Abstract
Our previous studies showed that anti-β2M monoclonal antibodies (mAbs) at high doses have direct apoptotic effects on myeloma cells, suggesting that anti-β2M mAbs might be developed as a novel therapeutic agent. In this study, we investigated the ability of the mAbs at much lower concentrations to indirectly kill myeloma cells by utilizing immune effector cells or molecules. Our results showed that anti-β2M mAbs effectively lysed MM cells via antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), which were correlated with and dependent on the surface expression of β2M on MM cells. The presence of MM bone marrow stromal cells or addition of IL-6 did not attenuate anti-β2M mAb-induced ADCC and CDC activities against MM cells. Furthermore, anti-β2M mAbs only showed limited cytotoxicity toward normal B cells and non-tumorous mesenchymal stem cells, indicating that the ADCC and CDC activities of the anti-β2M mAbs were more prone to the tumor cells. Lenalidomide potentiated in vitro ADCC activity against MM cells and in vivo tumor inhibition capacity induced by the anti-β2M mAbs by enhancing the activity of NK cells. These results support clinical development of anti-β2M mAbs, both as a monotherapy and in combination with lenalidomide, to improve MM patient outcome.
Keywords: β2-microglubulin, monoclonal antibody, antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, multiple myeloma
Introduction
Multiple myeloma (MM) is a clonal plasma cell neoplasm that utilizes the bone marrow (BM) microenvironment for survival and proliferation1-3. Current myeloma therapies such as hematopoietic cell transplantation and combinatorial chemotherapies are rarely curative and relapse is common. This implies that therapy-resistant myeloma-initiating cells exist and that new therapeutics must be developed to target and eradicate these chemoresistant myeloma cells.
Lenalidomide is a potent novel thalidomide analog which has demonstrated remarkable clinical activity in the treatment of MM4. The strong evidence-based clinical success of lenalidomide in MM patients has led to its approval by US-FDA under the trade name of Revlimid® capsules by Celgene Corporation. However, adverse effects and drug resistance have been observed in MM patients, which are great challenges for the extended application of lenalidomide5.
Targeted immunotherapy with monoclonal antibodies (mAbs) is an effective and safe method for the treatment of cancers. However, there is still no mAb-based cancer therapy approved to treat patients with MM. Early clinical trials of mAbs targeting CD20 and CD38 have conveyed only very limited benefit to the treatment of MM6-8. In recent years, efforts have been made to identify potential therapeutic mAbs by defining alternative or novel MM target antigens, i.e., CD409, 10, IL6R11, HM1.2412, 13, CD7414, CD4715, TRAIL-R116, CS117, as well as to conjugate mAbs with classic or novel drugs to specifically kill MM cells, i.e., CD56-maytansinoid (DM1)18, CD138-DM1/DM419. Development of mAbs with improved cytotoxicity, targeting new and known myeloma-associated antigens, continues to be an active research area.
β2-microglubulin (β2M) is a part of the major histocompatibility complex (MHC) class I molecule on the cell surface of nucleated cells20. We have recently demonstrated that human β2M is a potential target for MM treatment21. Our previous studies showed that anti-β2M mAbs have strong direct apoptotic effects on myeloma and other hematological malignancies with less toxicity on normal tissues and cells in vitro and in mouse models21, 22, suggesting that anti-β2M mAbs might be a novel therapeutic agent for MM. Furthermore, others have reported similar results by using anti-MHC class-1 single-chain Fv diabody or anti-β2M antibodies to induce apoptosis in human myeloma23, renal cell carcinoma24, and prostate cancer25.
Natural killer (NK) cell-mediated antibody-dependent cell-mediated cytotoxicity (ADCC) is a critical mechanism for many approved therapeutic mAbs26-28. FcγRIIIa, a member of the leukocyte receptor family FcγRs, is known to be a major triggering receptor of ADCC in NK cells. FcγRIIIa polymorphism status of NK cells from cancer patients plays a key role in the clinical outcome of patients receiving rituximab27, trastuzumab29, or cetuximab28. Complement-dependent cytotoxicity (CDC) is a cytolytic cascade mechanism by which complement proteins present in serum are activated by antigen-specific antibodies. CDC is triggered by the binding of C1q, a subunit of C1, to the CH2 domain of a cell-bound IgG antibody, leading to the formation of the membrane attack complex (MAC) and ultimately lysis of target cells30. Human IgG1 and IgG3 efficiently mediate effector function activities, while IgG2 and IgG4 are generally ineffective31, 32.
In this study, we evaluated anti-β2M mAb-mediated ADCC and CDC activities against established human MM cell lines and primary MM cells from patients. The ADCC and CDC activities of anti-β2M mAbs were more against tumor cells, and BM microenvironment could not protect MM cells from anti-β2M mAb-mediated ADCC and CDC activities. Lenalidomide enhanced in vitro and in vivo anti-β2M mAb-mediated ADCC activities.
Materials and Methods
Cell lines and primary cells
Human myeloma cell line ARP-1 and CAG were established at the University of Arkansas for Medical Sciences from BM aspirates of patients with MM33. MM.1S was kindly provided by Dr. Steven Rosen of Northwestern University (Chicago, IL). U266 WT and U266/R10R were generously provided by Dr. Robert Z. Orlowski of MD Anderson Cancer Center34 (Houston, TX). RPMI-8226 was purchased from ATCC. Human peripheral blood mononuclear cells (PBMCs) were isolated from blood of healthy donors by Ficoll-Hypaque density centrifugation. B cells were separated from fresh PBMCs using EasySep™ Human B Cell Enrichment Kit (Stem cell Technologies). CD138+ myeloma cells were purified from BM aspirates of MM patients using RoboSep™ Human Whole Blood and Bone Marrow CD138 Positive Selection Kit (Stem cell Technologies). Human bone marrow-derived mesenchymal stem cells (MSCs) were established from BM aspirates of patients with MM as previously described35. Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum and were maintained in 37°C with 5% CO2.
ADCC and CDC assays
β2M-specific D1 mAbs were generated as previously described21, and mouse IgG1 (mIgG1; BioLegend) was used as isotype control. ADCC and CDC were measured by 51Chromium (51Cr)-release assays. In ADCC assay, PBMCs from normal volunteers were used as effector cells, and in CDC assay guinea pig serum (Sigma-Aldrich) was used as the source of complements. Target cells (1 × 106) including myeloma cells, normal B cells or MSCs were incubated with 200 μCi of 51Cr for 1 hour at 37°C with gentle resuspension of pellet at 15 minute intervals. After washing, cells were plated at 10,000 cells/well in 96-well U-bottom plate with PBMCs or guinea pig serum. This was followed by the addition of anti-β2M mAbs at final concentrations ranging from 5 to 20 μg/ml. In some experiments, PBMCs were pretreated with lenalidomide (2 μM; Selleck Chem) or human IL-2 (10 U/ml; R&D Systems) for 48 hours before assay, or target cells were co cultured with human IL-6 (1 ng/ml; R&D Systems), or the plates were precoated with MSCs overnight before adding target cells. Cells were then incubated for 4 hours at 37°C, and cell-released 51Cr was measured using a gamma-Counter. Spontaneous release was determined from target cells without the addition of anti-β2M mAbs, PBMCs or guinea pig serum, and maximum release was determined from target cells with 6% Triton X-100 without the addition of the mAbs, PBMCs or guinea pig serum. Percent cytotoxicity was calculated as [(counts in sample − spontaneous release)/(maximum counts − spontaneous release)] ×100%. All experiments were performed in triplicate.
