Abstract
Summary
Background
The risk of major bleeding in patients who have completed anticoagulation therapy for unprovoked venous thromboembolism (VTE) is unknown.
Objective
To report the major bleeding and fatal bleeding rates in patients randomized to placebo or observation (i.e. no anticoagulation therapy) for the secondary prevention of recurrent VTE.
Patients and methods
We performed a systematic review and meta-analysis of the literature to summarize the rates of major bleeding and fatal bleeding in patients randomized to placebo or observation during the secondary prevention of VTE. Unrestricted searches of MEDLINE (January 1, 1950 to August 31, 2013), Embase (January 1, 1980 to August 31, 2013), and the Cochrane Register of Controlled Trials using the OVID interface were conducted. Publications from potentially relevant journals were also searched by hand. We used a random-effects model to pool study results and I2 testing to assess for heterogeneity.
Results
The analysis included 11 studies and 3965 patients who were followed for a median of 24 months. The overall pooled major bleeding rate was 0.45 per 100 patient-years (95% CI 0.29–0.64, I2 = 0%), and the overall pooled fatal bleeding rate was 0.14 per 100 patient-years (95% CI 0.057–0.26, I2 = 0%).
Conclusions
Patients not receiving anticoagulant therapy for the secondary prevention of VTE experience major bleeding events, and this may have an impact on recommendations for extended treatment in this patient population.
Keywords: anticoagulants; hemorrhage; review, systematic; venous thromboembolism; venous thrombosis
Introduction
The optimal duration of anticoagulation in patients with unprovoked venous thromboembolism (VTE) is controversial. In these patients, the risk of recurrent events during the first year after anticoagulation is discontinued is between 5% and 10% 1. The American College of Chest Physicians recommends a minimum of 3 months of anticoagulation therapy and to consider prolonged treatment in patients with low or moderate risk of major bleeding events 1. To counsel patients, clinicians need accurate estimates of the risks of recurrent VTE and of major bleeding episodes with different therapeutic strategies to ensure that the benefits outweigh the risks of long-term secondary prevention. The risk of major bleeding after completing anticoagulant therapy is unknown in this patient population. Therefore, the incremental risks of major bleeding with various treatment strategies over and above the risk with no anticoagulant therapy are unclear. This systematic review and meta-analysis evaluates the rate of major bleeding episodes in high-risk patients participating in trials for the secondary prevention of recurrent VTE who received placebo or observation only (i.e. no anticoagulant therapy).
Patients and methods
We searched MEDLINE (January 1, 1950 to August 31, 2013), EMBASE (January 1, 1980 to August 31, 2013), and the Cochrane Register of Controlled Trials using the OVID interface. Publications were also sought through a hand-search of potentially relevant journals. There were no restrictions on language, publication year, or type of publication. The search strategy included the MeSH terms venous thrombosis, pulmonary embolism, aspirin, warfarin, and acenocoumarol. The following terms were also added to the search strategy: recurrent venous thromboembolism, oral anticoagulants, dabigatran, rivaroxaban, apixaban, and ximelagatran. The outcomes were major bleeding and fatal bleeding as defined by ISTH 2 or per individual study. The methodological quality was evaluated using the Risk of Bias Assessment Tool from the Cochrane Handbook for randomized trials 3. The primary measurement was bleeding event rates reported per 100 patient-years stratified according to the underlying treatment strategy with its associated 95% CIs. Pooled measurements were calculated using a random-effects model within the Stats Direct software (version 2.7.9; StatsDirect Ltd, Cheshire, UK). Heterogeneity was assessed using the I2 test 4.
Results and discussion
Our systematic search identified 636 citations. A total of 11 trials assessing anticoagulation for secondary prevention after VTE were included in the analysis 5–15. Eight studies were randomized placebo-controlled trials 5–9,11,12,14, two studies compared vitamin K antagonist (VKA) treatment with observation 13,15, and one study compared different durations of VKA therapy (the period of observation for major bleeding and fatal bleeding events after completing a fixed duration of anticoagulation was included in this analysis) 9,10. All studies were judged to be at low risk of bias according to the risk of bias assessment tool.
