Abstract
cGMP is an important intracellular second messenger that mediates multiple tissue and cellular responses. The cGMP pathway is a key element in the pathophysiology of the heart and its modulation by drugs such as phosphodiesterase-5 (PDE-5) inhibitors and guanylate cyclase activators may represent a promising therapeutic approach for acute myocardial infarction, cardiac hypertrophy, heart failure and doxorubicin cardiotoxicity in patients. In addition, PDE-5 inhibitors may prove as innovative therapeutic agents for enhancing the chemosensitivity of doxorubicin while providing concurrent cardiac benefit.
Keywords: cGMP, Cardiomyocytes, Signalling, infarction, ischemia
Introduction
Cyclic guanosine 3’,5’-monophosphate (cGMP) is a critical intracellular second messenger regulating fundamental physiological processes in the myocardium, from acute contraction/relaxation to chronic gene expression, cell growth and apoptosis. Several studies have shown that cGMP inhibits hypertrophy, reduces ischemia-reperfusion (I/R) injury, regulates contractile function and cardiac remodeling (1–3). cGMP is generated from the cytosolic purine nucleotide GTP by guanylyl cylases (GCs) using Mg2+ or Mn2+ as cofactors. Two isoforms of GCs exist in vertebrate cells and tissues: a nitric oxide (NO)-sensitive cytosolic or soluble guanylyl cyclase (sGC) and natriuretic peptide (NP)-activated plasma membrane bound, particulate guanylyl cyclase (pGC). Once produced, the effects of cGMP occur through three main groups of cellular target molecules: cGMP-dependent protein kinases (PKGs), cGMP-gated cation channels and phosphodiesterases (PDEs). cGMP positively regulates PKG, but inhibits/activates PDEs, which are predominant in the cardiovascular system (4;5).
Regulation of cGMP by Phosphodiesterases
The cGMP pool in the cell is tightly controlled by PDEs which specifically cleave the 3', 5’-cyclic phosphate moiety of cAMP and/or cGMP to produce the corresponding 5' nucleotide. Currently 21 PDE genes have been cloned and are classified into 11 families according to their sequence of homology, biochemical and pharmacological properties (6). The PDEs vary in their substrate specificity for cAMP and cGMP: PDE-5, PDE-6 and PDE-9 are specific for cGMP; PDE-4, PDE-7 and PDE-8 are specific for cAMP; and PDE-1, PDE-2, PDE-3, PDE-10 and PDE-11 have mixed specificity for cAMP/cGMP (7). PDE-5 selectively hydrolyzes cGMP, and its inhibition increases cGMP bioavailability. The abundance of PDE-5 in smooth muscles and its role in regulating their contractile tone has made PDE-5 an important drug target for the treatment of erectile dysfunction (6), leading to the development of potent PDE-5 inhibitors, such as sildenafil (Viagra™ and Revatio™), vardenafil (Levitra™) and tadalafil (Cialis™ and Adcirca™). Revatio™ and Adcirca™ have also been approved for treatment of pulmonary hypertension. Earlier studies showed that PDE-5 is not present in normal cardiomyocytes (8;9) although later investigations revealed its expression in canine (10), mouse cardiomyocytes (1,11,12), and human heart (13,14). PDE5 expression is increased in hypertrophic human right ventricle, as well as failing left ventricular tissue (13–15). A gene silencing model also confirmed PDE-5 protein expression (16), while a recent report still questioned its presence in adult mouse cardiac myocytes (17). Since cGMP-hydrolytic activity is also attributable to PDE-1 and PDE-3, Vandeput et al. suggested that the effects of sildenafil on cGMP hydrolysis were due to inhibition of both PDE-5 and PDE-1 in the left ventricles of normal and failing mouse hearts (14).
cGMP in Pre and Post-Conditioning
Nitric oxide (NO) triggers various physiological responses by binding and activating sGC to produce cGMP from GTP (18). NO-cGMP-PKG signaling pathway is involved in the cardioprotective action in I/R injury as a survival signal (19;20). In cardiomyocytes, cGMP reduced the effects of myocyte stunning following simulated I/R (21). NO donor, S-nitroso-N-acetyl-L,L-penicillamine mimicked the preconditioning (PC)-like effect by inducing cGMP (22). Moreover, the activation of NO/cGMP/PKG pathway inhibited the elevation of intracellular Ca2+ concentrations by phosphorylating target proteins responsible for intracellular Ca2+ homeostasis during I/R injury in Chinese hamster ovary (CHO) cells (23).