β2M short-hairpin RNA transfection of myeloma cells by lentivirus
Myeloma cells were transfected using human β2M short-hairpin RNA (shRNA) lentiviral particles (Genecopoeia) according to the manufacturer's protocol to knockdown β2M expression.
Western blotting
Western blotting was conducted as previously described21. Mouse anti-β2M mAb was obtained from Santa Cruz Biotech. Rabbit anti-β-actin polyclonal antibody was obtained from Sigma-Aldrich. The experiments were carried out in triplicate.
Quantitative real-time PCR
The primers for amplification were as follows: β2M-F 5′-AAT TGA AAA AGT GGA GCA TTC AGA-3′; β2M-R 5′-GGC TGT GAC AAA GTC ACA TGG TT-3′; GAPDH-F 5′-CAC TCC TCC ACC TTT GAC G-3′; GAPDH-R 5′-ACC ACC CTG TTG CTG TAG C-3′. Gene expression levels in each cDNA sample were normalized to the internal GAPDH levels. The experiments were carried out in triplicate for each data point.
Cell proliferation
Cells were plated at a density of 1,000 cells/well in triplicate in 96-well culture plates. After two-day culture, cell proliferation was monitored by detecting absorbance at 490 nm with an automatic microplate reader using MTS assay (Promega). The experiments were carried out in triplicate.
Flow cytometry
APC-conjugated mAbs against human β2M, HLA-ABC, CD138, and isotype controls were obtained from BioLegend. FITC-labeled Annex in V antibody and PI were purchased from Life Technologies. Data were acquired with a flow cytometer (FACS Calibur; BD Biosciences). The experiments were carried out in triplicate.
Enzyme-linked immunosorbent assay
Cell culture supernatants were collected, and the amount of secreted β2M in the supernatants was quantified using human β2M Quantikine IVD ELISA Kit (R&D Systems). The experiments were carried out in triplicate.
In vivo tumor xenograft models
Six week old male SCID mice (Jackson Laboratory) were injected subcutaneously in the right flank with 1 × 106 APR-1 cells. Three to four weeks later when palpable tumors (5 mm in diameter) developed, mice (5 per group) were intraperitoneally injected with lenalidomide (25 mg/kg), anti-β2M mAbs (5 mg/kg) subcutaneously (around tumors) or in combination of both every 3 days. Control mice received equal amounts of mIgG1 or DMSO. Tumors were measured every 3 days with calipers and tumor volumes (mm3) were calculated as (width2 × length)/2. Mice were humanely sacrificed when moribund or when subcutaneous tumors reached 15 mm in diameter. All mice were maintained in American Association of Laboratory Animal Care-accredited facilities, and studies were approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center and Cleveland Clinic.
In situ apoptosis assay
In situ tumor cell apoptosis was determined using the TdT-mediated dUTP nick-end labeling (TUNEL) assay (Boehringer-Mannheim). Sectioned tumor tissue was embedded in paraffin. Three slides from each group were evaluated for the apoptotic cells. Six slide fields were randomly examined using a defined rectangular field area with × 200 magnification, and apoptotic cells were counted in each field.
Statistical Analysis
The Student t test was used to compare various experimental groups. A P value < 0.05 was considered statistically significant. Unless otherwise indicated, the values provided are means and standard deviations (SDs).
Results
Anti-β2M mAbs mediate ADCC activities against myeloma cells
The ADCC activity of anti-β2M mAbs was evaluated using PBMCs isolated from healthy donors as effector cells. As shown in Figure 1A, anti-β2M mAbs at low concentrations (5-20 μg/ml) were able to, in a dose-dependent manner, mediate significant ADCC activities against myeloma ARP-1 cells (P < 0.05 to P < 0.01, compared with mIgG1 control). Significant cell lysis could be observed at an E:T ratio of 40:1 (one myeloma cells: 40 PBMCs); about 40% of myeloma cells were lysed in the culture with the mAbs and only fewer than 10% in those with mIgG1 (P < 0.01). Next, the ADCC activity of anti-β2M mAbs was evaluated against a panel of MM cell lines including ARP-1, MM.1S, U266, CAG and RPMI-8226. Compared to mIgG1, anti-β2M mAbs induced effective lysis of MM cells (Figure 1B; P < 0.05 to P < 0.01). Maximal lysis induced by anti-β2M mAbs ranged from 30% to 50%, which were 2-fold higher over controls for all MM cell lines assayed. Furthermore, B cells and human bone marrow-derived MSCs were used to evaluate the side effects of mAbs treatment on normal cells. At 20 μg/ml of antibody concentration and an E:T ratio of 40:1, the lysis of normal B cells and MSCs was observed in 8.1% and 14.4% cells, respectively, compared with 45.4% of ARP-1 cells (Figure 1C; P < 0.05). These results indicated that anti-β2M mAb-mediated ADCC activity was more towards myeloma cells. In line with these results, purified CD138+ primary myeloma cells but not CD138− non-myeloma cells from the same patients were sensitive to anti-β2M mAb-mediated ADCC (Figure 1D; P < 0.01). Taken together, these results indicated that anti-β2M mAbs at low concentrations were effective at mediating ADCC activities against myeloma cells.
Fig. 1. ADCC activities of anti-β2M mAbs against MM cells.