A total of 3965 patients were included in our analysis; 3630 were treated with placebo and 335 patients were simply observed for bleeding events (Table 1). The total population was followed for a median of 23.9 months (range 6–40.3 months); the median follow-up for those receiving placebo was 14.3 months (6–25.2 months) and 37.2 months for those enrolled in observation without anticoagulation only arms (32.7–40.3 months). The majority of patients were male (median 57%, range 51%–63%); the mean age varied between 53 and 68 years; and most patients had unprovoked VTE (median 100%, range 57%–100%).
Table 1.
Characteristics of included studies
| Study | Patients | Treatment regimen (No. of patients) (group 1 vs. group 2 vs. group 3) | Study design | Follow-up duration (mean) | Mean age (years) | Men (%) | Unprovoked VTE (%) | Antiplatelet use (%) | Active malignancy (%) | Normal renal function* (%) | Major bleeding events (No.) | Fatal bleeding events (No.) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Group | Group | Group | Group | Group | Group | Group | |||||||||||||||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |||||
| DURAC 2 10 | All VTE | Fixed duration VKA† (n = 111) vs. extended duration VKA (n = 116) | Open label, randomized | Fixed duration group followed for 40.3 months | 65 | 64 | – | 63 | 59 | ─ | NR | NR | ─ | NR | NR | ─ | 0 | 0 | ─ | NR | NR | ─ | 2 | 10 | ─ | 1 | 2 | ─ |
| LAFIT 12 | All VTE | Placebo (n = 83) vs. VKA (n = 79) | Double blind, randomized | 10 months | 58 | 59 | ─ | 53 | 68 | ─ | 100 | 100 | ─ | NR | NR | ─ | NR | NR | ─ | NR | NR | ─ | 0 | 3 | ─ | 0 | 0 | ─ |
| WODIT DVT 13 | DVT only | Observation (n = 133) vs. VKA (n = 134) | Open label, randomized | 37.2 months | 68 | 67 | ─ | 61 | 55 | ─ | 100 | 100 | ─ | NR | NR | ─ | 0 | 0 | ─ | NR | NR | ─ | 2 | 4 | ─ | 2 | 0 | ─ |
| WODIT PE 15 | PE only | Observation (n = 91) vs. VKA (n = 90) | Open label, randomized | 32.7 months | 61 | 63 | ─ | NR | NR | ─ | 57 | 55 | ─ | NR | NR | ─ | 0 | 0 | ─ | NR | NR | ─ | 1 | 2 | ─ | 0 | 0 | ─ |
| PREVENT 14 | All VTE | Placebo (n = 253) vs. Low intensity VKA (n = 255) | Double blind, randomized | 2.1 years | 53‡ | 53‡ | ─ | 53 | 53 | ─ | 100 | 100 | ─ | NR | NR | ─ | NR | NR | ─ | NR | NR | ─ | 2 | 5 | ─ | 1 | 0 | ─ |
| THRIVE III 11 | All VTE | Placebo (n = 611) vs. ximelagatran 24 mg BID (n = 612) | Double blind, randomized | 505 days | 58 | 56 | ─ | 51 | 54 | ─ | NR | NR | ─ | NR | NR | ─ | 5 | 6 | ─ | 110§ | 114§ | ─ | 5 | 6 | ─ | 0 | 0 | ─ |
