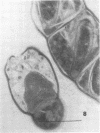
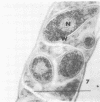
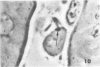
Abstract
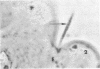
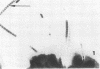
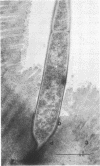
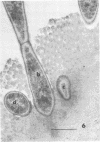
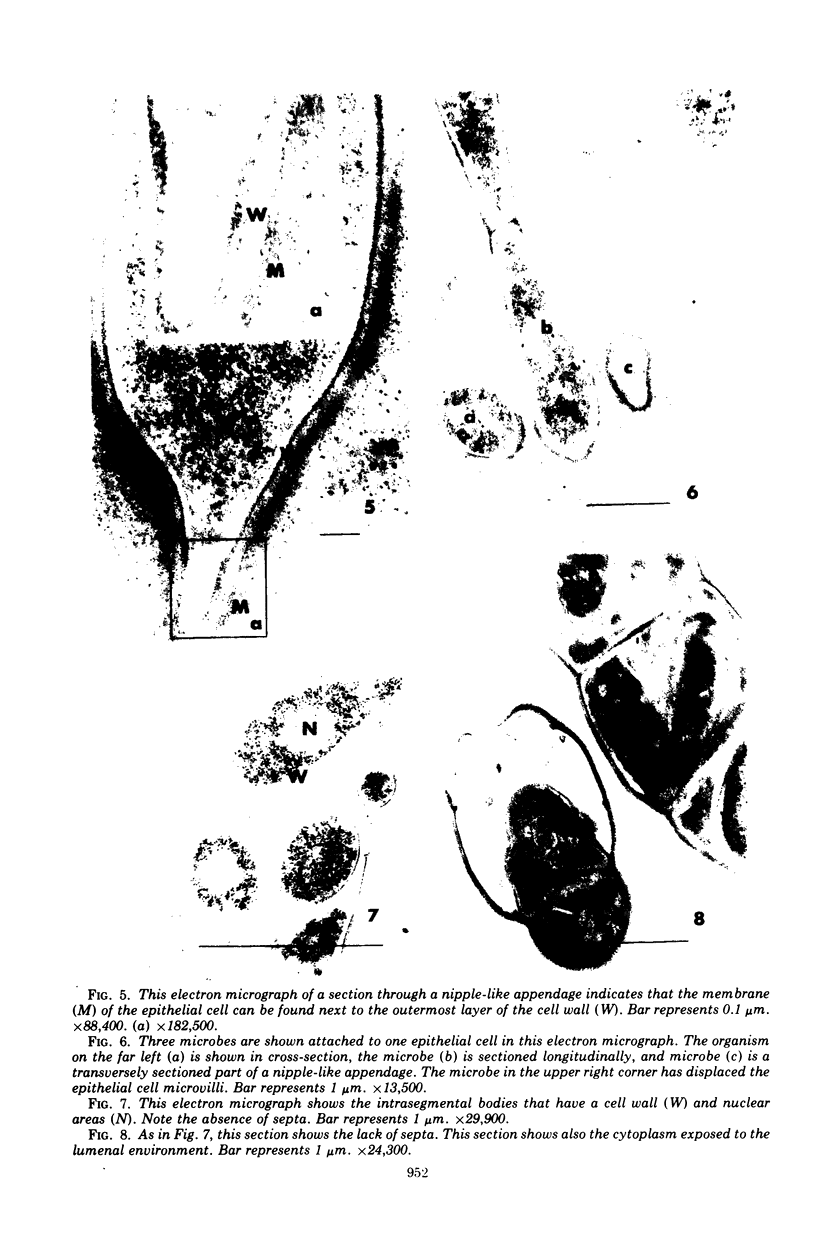
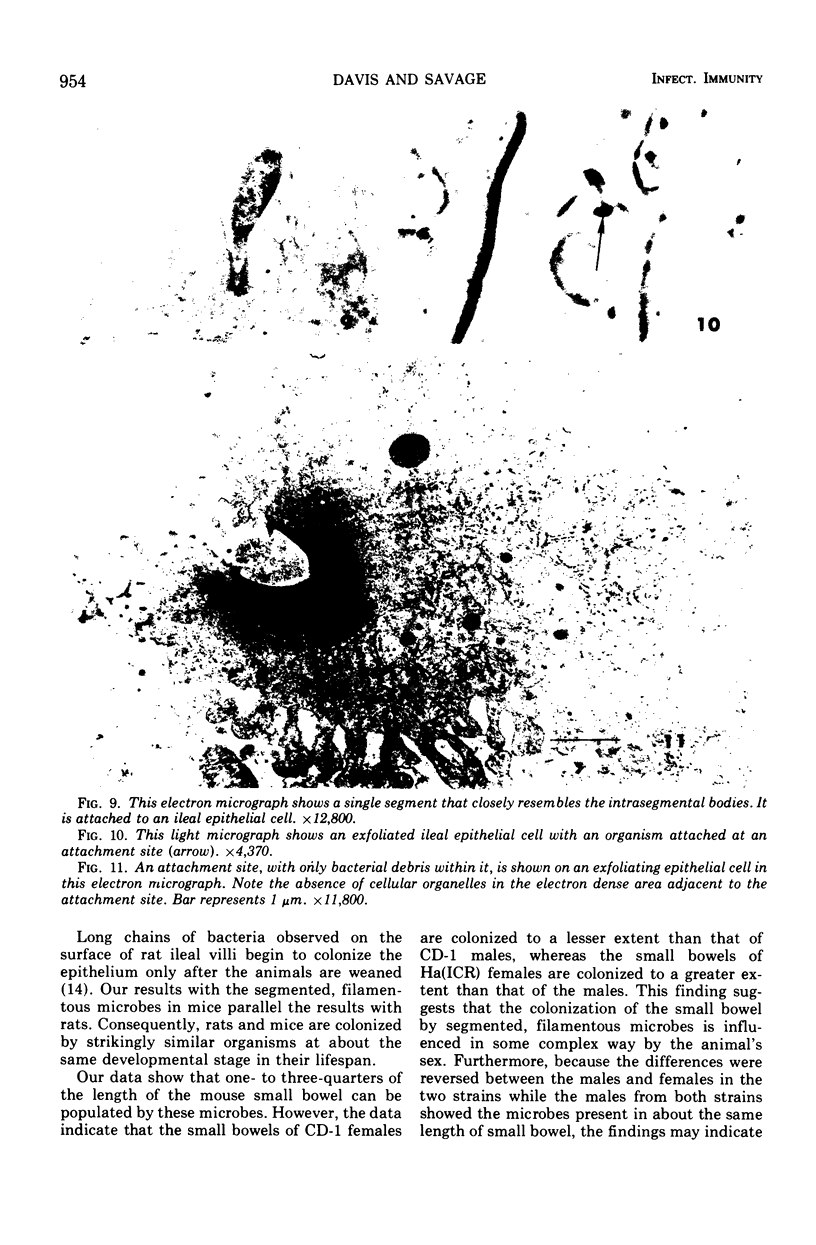
Some indigenous microorganisms localize on epithelial surfaces in various areas of the digestive tracts of animals. One of these, a segmented, filamentous microbe, localizes on the epithelium in the small bowels of mice and rats. These filamentous microbes colonize mice at weaning time and persist in adult animals for at least 2 months. Results of the study of light and electron micrographs suggest that the microorganisms are procaryotic, and that they interact with small bowel epithelial cells to form an attachment site. This site consists of modified epithelial cell membrane and apical cytoplasm adjacent to the attached bacterium. The microbe fills the site with part of its first segment. This segment has a nipple-like appendage on the end inserted into the epithelial cell. The other segments, which compose the rest of the filament, are usually separated by septa. Many of the individual segments contain intrasegmental bodies that appear to be procaryotic cells. Some of these intrasegmental bodies are similar in morphology to the first segment of each filament inserted into an epithelial cell. These intracellular bodies may be components in the life cycle of the microorganism. The organism has not yet been cultured in recognizable form. Therefore, such a hypothesis cannot be proved as yet, nor can the microbe be classified with certainty. Because it localizes in an epithelial habitat in the small bowel, however, it may be a particularly important microbial type in the gastrointestinal ecosystem of laboratory rodents.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS G. D., BAUER H., SPRINZ H. Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab Invest. 1963 Mar;12:355–364. [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEDLER R. W. The effect of the intestinal flora on the growth rate of mice, and on their susceptibility to experimental infections. J Exp Med. 1960 Mar 1;111:407–417. doi: 10.1084/jem.111.