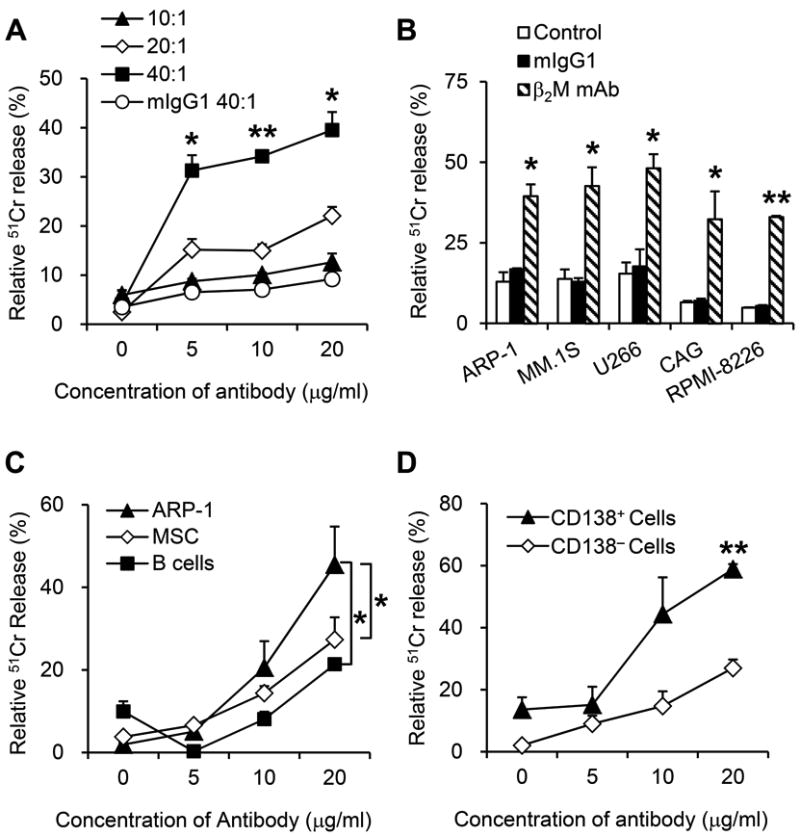
Myeloma cells were incubated with 51Cr for 1 hour, washed, and incubated with different numbers of PBMCs and anti-β2M mAbs D1 or mIgG1 for 4 hours. Shown is ADCC killing of (A) ARP-1, (B) different MM cell lines, (C) MSCs, B cells and ARP-1, and (D) CD138+ primary MM cells and CD138− nonmyeloma cells from MM patients, mediated by anti-β2M mAbs at different concentrations (A, C, and D) and at different E:T ratios (A). In B, C and D, an E: T ratio of 40:1 was used. Summarized data from three performed independent experiments are shown. *P < 0.05, **P < 0.01.
Anti-β2M mAb-mediated ADCC activities correlate with the expression of β2M on cell surface
To evaluate the significance of MM cell surface β2M expression in anti-β2M mAb-mediated ADCC effects, lentiviral systems were utilized to knockdown myeloma cell expression of β2M. The knockdown efficiency was evaluated by flow cytometry, western blotting, quantitative real-time PCR, and ELISA. The results showed that β2M shRNA reduced about 70% of surface expression of β2M (Figure 2A; P < 0.01, compared with controls) and HLA-ABC (Figure 2B; P < 0.01, compared with controls). Significant reduction in the total β2M protein (Figure 2C) and β2M mRNA (Figure 2D) was observed (P < 0.05, compared with controls). In addition, β2M-knockdown cells secreted significantly lower amount of soluble β2M as compared to control cells (Figure 2E; P < 0.05). Next, the ADCC activities mediated by anti-β2M mAbs in β2M-knockdown cells were examined. Compared with control myeloma cells in which about 40% of cells were lysed, only fewer than 20% of β2M-knockdown cells was killed (Figure 2F; P < 0.05), indicating that anti-β2M mAb-mediated ADCC activity depended on the expression of surface β2M on myeloma cells.
Fig. 2. ADCC activities of anti-β2M mAbs correlated with cell surface expression level of β2M.
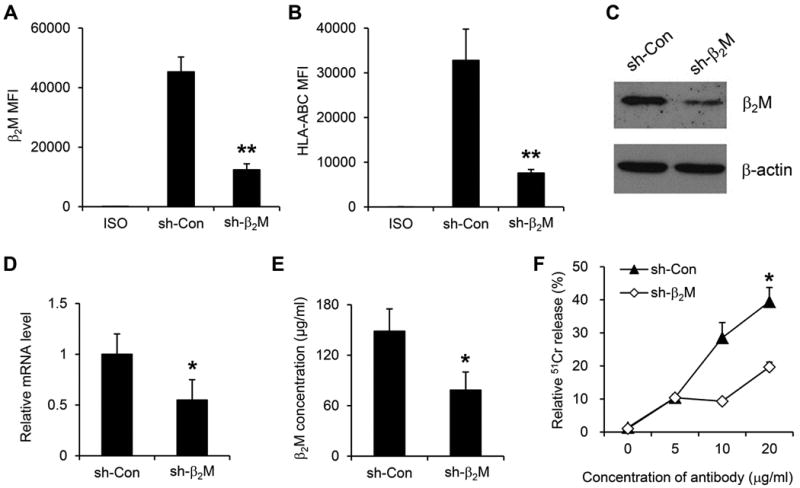
Cell surface expression of (A) β2M and (B) HLA-ABC in β2M-knockdown stably transfected cell line ARP-1. Numbers represent MFI (mean fluorescence intensity). (C) Western blotting analysis of the protein levels of β2M in the MM cells. β-actin was used as an internal control. (D) Quantitative real-time RT-PCR analysis of the relative mRNA levels of β2M in the MM cells. GAPDH was used as an internal control. (E) Secreted β2M concentration by the MM cells. (F) ADCC activities of anti-β2M mAbs against the MM cells. An E:T ratio of 40:1 was used. Summarized data from three performed independent experiments are shown. *P < 0.05, **P < 0.01.
BM microenvironment factors do not protect myeloma cells from anti-β2M mAb-mediated ADCC
Increasing evidence has shown that BMSCs in the myeloma tumor bed provide a tumor promoting microenvironment and protect MM cells from chemotherapy drug-induced apoptosis36. IL-6 is an important survival cytokine for MM37, and promotes MM cell survival under chemotherapy agent dexamethasone treatment38. Therefore, we investigated whether BMSCs and IL-6 were able to protect MM cells from anti-β2M mAb-induced ADCC. Our results showed that equally strong anti-β2M mAb-induced ADCC activities were seen against ARP-1 (Figure 3A) and U266 (Figure 3B) cells in cultures with or without IL-6 or MSCs. These findings suggested that the ADCC activity overcomes the protective effects of IL-6 and BMSCs on MM cells and that the mAbs may be effective at mediating ADCC activity against MM cells in their microenvironment.
Fig. 3. The effects of BM microenvironment or NK cell activators on anti-β2M mAb-mediated ADCC activities.