| RESONATE 9 | All VTE | Placebo (n = 659) vs. dabigatran 150 mg BID (n = 684) | Double blind, randomized | 6 months | 56 | 56 | ─ | 55 | 56 | ─ | 100 | 100 | ─ | NR | NR | ─ | < 1 | < 1 | ─ | 70 | 70 | ─ | 0 | 2 | ─ | 0 | 0 | ─ |
| EINSTEIN-EXT 5 | All VTE | Placebo (n = 590) vs. rivaroxaban 20 mg daily (n = 598) | Double blind, randomized | 265 days | 58 | 58 | ─ | 57 | 59 | ─ | 74 | 73 | ─ | NR | NR | ─ | 4.4 | 4.7 | ─ | 63 | 62 | ─ | 0 | 4 | ─ | 0 | 0 | ─ |
| AMPLIFY-EXT 8 | All VTE | Placebo (n = 826) vs. apixaban 5 mg BID (n = 811) vs. apixaban 2.5 mg BID (n = 840) | Double blind, randomized | 12 months | 57 | 56 | 57 | 57 | 58 | 58 | 91 | 91 | 93 | 13 | 11.8 | 14.3 | 2.2 | 1.1 | 1.8 | 68 | 71 | 71 | 4 | 1 | 2 | 0 | 0 | 0 |
| WARFASA 6 | All VTE | Placebo (n = 197) vs. ASA 100 mg daily (n = 205) | Double blind, randomized | 23.9 months | 62 | 62 | ─ | 62 | 66 | ─ | 100 | 100 | ─ | 0 | 100 | ─ | 0 | 0 | ─ | NR | NR | ─ | 1 | 1 | ─ | 0 | 0 | ─ |
| ASPIRE 7 | All VTE | Placebo (n = 411) vs. ASA 100 mg daily (n = 411) | Double blind, randomized | 37.2 months | 54 | 55 | ─ | 54 | 55 | ─ | 100 | 100 | ─ | 0 | 100 | ─ | 1 | 1 | ─ | NR | NR | ─ | 6 | 8 | ─ | 2 | 0 | ─ |
ASA, acetyl salicylic acid; DVT, deep vein thrombosis; NR, not reported; PE, pulmonary embolism; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Normal renal function is defined as creatinine clearance ≥ 80 mL min −1 (Cockcroft-Gault).
Standard-dose adjusted VKA unless otherwise indicated.
Median age.
Median creatinine clearance (Cockcroft-Gault).
The overall pooled major bleeding rate in patients who have completed anticoagulation for the secondary prevention of recurrent VTE (i.e. no longer on anticoagulant therapy) was 0.45 per 100 patient-years (95% CI 0.29–0.64, I2 = 0%). The pooled major bleeding rate for patients randomized to placebo was 0.42 per 100 patient-years (95% CI 0.25–0.62, I2 = 0%), and that for patients randomized to observation was 0.62 per 100 patient-years (95% CI 0.23–1.2, I2 = 0%) (Fig.1). Fatal bleeding events were rare with an overall pooled fatal bleeding rate of 0.14 per 100 patient-years (95% CI 0.057–0.26, I2 = 0%). In the studies reporting sites of major bleeding and fatal bleeding, intracranial hemorrhage (including subdural and subarachnoid bleeding) and gastrointestinal bleeding were most common.
Fig 1.
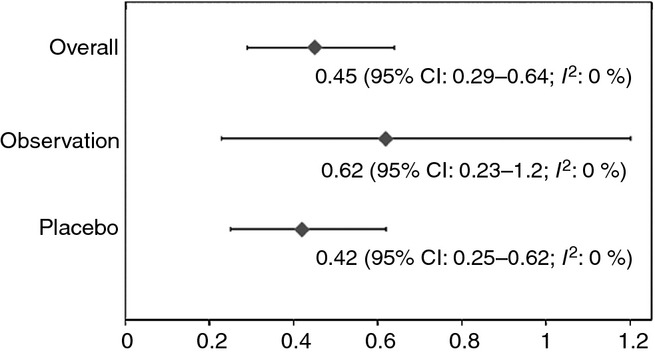
Forest plot of pooled proportions of major bleeding rates in patients randomized to placebo, observation, and overall (per 100 patient-years).