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOS R., SCHAEDLER R. W., COSTELLO R., HOET P. INDIGENOUS, NORMAL, AND AUTOCHTHONOUS FLORA OF THE GASTROINTESTINAL TRACT. J Exp Med. 1965 Jul 1;122:67–76. doi: 10.1084/jem.122.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., McAllister J. S., Savage D. C. Microbial colonization of the intestinal epithelium in suckling mice. Infect Immun. 1973 Apr;7(4):666–672. doi: 10.1128/iai.7.4.666-672.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., Mulcahy D., Takeuchi A., Savage D. C. Location and description of spiral-shaped microorganisms in the normal rat cecum. Infect Immun. 1972 Aug;6(2):184–192. doi: 10.1128/iai.6.2.184-192.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R., Turvey A. [Bacteria associated with the intestinal wall of the fowl (Gallus domesticus)]. J Appl Bacteriol. 1971 Sep;34(3):617–622. doi: 10.1111/j.1365-2672.1971.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton J. C., Rosario B. The attachment of microorganisms to epithelial cells in the distal ileum of the mouse. Lab Invest. 1965 Aug;14(8):1464–1481. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Gordon J., Dubos R. Enumeration of the oxygen sensitive bacteria usually present in the intestine of healthy mice. Nature. 1968 Dec 14;220(5172):1137–1139. doi: 10.1038/2201137a0. [DOI] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R., COSTELLO R. THE DEVELOPMENT OF THE BACTERIAL FLORA IN THE GASTROINTESTINAL TRACT OF MICE. J Exp Med. 1965 Jul 1;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., Dubos R. J. Localization of indigenous yeast in the murine stomach. J Bacteriol. 1967 Dec;94(6):1811–1816. doi: 10.1128/jb.94.6.1811-1816.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., Dubos R., Schaedler R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968 Jan 1;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C. Localization of certain indigenous microorganisms on the ileal villi of rats. J Bacteriol. 1969 Mar;97(3):1505–1506. doi: 10.1128/jb.97.3.1505-1506.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., McAllister J. S., Davis C. P. Anaerobic bacteria on the mucosal epithelium of the murine large bowel. Infect Immun. 1971 Oct;4(4):492–502. doi: 10.1128/iai.4.4.492-502.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., Chanock R. M. Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence. J Bacteriol. 1968 Sep;96(3):695–705. doi: 10.1128/jb.96.3.695-705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]