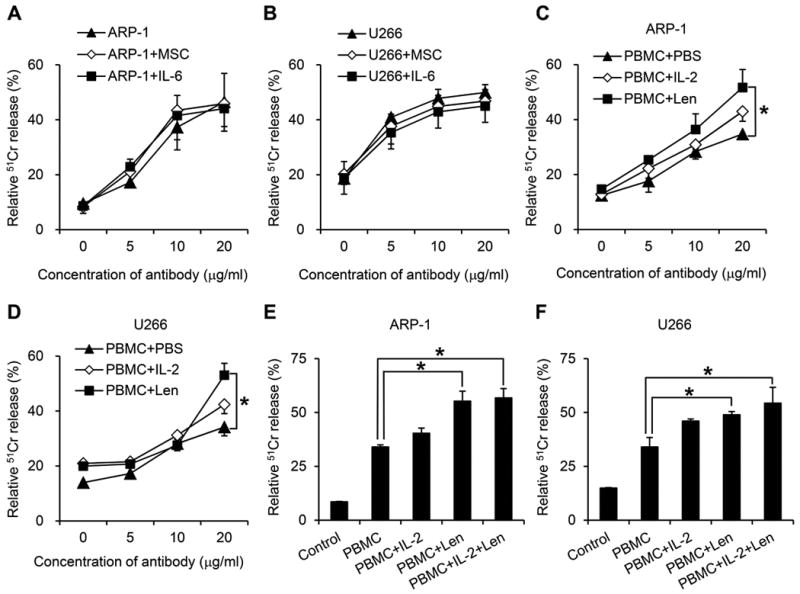
ADCC activities against (A) ARP-1 or (B) U266 cells mediated by anti-β2M mAbs in the presence or absence of MSCs and IL-6. IL-2 and lenalidomide enhance the ADCC activities of anti-β2M mAbs against (C) ARP-1 and (D) U266 cells. Combination of IL-2 and lenalidomide had no further additive or synergistic effect to enhance ADCC activities of anti-β2M mAbs against (E) ARP-1 and (F) U266 cells. An E:T ratio of 40:1 was used in these studies. Summarized data from three performed independent experiments are shown. *P < 0.05.
IL-2 and lenalidomide enhance anti-β2M mAb-mediated ADCC
IL-2 is an essential factor for the differentiation and activity of NK cell, and is involved in the adaptive immune responses39. Lenalidomide is an immunomodulatory drug that has been used effectively for the treatment of MM40, and is also known to increase the activity of NK cells9, 10. To investigate whether these agents may enhance anti-β2M mAb-mediated ADCC activity, PBMCs from healthy donors were preincubated with IL-2 or lenalidomide for 48 hours before assay. The results showed that pretreatment of effector cells with IL-2 or lenalidomide enhanced anti-β2M mAb-mediated ADCC against ARP-1 (Figure 3C) and U266 cells (Figure 3D), and the effect of lenalidomide was stronger than IL-2 (P < 0.05). Next we combined lenalidomide and IL-2 to determine whether there was synergistic effect of the two. The results (Figure 3E, 3F) showed that there was no further enhancing effect of combining lenalidomide and IL-2 compared with lenalidomide alone. These results indicated that NK cell activators lenalidomide and IL-2 could enhance anti-β2M mAb-mediated ADCC, and lenalidomide was more efficient than IL-2, whereas the two NK cells activators had no additive or synergistic effect on the ADCC activity.
Combination treatment of anti-β2M mAbs and lenalidomide in vitro and in vivo
To investigate whether there was a synergistic effect of lenalidomide and anti-β2M mAb, we analyzed ADCC activities of anti-β2M mAbs on lenalidomide-sensitive U266 WT and lenalidomide-resistant U266/R10R cell lines34. MTS assay was used and confirmed the drug sensitivity of the cell lines to lenalidomide (Figure 4A). As shown in Figure 4B, lenalidomide alone was only effective on U266 WT cells, while anti-β2M mAbs alone could induce ADCC activities on both lenalidomide-sensitive U266 WT and lenalidomide-resistant U266/R10R cell lines. Combination of both induced similar cell apoptosis in U266 WT and U266/R10R cells, which was more efficacious than either of the treatments alone (P < 0.05 to P < 0.01). These results indicated that anti-β2M mAbs was effective on both lenalidomide-resistant and -sensitive cells and could enhance the anti-tumor effects of lenalidomide.
Fig. 4. Lenalidomide enhances the ADCC activities of anti-β2M mAbs both in vitro and in vivo.
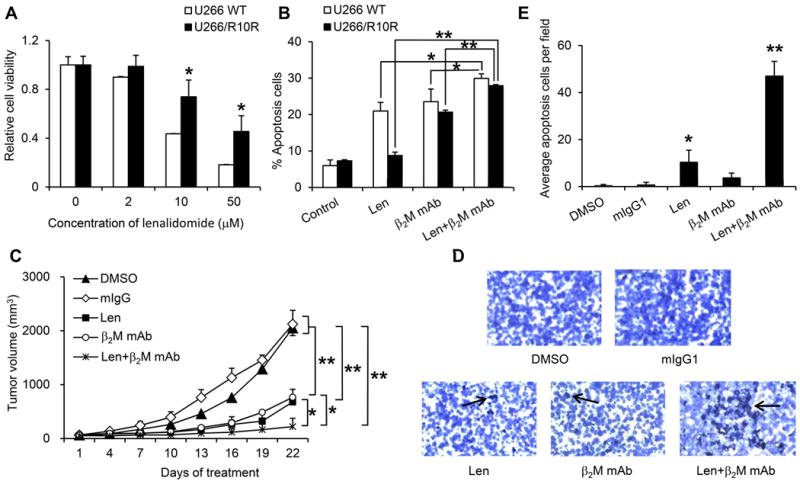
(A) Viability of lenalidomide-resistant U266/R10R and -sensitive U266 WT MM cells in culture with different concentrations of lenalidomide for 48 hours. (B) ADCC activity detected as apoptosis of lenalidomide-resistant U266/R10R and -sensitive U266 WT MM cells. The MM cells and normal PBMCs were first incubated separately with lenalidomide for 48 hours, followed by washing and incubating together with anti-β2M mAbs for 4 hours. Controls included medium alone (control), lenalidomide alone, and ADCC without lenalidomide. An E:T ratio of 40:1 was used. CD138+ MM cell apoptosis was detected using Annexin V and PI staining. (C) Tumor burden of ARP-1 tumor-bearing SCID mice (n=5) treated with lenalidomide alone, anti-β2M mAbs alone, or in combination. (D, E) In situ TUNEL assay was performed to detect cell apoptosis in the tumors of treated mice. Representative images were given in (D). Average numbers of apoptotic tumor cells from 6 randomly chosen fields were given in (E). Summarized data from three performed independent experiments are shown. *P < 0.05, **P < 0.01.
We next examined the therapeutic activities of anti-β2M mAbs in combination with lenalidomide in vivo in a xenograft myeloma model. SCID mice bearing ARP-1 subcutaneous tumors (n = 5 per group) were treated with lenalidomide, anti-β2M mAbs, or a combination of both every 3 days for a total of 3 weeks. DMSO and mIgG1 were used as controls. The doses of the treatments were chosen based on our preliminary studies (data not shown). As shown in Figure 4C, although treatment with anti-β2M mAbs or lenalidomide alone significantly reduced the tumor burdens in the mice (P < 0.05 to P < 0.01, compared with DMSO or mIgG1 controls), combinational treatment with anti-β2M mAbs and lenalidomide was more efficacious than either of the treatments alone (P < 0.05 and P = 0.104, respectively, compared with mAbs or lenalidomide alone; and P < 0.01, compared with DMSO or mIgG1 controls). We used TUNEL assay to detect tumor cell apoptosis in treated mice. As shown in Figure 4D and 4E, significantly higher numbers of apoptotic tumor cells were detected in mice treated with lenalidomide (P < 0.05) and with anti-β2M mAbs and lenalidomide (P < 0.01) compared with DMSO or mIgG1 controls. These data indicated that anti-β2M mAbs and lenalidomide displayed enhanced in vitro and in vivo therapeutic effects against MM.
