-d-glucuronopyranosyl-(1→2)-myo-inositol, an unusual extracellular oligosaccharide produced by cultured rose cells. Planta. 1999;210:150–156. doi: 10.1007/s004250050664. [DOI] [PubMed] [Google Scholar]
- Smith CK, Hewage CM, Fry SC, Sadler IH. α-d-mannopyranosyl-(1→4)-α-d-glucuronopyranosyl-(1→2)-myo-inositol, a new and unusual oligosaccharide from cultured rose cells. Phytochemistry. 1999;52:387–396. doi: 10.1007/s004250050664. [DOI] [PubMed] [Google Scholar]
- Smyth DA, Dugger WM. Effects of boron deficiency on rubidium uptake and photosynthesis in the diatom Cylindrotheca fusiformis. Plant Physiol. 1980;66:692–695. doi: 10.1104/pp.66.4.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling P, Franke S, Lüthje S, Heinz E. Are glucocerebrosides the predominant sphingolipids in plant plasma membranes? Plant Physiol. Biochem. 2005;43:1031–1038. doi: 10.1016/j.plaphy.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Ternes P, Feussner K, Werner S, Lerche J, Iven T, Heilmann I, Riezman H, Feussner I. Disruption of the ceramide synthase LOH1 causes spontaneous cell death in Arabidopsis thaliana. New Phytol. 2011;192:841–854. doi: 10.1111/j.1469-8137.2011.03852.x. [DOI] [PubMed] [Google Scholar]
- Voxeur A, Gilbert L, Rihouey C, Driouich A, Rothan C, Baldet P, Lerouge P. Silencing of the GDP-d-mannose 3,5-epimerase affects the structure and cross-linking of the pectic polysaccharide rhamnogalacturonan II and plant growth in tomato. J. Biol. Chem. 2011;286:8014–8020. doi: 10.1074/jbc.M110.198614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warington K. The effect of boric acid and borax on the broad bean and certain other plants. Ann. Bot. 1923;37:629–672. (old series), [Google Scholar]
- Wimmer MA, Lochnit G, Bassil E, Mühling KH, Goldbach HE. Membrane-associated, boron-interacting proteins isolated by boronate affinity chromatography. Plant Cell Physiol. 2009;50:1292–1304. doi: 10.1093/pcp/pcp073. [DOI] [PubMed] [Google Scholar]