Ischemic PC rapidly increased cGMP levels via sGC during ischemia leading to delayed protective effect (24 hrs later) against myocardial stunning and infarction in conscious rabbits (24). Bradykinin, one of the triggers of PC caused receptor-mediated production of NO resulting in cGMP production, activation of PKG, and opening of mitochondrial KATP (mitoKATP) channel in rabbit heart and cardiomyocytes (25). Opening of mitoKATP channels causes partial compensation of the membrane potential, which enables additional protons to be pumped out to form a H+ electrochemical gradient for both ATP synthesis and Ca2+ transport (26). The cGMP/PKG pathway also confers ischemic post conditioning protection in part by delaying normalization of pHi during reperfusion, probably via PKG-dependent inhibition of Na+/H+-exchanger in rat heart (27).
cGMP Modulatory Drugs for Cardioprotection
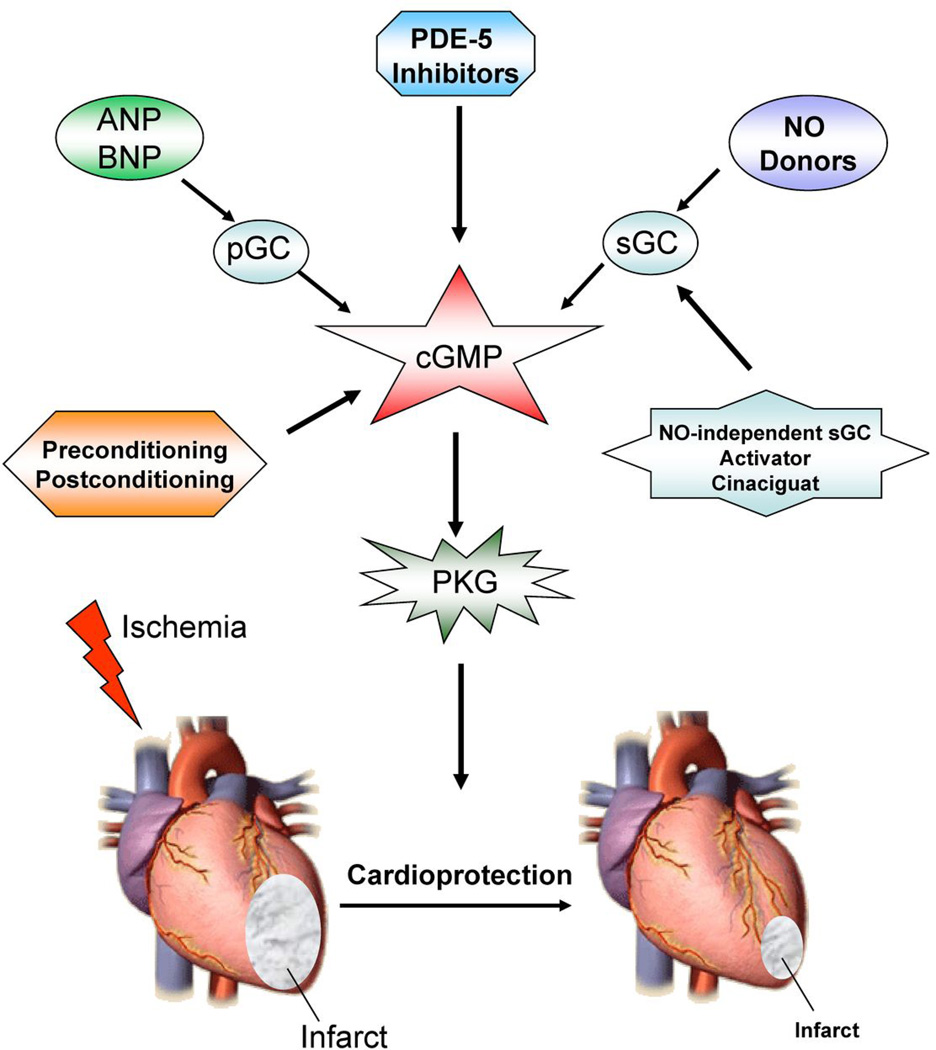
cGMP modulatory drugs induce cardioprotective effect through PKG as outlined in Figure 1. Natriuretic peptides (NP) exert biological effects by binding to membrane associated pGC. Atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP) and C-type natriuretic peptide (CNP) are three structurally related, but genetically distinct, signaling molecules that regulate the cardiovascular, skeletal, nervous, reproductive and other systems by activating pGC and elevating intracellular cGMP concentrations. ANP is primarily stored in atrial granules and secreted in response to atrial stretch. BNP is also in atrial granules but is found in highest level in ventricles of stressed hearts (28). CNP is found at lower concentration in vascular endothelium and is present in higher concentration in chondrocytes where it stimulates long bone growth (29).
Figure 1. Myocardial protection by upregulation of cGMP.
Cardioprotective modalities including preconditioning, post conditioning, ANP/BNP, NO donors and Cinaciguat generate cGMP through activation of sGC/pGC. cGMP exerts cardioprotective effect against ischemia/reperfusion injury through activation of PKG.
In the cardiovascular system, NPs have growth suppressive, antiproliferative and antihypertrophic actions on vascular smooth muscle cells, cardiomyocytes and fibroblasts (30). ANP/BNP exert myocardial protective effects against I/R injury through a cGMP-PKG-dependent modulation of mitoKATP channels (31). ANP also protects against reoxygenation-induced hypercontracture in cardiomyocytes by stimulating cGMP synthesis (32). Administration of ANP at reperfusion protected against I/R injury (33,34) exerted antiapoptotic effects in rat cardiomyocytes through cGMP-PKG and by inducing phosphatidylinositol 3-kinase-protein kinase B (PI3K/AKT) signaling (35). cGMP analog, 8-Br-cGMP, or elevation of intracellular cGMP concentration via the sGC activator NO or BNP exerted cardioprotective effects through PKG activation (36).