Anti-β2M mAbs mediate CDC activities against myeloma cells
Next, we evaluated anti-β2M mAb-mediated CDC activities using guinea pig serum as the source of complements. As shown in Figure 5A, anti-β2M mAbs mediated, in a dose-dependent manner, significant CDC activities against myeloma cells as compared with mIgG1 control (P < 0.05 to P < 0.01). Heat-inactivated guinea pig serum was used as a negative control, and no CDC activities were detected (data not shown). Anti-β2M mAb-induced CDC activity was evaluated in a panel of MM cell lines including ARP-1, MM.1S, U266, CAG and RPMI-8226. Compared to mIgG1, anti-β2M mAbs effectively lysed all MM cells via mediating CDC (Figure 5B; P < 0.05 to P < 0.01). We examined anti-β2M mAb-mediated CDC activities against normal B cells, MSCs and ARP-1 cells. The maximal lysis of B cells was 12.8%, MSC cells was 11.1%, and ARP-1 cells was 42.7% at mAb concentration of 20 μg/ml (Figure 5C), which indicated that the CDC activity of anti-β2M mAbs was more towards myeloma cells. Similarly, anti-β2M mAbs induced strong CDC lysis of MM patient-derived CD138+ primary tumor cells (Figure 5D; P < 0.05). These results indicated that anti-β2M mAbs induced CDC activities against human MM cell lines and primary tumor cells from MM patients.
Fig. 5. CDC activities of anti-β2M mAbs in MM cells.
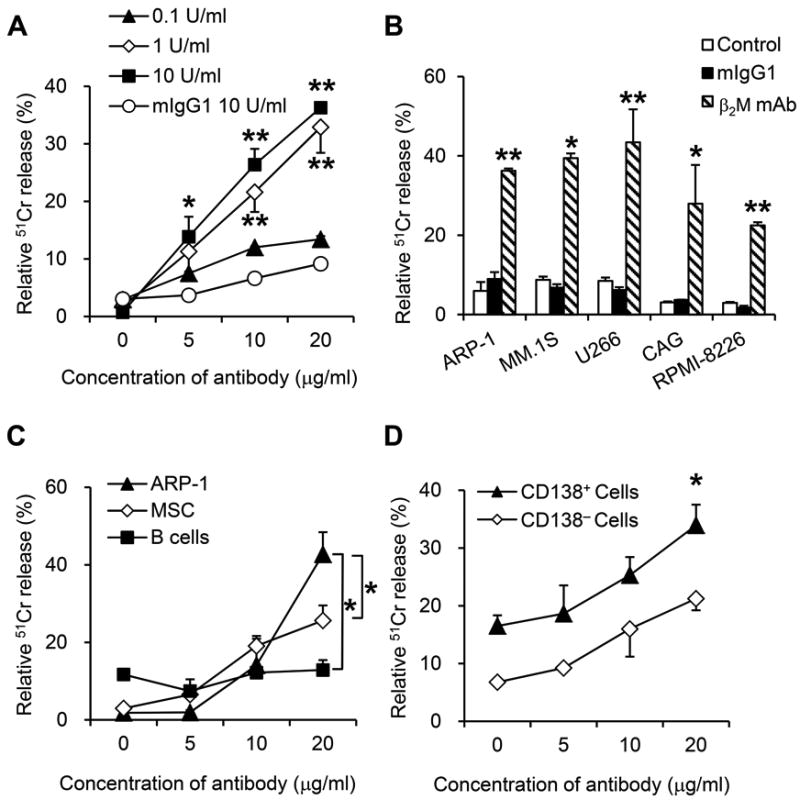
Myeloma cells were incubated with 51Cr for 1 hour, followed by washing, addition of different concentrations of guinea pig serum and anti-β2M mAbs or mIgG1, and incubation for 4 hours. Shown is CDC killing of (A) ARP-1, (B) different MM cell lines, (C) MSCs, B cells and ARP-1, and (D) CD138+ primary MM cells and CD138− nonmyeloma cells from MM patients, mediated by different concentrations of anti-β2M mAbs (A, C, and D) and different concentrations of guinea pig serum (A). In B, C and D, a concentration of guinea pig serum at 10 U/ml was used. Summarized data from three performed independent experiments are shown. *P < 0.05, **P < 0.01.
We examined whether myeloma-supporting stromal cells and IL-6 could protect MM cells against CDC-mediated lysis. In the studies, IL-6 or MSCs were cocultured with myeloma cells during CDC assay. Our results showed that strong anti-β2M mAb-induced CDC activity was seen against ARP-1 (Figure 6A) and U266 (Figure 6B) cells in the absence or presence of MSCs or IL-6, suggesting that anti-β2M mAbs still triggered CDC lysis of MM cells in the presence of BMSCs and IL-6. Anti-β2M mAb-mediated CDC activity also depended on the expression of β2M on the cell surface, because fewer β2M-knockdown myeloma cells were killed as compared with control myeloma cells (Figure 6C; P < 0.05). Finally, we analyzed anti-β2M mAb-mediated CDC activity on lenalidomide-sensitive cells U266 WT and lenalidomide-resistant cells U266/R10R, and the results showed that both cell lines were equally sensitive to the killing (Figure 6D).
Fig. 6. CDC activities of anti-β2M mAbs.
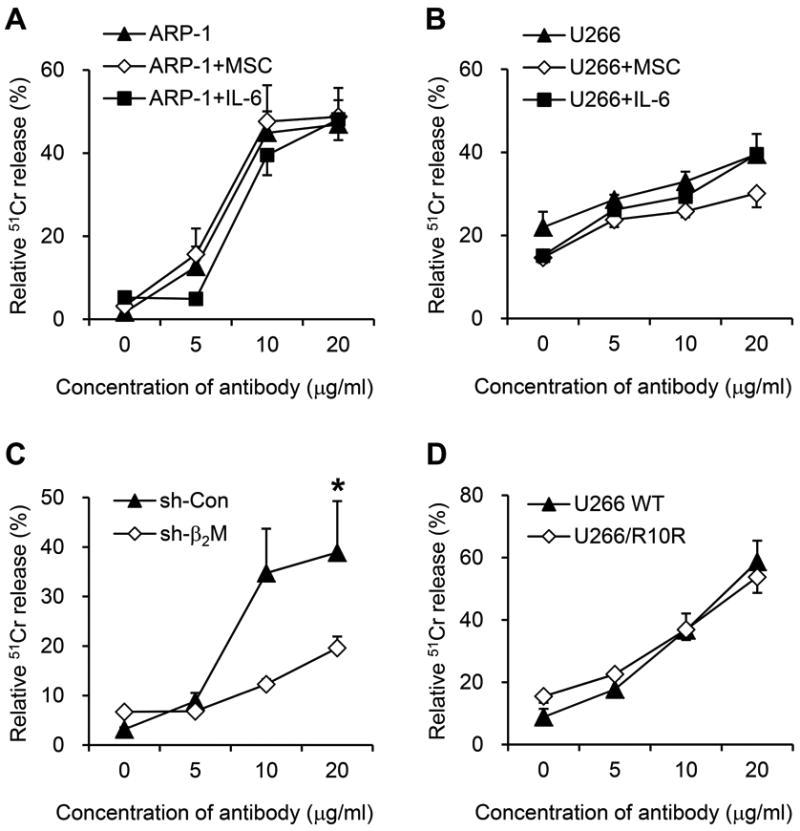
CDC activities of anti-β2M mAbs against (A) ARP-1 and (B) U266 cells in the presence or absence of MSCs and IL-6. (C) CDC activities of anti-β2M mAbs against β2M-knockdown stably transfected cell line ARP-1. (D) CDC activities of anti-β2M mAbs against both lenalidomide-resistant U266/R10R and -sensitive U266 WT cells. A concentration of guinea pig serum at 10 U/ml was used in these studies. Summarized data from three performed independent experiments are shown. *P < 0.05.