Our systematic review demonstrates that patients not receiving anticoagulant or antiplatelet therapy for the secondary prevention of recurrent VTE also experience major bleeding events. This event rate needs to be accounted for and included in the risk-benefit ratio, along with the previously reported risk of recurrent VTE, on and off anticoagulation, and risk of major bleeding on anticoagulant therapy, when clinicians are deciding on the length of anticoagulation therapy for patients with unprovoked VTE 16–20.
The rate of major bleeding events for patients with VTE on oral anticoagulation has previously been reported to be approximately 2.1 per 100 patient-years (95% CI 1.6–2.6) 17. Our review reported a risk of major bleeding of 0.45 per 100 patient-years (95% CI 0.29–0.64) for similar patients not receiving anticoagulant therapy. Therefore, the incremental risk of major bleeding associated with anticoagulant therapy is approximately 1.5 per 100 patient-years. A recent systematic review and network meta-analysis reported that the use of VKA was associated with the greatest risk of major bleeding events in comparison to placebo or observation (OR 5.24, 95% credible interval [CrI] 1.78–18.25) 20. In that review, the new oral anticoagulant apixaban, at two different treatment doses, was associated with the lowest risk of major bleeding events relative to placebo or observation.
It is interesting to note that the rates of major bleeding are higher in patients randomized to observation than those on placebo, although this finding is not significant. This may be attributed to different definitions of major bleeding used among the trials. All three trials randomizing patients to observation were published between 1997 and 2003―that is, before the ISTH criteria were established.
Our review has several strengths and limitations. First, all bleeding events were independently adjudicated; second, there was no heterogeneity identified between studies. Limitations of this study include the relatively small sample size of patients randomized to observation and generalizability to clinical practice. With respect to this latter concern, the patient population was extracted from RCTs. These patients are selected for participation, are likely younger, and generally do not have a history of bleeding events and severe comorbidities as they are often excluded from such studies, which may underestimate the true “real-world” bleeding risk and make our reported rate a conservative estimate. Timing of major bleeding events and known risk factors for major bleeding (antiplatelet use, moderate renal dysfunction, active malignancy, etc.) were not consistently reported in all trials, and therefore subgroup analyses could not be conducted. Last, observational prospective cohort studies were not included in the review. Although this could provide more insight into “real-world” bleeding risks, it may have significantly increased heterogeneity given the potential lack of independent adjudication or the use of standardized definitions of major bleeding.
In conclusion, our review suggests that the incidence of major bleeding events is non-negligible in patients receiving no treatment and may change the estimate of the incremental harm-benefit ratio clinicians consider when discussing anticoagulation for secondary prevention of recurrent VTE with patients.
Addendum
L. Castellucci and M. Carrier designed the study; performed the research; collected, analyzed, and interpreted the data; performed statistical analysis; and wrote the manuscript. G. Le Gal and M. Rodger analyzed and interpreted data, provided vital reviews to the manuscript, and wrote the manuscript.
Acknowledgments
M. Carrier is a recipient of a New Investigator Award from the Heart and Stroke Foundation of Canada and of a Tier 2 Research Chair in Thrombosis and Cancer. M. Rodger is a recipient of a Career Investigator Award from the Heart and Stroke Foundation of Canada and of a Faculty of Medicine, Clinical Research Chair in Venous Thrombosis and Thrombophilia.
Disclosure of Conflicts of Interest
The authors state that they have no conflicts of interest.