Cinaciguat (BAY 58-2667) activates sGC independent of NO (37). This drug preferentially activates sGC when the heme iron is oxidized (Fe3+) or the heme moiety is missing, which makes this enzyme insensitive to both its endogenous ligand NO and exogenous nitrovasodilators (37). These unique attributes make Cinaciguat an attractive molecule for protection against reperfusion injury. Indeed, Cinaciguat induces pre- or post-conditioning like effect against I/R in rabbit, rat (38), mouse (39) and dog heart (40) by activating PKG and opening of the mitoKATP channels (39). Intriguingly, this drug caused PKG-dependent generation of hydrogen sulfide in mouse cardiomyocytes and protected against simulated ischemia/reoxygenation (SI-RO) injury, similar to tadalafil in the heart (3). Hydrogen sulfide causes cardioprotection through opening of sarcolemmal KATP channels in rat heart and cardiomyocytes (41).
PDE-5 inhibitors are promising drugs for cardiovascular protection. Our laboratory first demonstrated the cardioprotective effect of sildenafil against I/R injury (42). Rabbits treated with sildenafil before ischemia showed significant reduction of infarct size, which was mediated by opening of mitoKATP channels (42). Sildenafil also reduced cell death due to necrosis and apoptosis in isolated cardiomyocytes suggesting that the cytoprotective effect of this drug was independent of its vascular/hypotensive effect (11). Vardenafil is 20-fold more potent than sildenafil for inhibiting purified PDE-5 (43) and it also had similar protective effect against I/R injury in rabbits (44). Moreover, both drugs reduced infarct size when infused at reperfusion through opening of mitoKATP channel (45). Tadalafil is a long-acting PDE-5A inhibitor, which has a half-life of 17.5 h (46) and is effective for up to 36 hours for improving erectile function. Tadalafil also reduced infarct size and improved cardiac function following I/R in mice (3).
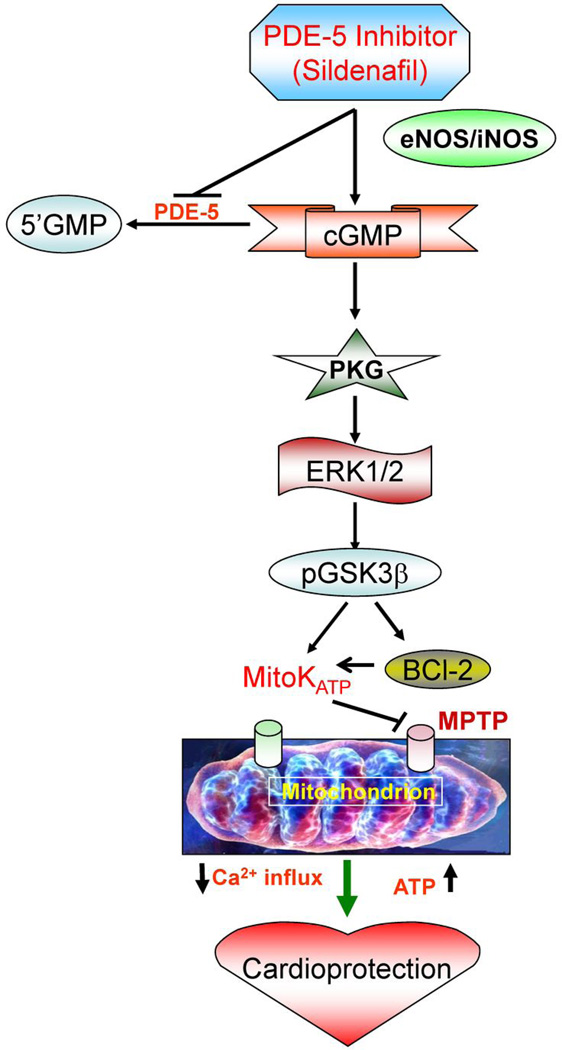
Mechanistically, the cardioprotective effect of sildenafil was dependent on enhanced NO generation through eNOS/iNOS (47), activation of protein kinase C (PKC) (48), and opening of mitoKATP channels (42). Sildenafil also increased cGMP accumulation and PKG activation in mouse cardiomyocytes and heart (11,47). The PKG-dependent cytoprotective mechanism of sildenafil involves phosphorylation of ERK and GSK3β, induction of Bcl-2 and opening of mitoKATP channels (49) (Figure 2). Interestingly, sildenafil increased Bcl-2 expression which was absent in iNOS-deficient cardiomyocytes thereby suggesting a link of NO signaling with the expression of antiapoptotic protein (49). Overexpression of PKG1α also reduced in adult rat cardiomyocyte injury after ischemia which involved inhibition of active caspase-3, phosphorylation of Akt, ERK, and JNK, and increased expression of NOS and Bcl-2 as well as decreased Bax expression (50).
Figure 2. Mechanism of cardioprotection by PDE-5 inhibitor, sildenafil.