Discussion
A large number of antibody therapeutics target surface antigens on tumor cells while simultaneously recruiting immune effector cells to specifically destroy the malignant cells. mAbs are emerging as a major new class of drugs that confer great benefits to cancer patients. Enhancing ADCC and CDC activities is one of the most promising ways to improve the clinical efficacy of already-approved antibodies, and this concept is actively being examined in the clinic, especially in the field of hematological malignancy treatment41, 42. In this study, we observed ADCC and CDC activities of anti-β2M mAbs against established human MM cell lines and primary MM cells from patients. More importantly, lenalidomide enhanced anti-β2M mAb-mediated ADCC activities through increasing the activity of NK cells.
The potent ADCC and CDC activities of anti-β2M mAbs were more prone to the tumor cells and had lower effects on normal B cells and BMSCs. These findings indicated the potential of an immunotherapeutic strategy against MM by anti-β2M mAbs with low side effects. The ADCC and CDC activities of anti-β2M mAbs were dependent on the level of surface expression of β2M. Anti-β2M mAbs were also able to induce significant ADCC and CDC activities against MM cells with low β2M surface expression. Therefore, anti-β2M mAb may impact a larger and heterogeneous β2M-expressing cancer patient population.
Previous research has shown that BMSCs in MM BM play a crucial role in MM drug resistance43. IL-6 promotes myeloma cell proliferation and drug resistance by activating PI3K-Akt pathway44. Our results showed strong ADCC and CDC activities of anti-β2M mAbs on MM cells in the presence of BMSCs or IL-6, indicating its ability to overcome the MM growth and survival advantages conferred by the BM microenvironment. These findings suggested that anti-β2M mAb has potent anti-MM activity and may be used to treat MM patients who have become resistant to conventional chemotherapy drugs.
IL-2 and lenalidomide treatment of NK cells could augment the activities of NK cells10, 45. IL-2 has been assessed, alone or in combination with IL-2-activated killer cells (“adoptive immunotherapy”), for its anticancer potential in several animal models and in patients with various forms of advanced cancers46. Lenalidomide has been shown to modulate the activity of NK cells and macrophages in vitro and in vivo, providing the scientific rationale to combine it with mAb-based cancer therapies10, 17, 47. Anti-β2M mAb, with its enhanced effector cell interaction capability, is expected to have superior anti-MM activity in combination with IL-2 and lenalidomide. Our results showed that IL-2 and lenalidomide pretreatment of effector cells significantly augmented anti-β2M mAb-induced ADCC against ARP-1 and U266 MM cells. Synergy between anti-β2M mAb and lenalidomide could also be found in vivo, underscoring a potential clinical development strategy for combining anti-β2M mAb with lenalidomide to treat patients.
In our ADCC assays, the MM cell lines were allogeneic to the effector PBMCs or T cells. However, no significant lyse was observed in MM cells in cultures of allogeneic PBMCs without the mAbs, indicating that the alloreactivity did not affect the evaluation of anti-β2M mAb-induced ADCC in the target cells. As the ADCC assay is a 4-hour assay, this is too short for alloreactivity of allogeneic PBMCs/T cells to be activated and observed.
ADCC is dependent on the interaction of the IgG Fc domain with FcγRs on effector cells. In this study, we used mouse anti-human β2M-specific IgG1 mAbs to generate ADCC activities with human FcγRs on human NK cells. It is known that there is a cross-reaction between mouse IgGs and human FcγRs on human effector cells48, 49. However, for the therapeutic application, humanized mAbs will be developed and used to reduce the risk for immunogenicity of the mAbs in patients.
In conclusion, this study has demonstrated the significantly enhanced ADCC and CDC activities of anti-β2M mAbs on myeloma but not normal cells, suggesting that anti-β2M mAbs may be a more promising next-generation immunotherapeutic for the treatment of MM. Moreover, lenalidomide potentiated anti-β2M mAb-induced MM cell killing via NK-mediated ADCC, which provides a rationale to combine these drugs to improve patient outcome in MM.
What's new?
anti-β2M mAb-mediated ADCC and CDC activities were correlated with the expression of β2M on the cell surface. anti-β2M mAbs only showed limited cytotoxicity toward normal B cells or non-tumoric MSCs, indicating that the ADCC and CDC activities of anti-β2M mAbs were more prone to the tumor cells. Lenalidomide synergistically enhanced in vitro ADCC against MM cells and in vivo tumor inhibition induced by anti-β2M mAbs through increasing the activity of NK cells. These results support clinical development of anti-β2M mAbs, both as a monotherapy and in combination with lenalidomide, to improve patient outcome of MM.
Acknowledgments
We would like to thank the University of Texas MD Anderson Cancer Center Myeloma Tissue Bank for providing patient samples. This work was supported by grants from the National Cancer Institute (R01 CA138402, R01 CA138398, R01 CA163881, and P50 CA142509), the Leukemia and Lymphoma Society, and the Multiple Myeloma Research Foundation.