References
- 1.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S–94S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 3.Higgins JPT. Cochrane handbook for systematic reviews of interventions version 5.0.2. Cochrane Database Syst Rev. 2009 [Google Scholar]
- 4.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 5.Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P, Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F, Schellong S. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 6.Becattini C, Agnelli G, Schenone A, Eichinger S, Bucherini E, Silingardi M, Bianchi M, Moia M, Ageno W, Vandelli MR, Grandone E, Prandoni P. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med. 2012;366:1959–67. doi: 10.1056/NEJMoa1114238. [DOI] [PubMed] [Google Scholar]
- 7.Brighton TA, Eikelboom JW, Mann K, Mister R, Gallus A, Ockelford P, Gibbs H, Hague W, Xavier D, Diaz R, Kirby A, Simes J. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med. 2012;367:1979–87. doi: 10.1056/NEJMoa1210384. [DOI] [PubMed] [Google Scholar]
- 8.Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Porcari A, Raskob GE, Weitz JI. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699–708. doi: 10.1056/NEJMoa1207541. [DOI] [PubMed] [Google Scholar]
- 9.Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, Kvamme AM, Friedman J, Mismetti P, Goldhaber SZ. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709–18. doi: 10.1056/NEJMoa1113697. [DOI] [PubMed] [Google Scholar]
- 10.Schulman S, Granqvist S, Holmstrom M, Carlsson A, Lindmarker P, Nicol P, Eklund SG, Nordlander S, Larfars G, Leijd B, Linder O, Loogna E. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. The Duration of Anticoagulation Trial Study Group. N Engl J Med. 1997;336:393–8. doi: 10.1056/NEJM199702063360601. [DOI] [PubMed] [Google Scholar]
- 11.Schulman S, Wahlander K, Lundstrom T, Clason SB, Eriksson H. Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. N Engl J Med. 2003;349:1713–21. doi: 10.1056/NEJMoa030104. [DOI] [PubMed] [Google Scholar]
- 12.Kearon C, Gent M, Hirsh J, Weitz J, Kovacs MJ, Anderson DR, Turpie AGG, Green D, Ginsberg JS, Wells PS, MacKinnon B, Julian JA. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. 1999;340:901–7. doi: 10.1056/NEJM199903253401201. [DOI] [PubMed] [Google Scholar]
- 13.Agnelli G, Prandoni P, Santamaria MG, Bagatella P, Iorio A, Bazzan M, Moia M, Guazzaloca G, Bertoldi A, Tomasi C, Scannapieco G, Ageno W. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med. 2001;345:165–9. doi: 10.1056/NEJM200107193450302. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Goldhaber SZ, Danielson E, Rosenberg Y, Eby CS, Deitcher SR, Cushman M, Moll S, Kessler CM, Elliott CG, Paulson R, Wong T, Bauer KA, Schwartz BA, Miletich JP, Bounameaux H, Glynn RJ. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348:1425–34. doi: 10.1056/NEJMoa035029. [DOI] [PubMed] [Google Scholar]
- 15.Agnelli G, Prandoni P, Becattini C, Silingardi M, Taliani MR, Miccio M, Imberti D, Poggio R, Ageno W, Pogliani E, Porro F, Zonzin P. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med. 2003;139:19–25. doi: 10.7326/0003-4819-139-1-200307010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Douketis JD, Gu CS, Schulman S, Ghirarduzzi A, Pengo V, Prandoni P. The risk for fatal pulmonary embolism after discontinuing anticoagulant therapy for venous thromboembolism. Ann Intern Med. 2007;147:766–74. doi: 10.7326/0003-4819-147-11-200712040-00007. [DOI] [PubMed] [Google Scholar]
- 17.Carrier M, Le Gal G, Wells PS, Rodger MA. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010;152:578–89. doi: 10.7326/0003-4819-152-9-201005040-00008. [DOI] [PubMed] [Google Scholar]
- 18.Boutitie F, Pinede L, Schulman S, Agnelli G, Raskob G, Julian J, Hirsh J, Kearon C. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants' data from seven trials. BMJ. 2011;342:d3036. doi: 10.1136/bmj.d3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med. 2003;139:893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [DOI] [PubMed] [Google Scholar]
- 20.Castellucci LA, Cameron C, Le Gal G, Rodger MA, Coyle D, Wells PS, Clifford T, Gandara E, Wells G, Carrier M. Efficacy and safety outcomes of oral anticoagulants and antiplatelet drugs in the secondary prevention of venous thromboembolism: systematic review and network meta-analysis. BMJ. 2013;347:f5133. doi: 10.1136/bmj.f5133. [DOI] [PMC free article] [PubMed] [Google Scholar]