Sildenafil treatment triggers signaling cascade resulting in increased expression of eNOS/iNOS and activation of cGMP dependent PKG. PKG subsequently causes phosphorylation of ERK1/2 and GSK3β in conjunction with increase in the Bcl-2, which inhibits apoptosis through attenuation in cytochrome C release and inhibition of the mitochondrial permeability transition pore (MPTP). PKG also opens mitoKATP channels which limits against ischemia/reperfusion injury through preservation of ATP and decrease in Ca2+ influx in the mitochondria.
cGMP Signaling in Hypertrophy and Heart Failure
Sildenafil exerts anti-hypertrophic effects in mice with pressure-overload in the absence of vascular unloading (1). The antihypertrophic effects coexisted with PKG activation, and its targets included RGS2 (regulator of G protein-coupled signaling 2) (51), as well as calcineurin-NFAT-TRPC6 (52). In the hypertrophied right ventricular myocardium, PDE-5 is upregulated, PKG activity is inhibited and cGMP is preferentially shifted to inhibition of PDE-3 (15). This leads to increase in cAMP, protein kinase A activation, increased intracellular calcium and increased contractility. The increased PDE-5 expression predisposed mice to adverse LV remodeling after myocardial infarction (MI). LV systolic and diastolic dysfunction were more marked in PDE5-TG than in wild-type mice, associated with enhanced hypertrophy and reduced contractile function in isolated cardiomyocytes from remote myocardium (13). Chronic treatment with sildenafil immediately following MI or beginning 3 days post MI attenuated ischemic cardiomyopathy (53) suggesting that PDE-5 inhibition may be a promising therapeutic tool for advanced HF in patients. Interestingly, PKG activation with sildenafil was associated with the inhibition of Rho kinase (54), which is known to suppress LV remodeling post MI in mice (55).
Cardiac Dysfunction in Duchenne Muscular Dystrophy
Duchenne muscular dystrophy (DMD) is a progressive and fatal genetic disorder of muscle degeneration. Patients with DMD lack dystrophin as a result of mutations in the X-linked dystrophin gene. The loss of dystrophin leads to severe skeletal muscle pathologies and cardiomyopathy, a delayed symptom of the disease that usually develops by the second decade of life, with more than 90% of patients presenting clinical symptoms by 18 y of age (56). Reduced NO-cGMP signaling is a key contributor to DMD cardiac pathogenesis. Dystrophin-deficient mice (mdx mice) show cardiac dysfunction with decrease in diastolic function followed by systolic dysfunction later in life. Loss of dystrophin prevents normal nNOS expression and/or signaling in all (skeletal, smooth and cardiac) muscle systems (57). The stimulation of cGMP synthesis by overexpression of cardiac-specific nNOS reduced impulse-conduction defects in dystrophin-deficient (mdx) mice (58;59). Similarly, increased pGC activity in young mdx mice decreased susceptibility to cardiac damage during sympathetic stress (60). Chronic treatment with sildenafil reduced functional deficits in cardiac performance of aged mdx mice without any effect on normal cardiac function in wild type controls (57). When sildenafil treatment was started after cardiomyopathy had developed, the established symptoms were rapidly reversed within a few days. These results suggest that PDE-5 inhibitors may be useful in treatment of cardiomyopathy in DMD patients.
cGMP and Doxorubicin-Induced Cardiotoxicity
Doxorubicin (DOX) is one of the most powerful and widely used anti-cancer drugs in clinics. Specially, the cumulative doses over 550 mg/m2 increase the risk of developing cardiac side effects, including congestive heart failure and dilated cardiomyopathy (61). DOX cardiotoxicity involves increased oxidative stress, inhibition of nucleic acid and protein synthesis, release of vasoactive amines, alteration of mitochondrial energetics and altered adrenergic function. Reduction in fractional shortening and abnormalities in the nonspecific T wave and ST segment of EKG are typically observed in DOX-induced ventricular dysfunction (62). Treatment with sildenafil prior to DOX inhibited cardiomyocyte apoptosis, preserved mitochondrial membrane potential (Δψm), myofibrillar integrity and prevented left ventricular dysfunction as well as ST segment prolongation (63). Similarly, Tadalafil also improved left ventricular function and prevented cardiomyocyte apoptosis in DOX-induced cardiomyopathy through mechanisms involving up-regulation of cGMP, PKG activity, and MnSOD level without interfering with the chemotherapeutic benefits of DOX (64). Thus, prophylactic treatment with PDE-5 inhibitors might become a promising therapeutic intervention for managing the clinical concern of DOX-induced cardiotoxicity in patients.
cGMP in Cancer Chemotherapy
Sildenafil and vardenafil induce caspase-dependent apoptosis and antiproliferation effect in B-cell chronic lymphatic leukemia (65). Vardenafil and DOX combination significantly improved survival and reduced the tumor size in brain-tumor-bearing rats (66). Oral administration of vardenafil and sildenafil increased the rate of transport of compounds across the blood-tumor-brain and improved the efficacy of DOX in treatment of brain tumors. We recently reported that sildenafil is both a powerful sensitizer of DOX-induced killing of prostate cancer and provides concurrent cardioprotective benefit (67). Co-treatment with sildenafil and DOX enhanced apoptosis in PC-3 and DU145 prostate cancer cells through enhancing ROS generation as compared to normal prostate cells where such combination attenuated DOX-induced ROS generation. The basic difference in mitochondrial respiration between normal and cancer cells (which produce large amount of lactate regardless of the availability of oxygen) appears to make cancer cells more sensitive to oxidative stress (68). DOX-induced apoptosis is mainly initiated by oxidative DNA damage although this apoptosis may involve topoisomerase II inhibition as well. The increased apoptosis by sildenafil and DOX was associated with enhanced expression of proapoptotic proteins Bad and Bax and suppression of antiapoptotic protein, Bcl-2 and Bcl-xL. Moreover, treatment with sildenafil and DOX in mice bearing prostate tumor xenografts resulted in significant inhibition of tumor growth (67).