Abbreviations
- β2M
β2-microglubulin
- MM
multiple myeloma
- mAb
monoclonal antibody
- ADCC
antibody-dependent cell-mediated cytotoxicity
- CDC
complement-dependent cytotoxicity
- BMSCs
bone marrow stromal cells
- MSCs
mesenchymal stem cells
- IL-6
Interleukin-6
- NK cell
Natural killer cell
- MHC
major histocompatibility complex
- MAC
membrane attack complex
- PBMCs
peripheral blood mononuclear cells
- TUNEL
TdT-mediated dUTP nick-end labeling
- 51Cr
51Chromium
- shRNA
short-hairpin RNA
- SDs
standard deviations
Footnotes
Authorship: Contribution: MZ and QY initiated the work, designed the experiments, and wrote the paper. JQ, YLan, YLu, and HL performed the experiments and statistical analyses, BH, YZ, JH, and JY provided samples and critical suggestion to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Yaccoby S, Barlogie B, Epstein J. Primary myeloma cells growing in SCID-hu mice: a model for studying the biology and treatment of myeloma and its manifestations. Blood. 1998;92:2908–13. [PubMed] [Google Scholar]
- 2.Kirshner J, Thulien KJ, Martin LD, Debes Marun C, Reiman T, Belch AR, Pilarski LM. A unique three-dimensional model for evaluating the impact of therapy on multiple myeloma. Blood. 2008;112:2935–45. doi: 10.1182/blood-2008-02-142430. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Rajkumar SV. Multiple myeloma. The New England journal of medicine. 2004;351:1860–73. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 4.Tariman JD. Lenalidomide: a new agent for patients with relapsed or refractory multiple myeloma. Clinical journal of oncology nursing. 2007;11:569–74. doi: 10.1188/07.CJON.569-574. [DOI] [PubMed] [Google Scholar]
- 5.Rao KV. Lenalidomide in the treatment of multiple myeloma. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2007;64:1799–807. doi: 10.2146/ajhp070029. [DOI] [PubMed] [Google Scholar]
- 6.Treon SP, Pilarski LM, Belch AR, Kelliher A, Preffer FI, Shima Y, Mitsiades CS, Mitsiades NS, Szczepek AJ, Ellman L, Harmon D, Grossbard ML, et al. CD20-directed serotherapy in patients with multiple myeloma: biologic considerations and therapeutic applications. Journal of immunotherapy. 2002;25:72–81. doi: 10.1097/00002371-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Korte W, Jost C, Cogliatti S, Hess U, Cerny T. Accelerated progression of multiple myeloma during anti-CD20 (Rituximab) therapy. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 1999;10:1249–50. doi: 10.1023/a:1008310819049. [DOI] [PubMed] [Google Scholar]
- 8.Ellis JH, Barber KA, Tutt A, Hale C, Lewis AP, Glennie MJ, Stevenson GT, Crowe JS. Engineered anti-CD38 monoclonal antibodies for immunotherapy of multiple myeloma. Journal of immunology. 1995;155:925–37. [PubMed] [Google Scholar]
- 9.Tai YT, Li X, Tong X, Santos D, Otsuki T, Catley L, Tournilhac O, Podar K, Hideshima T, Schlossman R, Richardson P, Munshi NC, et al. Human anti-CD40 antagonist antibody triggers significant antitumor activity against human multiple myeloma. Cancer research. 2005;65:5898–906. doi: 10.1158/0008-5472.CAN-04-4125. [DOI] [PubMed] [Google Scholar]
- 10.Tai YT, Li XF, Catley L, Coffey R, Breitkreutz I, Bae J, Song W, Podar K, Hideshima T, Chauhan D, Schlossman R, Richardson P, et al. Immunomodulatory drug lenalidomide (CC-5013, IMiD3) augments anti-CD40 SGN-40-induced cytotoxicity in human multiple myeloma: clinical implications. Cancer research. 2005;65:11712–20. doi: 10.1158/0008-5472.CAN-05-1657. [DOI] [PubMed] [Google Scholar]
- 11.Tassone P, Galea E, Forciniti S, Tagliaferri P, Venuta S. The IL-6 receptor super-antagonist Sant7 enhances antiproliferative and apoptotic effects induced by dexamethasone and zoledronic acid on multiple myeloma cells. International journal of oncology. 2002;21:867–73. [PubMed] [Google Scholar]
- 12.Ozaki S, Kosaka M, Wakahara Y, Ozaki Y, Tsuchiya M, Koishihara Y, Goto T, Matsumoto T. Humanized anti-HM1.24 antibody mediates myeloma cell cytotoxicity that is enhanced by cytokine stimulation of effector cells. Blood. 1999;93:3922–30. [PubMed] [Google Scholar]
- 13.Tai YT, Horton HM, Kong SY, Pong E, Chen H, Cemerski S, Bernett MJ, Nguyen DH, Karki S, Chu SY, Lazar GA, Munshi NC, et al. Potent in vitro and in vivo activity of an Fc-engineered humanized anti-HM1.24 antibody against multiple myeloma via augmented effector function. Blood. 2012;119:2074–82. doi: 10.1182/blood-2011-06-364521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein R, Mattes MJ, Cardillo TM, Hansen HJ, Chang CH, Burton J, Govindan S, Goldenberg DM. CD74: a new candidate target for the immunotherapy of B-cell neoplasms. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:5556s–63s. doi: 10.1158/1078-0432.CCR-07-1167. [DOI] [PubMed] [Google Scholar]
- 15.Kim D, Wang J, Willingham SB, Martin R, Wernig G, Weissman IL. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. 2012;26:2538–45. doi: 10.1038/leu.2012.141. [DOI] [PubMed] [Google Scholar]
- 16.Menoret E, Gomez-Bougie P, Geffroy-Luseau A, Daniels S, Moreau P, Le Gouill S, Harousseau JL, Bataille R, Amiot M, Pellat-Deceunynck C. Mcl-1L cleavage is involved in TRAIL-R1- and TRAIL-R2-mediated apoptosis induced by HGS-ETR1 and HGS-ETR2 human mAbs in myeloma cells. Blood. 2006;108:1346–52. doi: 10.1182/blood-2005-12-007971. [DOI] [PubMed] [Google Scholar]
- 17.Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, Lee AI, Podar K, Hideshima T, Rice AG, van Abbema A, Jesaitis L, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008;112:1329–37. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tassone P, Gozzini A, Goldmacher V, Shammas MA, Whiteman KR, Carrasco DR, Li C, Allam CK, Venuta S, Anderson KC, Munshi NC. In vitro and in vivo activity of the maytansinoid immunoconjugate huN901-N2'-deacetyl-N2'-(3-mercapto-1-oxopropyl)-maytansine against CD56+ multiple myeloma cells. Cancer research. 2004;64:4629–36. doi: 10.1158/0008-5472.CAN-04-0142. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda H, Hideshima T, Fulciniti M, Lutz RJ, Yasui H, Okawa Y, Kiziltepe T, Vallet S, Pozzi S, Santo L, Perrone G, Tai YT, et al. The monoclonal antibody nBT062 conjugated to cytotoxic Maytansinoids has selective cytotoxicity against CD138-positive multiple myeloma cells in vitro and in vivo. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:4028–37. doi: 10.1158/1078-0432.CCR-08-2867. [DOI] [PubMed] [Google Scholar]