Concluding Comments
It is clear from the above summarized studies that NO-cGMP-PKG pathway plays a key role in protection against MI, pre- and post-conditioning, hypertrophy, heart failure and DOX-induced cardiotoxicity. A number of clinically relevant therapeutic modalities including GC activators and PDE-5 inhibitors are promising agents in modulating the cGMP pathway in these disease states. Research over the past 9 years on the cardiac uses of PDE-5 inhibitors have helped initiate human trials including NIH multicenter trial (RELAX: Evaluating the Effectiveness of sildenafil at Improving Health Outcomes and Exercise Ability in People With Diastolic Heart Failure; NCT00763867) in patients with heart failure; clinical trial of sildenafil (Revatio) (REVERSE-DMD, NCT01168908) to treat Duchenne Muscular Dystrophy and Becker Muscular Dystrophy patients with cardiac disease, which is currently recruiting patients at the Johns Hopkins Medical Institutions. The role of sildenafil in enhancing the chemotherapeutic efficacy of DOX in prostate and other cancer cell lines, while alleviating the cardiotoxic effects of DOX, suggests that a new paradigm may be emerging for a safer use of this agent in the treatment of various types of cancer.
Table 1.
Pharmacological agents that target the cGMP signaling pathways
| Drug/Agent | Target | Model/species | Results | Reference |
|---|---|---|---|---|
| Atrial natriuretic peptide (ANP) | pGC | Rabbit and rat heart, adult rat ventricular myocytes | Protects against I/R injury and RO-induced hypercontracture by cGMP-dependent nuclear accumulation of zyxin and Akt. | (32–35) |
| Bradykinin | B2 receptor | Rabbit heart and cardiomyocytes | Mimics ischemic preconditioning by NO-cGMP-PKG dependent opening of mitoKATP channels | (25) |
| B-type natriuretic peptide (BNP) | pGC | Rat heart | Protects against I/R injury through cGMP-PKG dependent modulation of mitoKATP channels | (31) |
| 8-Br-cGMP | cGMP analog | Neonatal rat ventricular myocytes | Protects against SI-RO via cGMP-dependent protein kinase (PKG) | (36) |
| Cinaciguat (BAY 58-2667) | sGC | Rabbit, rat, mouse and dog heart | Induces pre- or post-conditioning like effect against I/R by PKG mediated opening of mitoKATP channels | (38–40) |
| Sildenafil | PDE-5 | Rabbit and mice heart, and adult mouse cardiomyocytes Mice, adult mouse cardiomyocytes Mdx mice (dystrophin-deficient mice) Mice prostate cancer xenograft |
Protects against I/R and SI-RO injury through eNOS/iNOS, activation of PKC, increased accumulation of cGMP, activation of PKG, phosphorylation of ERK and opening of mitoKATP channels Inhibits DOX-induced cardiomyocytes apoptosis, preserved mitochondrial membrane potential (Δψm), myofibrillar integrity and prevents DOX-induced left ventricular dysfunction Reduced functional deficits in the cardiac performance of aged mdx mice Sensitizes DOX-induced tumor reduction and provides concurrent cardioprotective benefits. |
(2;11;42;47–49;53) (57;63) (57) (67) |
| S-nitroso-N-acetyl-L,L-penicillamine (SNAP) | NO donor | Neonatal rat ventricular myocytes | Mimics preconditioning effect against SI-RO, which is cGMP dependent but independent of PKC or KATP channels | (22) |
| Vardenafil | PDE-5 | Rabbit heart Brain-tumor-bearing rat |
Protects against I/R injury via opening of mitoKATP channels Improved survival and reduced DOX-induced tumor size |
(3;45) (66) |
| Tadalafil | PDE-5 | Mice heart | Limit I/R injury by hydrogen sulfide (H2S) signaling in a PKG-dependent fashion. Prevents DOX-induced cardiomyopathy and improved left ventricular function through up-regulation of cGMP, PKG activity and MnSOD level without interfering with chemotherapeutic benefits of doxorubicin. |
(3) (57;64) |
Cyclic guanosine 3’,5’-monophosphate, cGMP; cGMP-dependent protein kinases, PKG; Doxorubicin, DOX; Ischemia-reperfusion, I/R; Nitric oxide, NO; Nitric oxide synthase, NOS; Mitochondrial KATP channel, MitoKATP channel; Manganese superoxide dismutase, MnSOD; Phosphodiesterase, PDE-5; Simulated ischemia-reoxygenation, SI-RO.
Acknowledgement
This study was supported by grants from National Institutes of Health (HL51045, HL79424 and HL93685) to Rakesh C. Kukreja and National Scientist Development Grant from the American Heart Association (10SDG3770011) to Fadi N. Salloum.