- 20.Bjorkman PJ, Burmeister WP. Structures of two classes of MHC molecules elucidated: crucial differences and similarities. Current opinion in structural biology. 1994;4:852–6. doi: 10.1016/0959-440x(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Qian J, Wezeman M, Wang S, Lin P, Wang M, Yaccoby S, Kwak LW, Barlogie B, Yi Q. Targeting beta2-microglobulin for induction of tumor apoptosis in human hematological malignancies. Cancer cell. 2006;10:295–307. doi: 10.1016/j.ccr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Cao Y, Hong S, Li H, Qian J, Kwak LW, Yi Q. Human-like mouse models for testing the efficacy and safety of anti-beta2-microglobulin monoclonal antibodies to treat myeloma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:951–9. doi: 10.1158/1078-0432.CCR-08-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekimoto E, Ozaki S, Ohshima T, Shibata H, Hashimoto T, Abe M, Kimura N, Hattori K, Kawai S, Kinoshita Y, Yamada-Okabe H, Tsuchiya M, et al. A single-chain Fv diabody against human leukocyte antigen-A molecules specifically induces myeloma cell death in the bone marrow environment. Cancer research. 2007;67:1184–92. doi: 10.1158/0008-5472.CAN-06-2236. [DOI] [PubMed] [Google Scholar]
- 24.Nomura T, Huang WC, Seo S, Zhau HE, Mimata H, Chung LW. Targeting beta2-microglobulin mediated signaling as a novel therapeutic approach for human renal cell carcinoma. The Journal of urology. 2007;178:292–300. doi: 10.1016/j.juro.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Huang WC, Wu D, Xie Z, Zhau HE, Nomura T, Zayzafoon M, Pohl J, Hsieh CL, Weitzmann MN, Farach-Carson MC, Chung LW. beta2-microglobulin is a signaling and growth-promoting factor for human prostate cancer bone metastasis. Cancer research. 2006;66:9108–16. doi: 10.1158/0008-5472.CAN-06-1996. [DOI] [PubMed] [Google Scholar]
- 26.Repka T, Chiorean EG, Gay J, Herwig KE, Kohl VK, Yee D, Miller JS. Trastuzumab and interleukin-2 in HER2-positive metastatic breast cancer: a pilot study. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:2440–6. [PubMed] [Google Scholar]
- 27.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Gordon M, Schultheis AM, Yang DY, Nagashima F, Azuma M, Chang HM, Borucka E, Lurje G, Sherrod AE, Iqbal S, Groshen S, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25:3712–8. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 29.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, Neri TM, Ardizzoni A. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:1789–96. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 30.Natsume A, Niwa R, Satoh M. Improving effector functions of antibodies for cancer treatment: Enhancing ADCC and CDC. Drug design, development and therapy. 2009;3:7–16. [PMC free article] [PubMed] [Google Scholar]
- 31.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nature biotechnology. 2005;23:1147–57. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 32.Norris DA, Lee LA. Antibody-dependent cellular cytotoxicity and skin disease. The Journal of investigative dermatology. 1985;85:165s–75s. doi: 10.1111/1523-1747.ep12276370. [DOI] [PubMed] [Google Scholar]
- 33.Hardin J, MacLeod S, Grigorieva I, Chang R, Barlogie B, Xiao H, Epstein J. Interleukin-6 prevents dexamethasone-induced myeloma cell death. Blood. 1994;84:3063–70. [PubMed] [Google Scholar]
- 34.Bjorklund CC, Ma W, Wang ZQ, Davis RE, Kuhn DJ, Kornblau SM, Wang M, Shah JJ, Orlowski RZ. Evidence of a role for activation of Wnt/beta-catenin signaling in the resistance of plasma cells to lenalidomide. The Journal of biological chemistry. 2011;286:11009–20. doi: 10.1074/jbc.M110.180208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Y, Yang J, Qian J, Zhang L, Lu Y, Li H, Lin H, Lan Y, Liu Z, He J, Hong S, Thomas S, et al. Novel phosphatidylinositol 3-kinase inhibitor NVP-BKM120 induces apoptosis in myeloma cells and shows synergistic anti-myeloma activity with dexamethasone. Journal of molecular medicine. 2012;90:695–706. doi: 10.1007/s00109-011-0849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitsiades CS, McMillin DW, Klippel S, Hideshima T, Chauhan D, Richardson PG, Munshi NC, Anderson KC. The role of the bone marrow microenvironment in the pathophysiology of myeloma and its significance in the development of more effective therapies. Hematology/oncology clinics of North America. 2007;21:1007–34. vii–viii. doi: 10.1016/j.hoc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Gado K, Domjan G, Hegyesi H, Falus A. Role of INTERLEUKIN-6 in the pathogenesis of multiple myeloma. Cell biology international. 2000;24:195–209. doi: 10.1006/cbir.2000.0497. [DOI] [PubMed] [Google Scholar]
- 38.Frassanito MA, Cusmai A, Iodice G, Dammacco F. Autocrine interleukin-6 production and highly malignant multiple myeloma: relation with resistance to drug-induced apoptosis. Blood. 2001;97:483–9. doi: 10.1182/blood.v97.2.483. [DOI] [PubMed] [Google Scholar]
- 39.Taguchi T. [Interleukin-2 and cancer treatment]. Gan to kagaku ryoho. Cancer & chemotherapy. 1986;13:1–10. [PubMed] [Google Scholar]
- 40.Sirohi B, Powles R. Lenalidomide in multiple myeloma. Expert review of anticancer therapy. 2009;9:1559–70. doi: 10.1586/era.09.123. [DOI] [PubMed] [Google Scholar]
- 41.Kern DJ, James BR, Blackwell S, Gassner C, Klein C, Weiner GJ. GA101 induces NK-cell activation and antibody-dependent cellular cytotoxicity more effectively than rituximab when complement is present. Leukemia & lymphoma. 2013 doi: 10.3109/10428194.2013.781169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Treon SP, Hansen M, Branagan AR, Verselis S, Emmanouilides C, Kimby E, Frankel SR, Touroutoglou N, Turnbull B, Anderson KC, Maloney DG, Fox EA. Polymorphisms in FcgammaRIIIA (CD16) receptor expression are associated with clinical response to rituximab in Waldenstrom's macroglobulinemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:474–81. doi: 10.1200/JCO.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 43.Dalton WS. Drug resistance and drug development in multiple myeloma. Seminars in oncology. 2002;29:21–5. doi: 10.1053/sonc.2002.34073. [DOI] [PubMed] [Google Scholar]
- 44.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 45.Henney CS, Kuribayashi K, Kern DE, Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981;291:335–8. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- 46.Borradori L, Knellwolf M, Morell A. Interleukin-2: molecular, physiological and pathophysiological bases and possible significance for clinical practice. Schweizerische medizinische Wochenschrift. 1987;117:945–51. [PubMed] [Google Scholar]
- 47.Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, Schafer P, Bartlett JB. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:4650–7. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 48.Ralph P, Nakoinz I, Diamond B, Yelton D. All classes of murine IgG antibody mediate macrophage phagocytosis and lysis of erythrocytes. Journal of immunology. 1980;125:1885–8. [PubMed] [Google Scholar]
- 49.Tax WJ, Hermes FF, Willems RW, Capel PJ, Koene RA. Fc receptors for mouse IgG1 on human monocytes: polymorphism and role in antibody-induced T cell proliferation. Journal of immunology. 1984;133:1185–9. [PubMed] [Google Scholar]