List of Abbreviations
- cGMP
Cyclic guanosine 3’, 5’-monophosphate
- GC
Guanylyl cyclase
- PKG
cGMP-dependent protein kinases
- DOX
Doxorubicin
- DMD
Duchenne muscular dystrophy
- I/R
Ischemia-reperfusion
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- MitoKATP
Mitochondrial KATP
- PDE
Phosphodiesterase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Takimoto E, Champion HC, Li M, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 2.Das A, Xi L, Kukreja RC. Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem. 2008;283:29572–29585. doi: 10.1074/jbc.M801547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salloum FN, Chau VQ, Hoke NN, et al. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation. 2009;120:S31–S36. doi: 10.1161/CIRCULATIONAHA.108.843979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmann F, Ammendola A, Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J Cell Sci. 2000;113(Pt 10):1671–1676. doi: 10.1242/jcs.113.10.1671. [DOI] [PubMed] [Google Scholar]
- 5.Smolenski A, Poller W, Walter U, Lohmann SM. Regulation of human endothelial cell focal adhesion sites and migration by cGMP-dependent protein kinase I. J Biol Chem. 2000;275:25723–25732. doi: 10.1074/jbc.M909632199. [DOI] [PubMed] [Google Scholar]
- 6.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 7.Rotella DP. Phosphodiesterase 5 inhibitors: current status and potential applications. Nat Rev Drug Discov. 2002;1:674–682. doi: 10.1038/nrd893. [DOI] [PubMed] [Google Scholar]
- 8.Wallis RM, Corbin JD, Francis SH, Ellis P. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol. 1999;83:3C–12C. doi: 10.1016/s0002-9149(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 9.Cheitlin MD, Hutter AM, Jr, Brindis RG, et al. ACC/AHA expert consensus document. Use of sildenafil (Viagra) in patients with cardiovascular disease. American College of Cardiology/American Heart Association. J Am Coll Cardiol. 1999;33:273–282. doi: 10.1016/s0735-1097(98)00656-1. [DOI] [PubMed] [Google Scholar]
- 10.Senzaki H, Smith CJ, Juang GJ, et al. Cardiac phosphodiesterase 5 (cGMP-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J. 2001;15:1718–1726. doi: 10.1096/fj.00-0538com. [DOI] [PubMed] [Google Scholar]
- 11.Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem. 2005;280:12944–12955. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- 12.Takimoto E, Champion HC, Belardi D, et al. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res. 2005;96:100–109. doi: 10.1161/01.RES.0000152262.22968.72. [DOI] [PubMed] [Google Scholar]
- 13.Pokreisz P, Vandenwijngaert S, Bito V, et al. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation. 2009;119:408–416. doi: 10.1161/CIRCULATIONAHA.108.822072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandeput F, Krall J, Ockaili R, et al. cGMP-Hydrolytic Activity and Its Inhibition by Sildenafil in Normal and Failing Human and Mouse Myocardium. Journal of Pharmacology and Experimental Therapeutics. 2009;330:884–891. doi: 10.1124/jpet.109.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagendran J, Archer SL, Soliman D, et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 16.Zhang M, Koitabashi N, Nagayama T, et al. Expression, activity, and pro-hypertrophic effects of PDE5A in cardiac myocytes. Cell Signal. 2008;20:2231–2236. doi: 10.1016/j.cellsig.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukowski R, Rybalkin SD, Loga F, Leiss V, Beavo JA, Hofmann F. Cardiac hypertrophy is not amplified by deletion of cGMP-dependent protein kinase I in cardiomyocytes. Proc Natl Acad Sci U S A. 2010;107:5646–5651. doi: 10.1073/pnas.1001360107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russwurm M, Koesling D. NO activation of guanylyl cyclase. Embo Journal. 2004;23:4443–4450. doi: 10.1038/sj.emboj.7600422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burley DS, Ferdinandy P, Baxter GF. Cyclic GMP and protein kinase-G in myocardial ischaemia-reperfusion: opportunities and obstacles for survival signaling. Br J Pharmacol. 2007;152:855–869. doi: 10.1038/sj.bjp.0707409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kukreja RC, Salloum F, Das A, et al. Pharmacological preconditioning with sildenafil: Basic mechanisms and clinical implications. Vascul Pharmacol. 2005;42:219–232. doi: 10.1016/j.vph.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Gandhi A, Yan L, Scholz PM, Huang MW, Weiss HR. Cyclic GMP reduces ventricular myocyte stunning after simulated ischemia-reperfusion. Nitric Oxide. 1999;3:473–480. doi: 10.1006/niox.1999.0263. [DOI] [PubMed] [Google Scholar]
- 22.Rakhit RD, Edwards RJ, Mockridge JW, et al. Nitric oxide-induced cardioprotection in cultured rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2000;278:H1211–H1217. doi: 10.1152/ajpheart.2000.278.4.H1211. [DOI] [PubMed] [Google Scholar]
- 23.Ruth P, Wang GX, Boekhoff I, et al. Transfected cGMP-dependent protein kinase suppresses calcium transients by inhibition of inositol 1,4,5-trisphosphate production. Proc Natl Acad Sci U S A. 1993;90:2623–2627. doi: 10.1073/pnas.90.7.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kodani E, Xuan YT, Takano H, Shinmura K, Tang XL, Bolli R. Role of cyclic guanosine monophosphate in late preconditioning in conscious rabbits. Circulation. 2002;105:3046–3052. doi: 10.1161/01.cir.0000019408.67709.b5. [DOI] [PubMed] [Google Scholar]
- 25.Oldenburg O, Qin Q, Krieg T, et al. Bradykinin induces mitochondrial ROS generation via, NO cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol Heart Circ Physiol. 2004;286:H468–H476. doi: 10.1152/ajpheart.00360.2003. [DOI] [PubMed] [Google Scholar]
- 26.Szewczyk A, Mikolajek B, Pikula S, Nalecz MJ. ATP-sensitive K+ channel in mitochondria. Acta Biochim Pol. 1993;40:329–336. [PubMed] [Google Scholar]
- 27.Inserte J, Barba I, Poncelas-Nozal M, et al. cGMP/PKG pathway mediates myocardial post conditioning protection in rat hearts by delaying normalization of intracellular acidosis during reperfusion. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Potter LR. Natriuretic Peptide Metabolism, Clearance and Degradation. FEBS J. 2011 doi: 10.1111/j.1742-4658.2011.08082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chusho H, Tamura N, Ogawa Y, et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci U S A. 2001;98:4016–4021. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res. 2006;69:318–328. doi: 10.1016/j.cardiores.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 31.D'Souza SP, Yellon DM, Martin C, et al. B-type natriuretic peptide limits infarct size in rat isolated hearts via KATP channel opening. Am J Physiol Heart Circ Physiol. 2003;284:H1592–H1600. doi: 10.1152/ajpheart.00902.2002. [DOI] [PubMed] [Google Scholar]
- 32.Hempel A, Friedrich M, Schluter KD, Forssmann WG, Kuhn M, Piper HM. ANP protects against reoxygenation-induced hypercontracture in adult cardiomyocytes. Am J Physiol. 1997;273:H244–H249. doi: 10.1152/ajpheart.1997.273.1.H244. [DOI] [PubMed] [Google Scholar]
- 33.Sangawa K, Nakanishi K, Ishino K, Inoue M, Kawada M, Sano S. Atrial natriuretic peptide protects against ischemia-reperfusion injury in the isolated rat heart. Ann Thorac Surg. 2004;77:233–237. doi: 10.1016/s0003-4975(03)01493-0. [DOI] [PubMed] [Google Scholar]
- 34.Yang XM, Philipp S, Downey JM, Cohen MV. Atrial natriuretic peptide administered just prior to reperfusion limits infarction in rabbit hearts. Basic Res Cardiol. 2006;101:311–318. doi: 10.1007/s00395-006-0587-2. [DOI] [PubMed] [Google Scholar]
- 35.Kato T, Muraski J, Chen Y, et al. Atrial natriuretic peptide promotes cardiomyocyte survival by cGMP-dependent nuclear accumulation of zyxin and Akt. J Clin Invest. 2005;115:2716–2730. doi: 10.1172/JCI24280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorbe A, Giricz Z, Szunyog A, et al. Role of cGMP-PKG signaling in the protection of neonatal rat cardiac myocytes subjected to simulated ischemia/reoxygenation. Basic Res Cardiol. 2010;105:643–650. doi: 10.1007/s00395-010-0097-0. [DOI] [PubMed] [Google Scholar]
- 37.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HHHW, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nature Reviews Drug Discovery. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krieg T, Liu Y, Rutz T, et al. BAY 58-2667, a nitric oxide-independent guanylyl cyclase activator, pharmacologically post-conditions rabbit and rat hearts. Eur Heart J. 2009;30:1607–1613. doi: 10.1093/eurheartj/ehp143. [DOI] [PubMed] [Google Scholar]
- 39.Salloum FN, Das A, Chau VQ, et al. BAY58-2667, a Novel NO-Independent Activator of Soluble Guanylate Cyclase, Protects Against Ischemia/Reperfusion Injury: Potential Role of Hydrogen Sulfide Signaling. [abstr] FASEB J. 2010;24:787.4. [Google Scholar]
- 40.Radovits T, Korkmaz S, Miesel-Groschel C, et al. Pre-conditioning with the soluble guanylate cyclase activator Cinaciguat reduces ischaemia-reperfusion injury after cardiopulmonary bypass. Eur J Cardiothorac Surg. 2011;39:248–255. doi: 10.1016/j.ejcts.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 41.Bian JS, Yong QC, Pan TT, et al. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. Journal of Pharmacology and Experimental Therapeutics. 2006;316:670–678. doi: 10.1124/jpet.105.092023. [DOI] [PubMed] [Google Scholar]
- 42.Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K(ATP) channels in rabbits. Am J Physiol Heart Circ Physiol. 2002;283:H1263–H1269. doi: 10.1152/ajpheart.00324.2002. [DOI] [PubMed] [Google Scholar]
- 43.Porst H, Rosen R, Padma-Nathan H, et al. The efficacy and tolerability of vardenafil, a new, oral, selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction: the first at-home clinical trial. Int J Impot Res. 2001;13:192–199. doi: 10.1038/sj.ijir.3900713. [DOI] [PubMed] [Google Scholar]
- 44.Salloum FN, Ockaili RA, Wittkamp M, Marwaha VR, Kukreja RC. Vardenafil: a novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia/reperfusion injury via opening of mitochondrial K(ATP) channels in rabbits. J Mol Cell Cardiol. 2006;40:405–411. doi: 10.1016/j.yjmcc.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Salloum FN, Takenoshita Y, Ockaili RA, et al. Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial K(ATP) channels when administered at reperfusion following ischemia in rabbits. J Mol Cell Cardiol. 2007;42:453–458. doi: 10.1016/j.yjmcc.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor J, Baldo OB, Storey A, Cartledge J, Eardley I. Differences in side-effect duration and related bother levels between phosphodiesterase type 5 inhibitors. BJU Int. 2009;103:1392–1395. doi: 10.1111/j.1464-410X.2008.08328.x. [DOI] [PubMed] [Google Scholar]
- 47.Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res. 2003;92:595–597. doi: 10.1161/01.RES.0000066853.09821.98. [DOI] [PubMed] [Google Scholar]
- 48.Das A, Ockaili R, Salloum F, Kukreja RC. Protein kinase C plays an essential role in sildenafil-induced cardioprotection in rabbits. Am J Physiol Heart Circ Physiol. 2004;286:H1455–H1460. doi: 10.1152/ajpheart.01040.2003. [DOI] [PubMed] [Google Scholar]
- 49.Das A, Salloum FN, Xi L, Rao YJ, Kukreja RC. ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol. 2009;296:H1236–H1243. doi: 10.1152/ajpheart.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Das A, Smolenski A, Lohmann SM, Kukreja RC. Cyclic GMP-dependent protein kinase Ialpha attenuates necrosis and apoptosis following ischemia/reoxygenation in adult cardiomyocyte. J Biol Chem. 2006;281:38644–38652. doi: 10.1074/jbc.M606142200. [DOI] [PubMed] [Google Scholar]
- 51.Takimoto E, Koitabashi N, Hsu S, et al. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J Clin Invest. 2009;119:408–420. doi: 10.1172/JCI35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koitabashi N, Aiba T, Hesketh GG, et al. Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation Novel mechanism of cardiac stress modulation by PDE5 inhibition. J Mol Cell Cardiol. 2010;48:713–724. doi: 10.1016/j.yjmcc.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salloum FN, Abbate A, Das A, et al. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol. 2008;294:H1398–H1406. doi: 10.1152/ajpheart.91438.2007. [DOI] [PubMed] [Google Scholar]
- 54.Chau VQ, Salloum FN, Hoke NN, Abbate A, Kukreja RC. Mitigation of the progression of heart failure with sildenafil involves inhibition of RhoA/Rho-kinase pathway. Am J Physiol Heart Circ Physiol. 2011;300:H2272–H2279. doi: 10.1152/ajpheart.00654.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hattori T, Shimokawa H, Higashi M, et al. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation. 2004;109:2234–2239. doi: 10.1161/01.CIR.0000127939.16111.58. [DOI] [PubMed] [Google Scholar]
- 56.Finsterer J, Stollberger C. The heart in human dystrophinopathies. Cardiology. 2003;99:1–19. doi: 10.1159/000068446. [DOI] [PubMed] [Google Scholar]
- 57.Adamo CM, Dai DF, Percival JM, et al. Sildenafil reverses cardiac dysfunction in the mdx mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2010;107:19079–19083. doi: 10.1073/pnas.1013077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wehling-Henricks M, Jordan MC, Roos KP, Deng B, Tidball JG. Cardiomyopathy in dystrophin-deficient hearts is prevented by expression of a neuronal nitric oxide synthase transgene in the myocardium. Hum Mol Genet. 2005;14:1921–1933. doi: 10.1093/hmg/ddi197. [DOI] [PubMed] [Google Scholar]
- 59.Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khairallah M, Khairallah RJ, Young ME, et al. Sildenafil and cardiomyocyte-specific cGMP signaling prevent cardiomyopathic changes associated with dystrophin deficiency. Proc Natl Acad Sci U S A. 2008;105:7028–7033. doi: 10.1073/pnas.0710595105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 62.Friess GG, Boyd JF, Geer MR, Garcia JC. Effects of first-dose doxorubicin on cardiac rhythm as evaluated by continuous 24-hour monitoring. Cancer. 1985;56:2762–2764. doi: 10.1002/1097-0142(19851215)56:12<2762::aid-cncr2820561207>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 63.Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–1610. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- 64.Koka S, Das A, Zhu SG, Durrant D, Xi L, Kukreja RC. Long-acting phosphodiesterase-5 inhibitor tadalafil attenuates doxorubicin-induced cardiomyopathy without interfering with chemotherapeutic effect. J Pharmacol Exp Ther. 2010;334:1023–1030. doi: 10.1124/jpet.110.170191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu B, Vemavarapu L, Thompson WJ, Strada SJ. Suppression of cyclic GMP-specific phosphodiesterase 5 promotes apoptosis and inhibits growth in HT29 cells. Journal of Cellular Biochemistry. 2005;94:336–350. doi: 10.1002/jcb.20286. [DOI] [PubMed] [Google Scholar]
- 66.Black KL, Yin DL, Ong JM, et al. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Research. 2008;1230:290–302. doi: 10.1016/j.brainres.2008.06.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Das A, Durrant D, Mitchell C, et al. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18202–18207. doi: 10.1073/pnas.1006965107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]