Abstract
Background
Tadalafil is a novel long acting inhibitor of phosphodiesterase-5. Since cGMP-dependent protein kinase (PKG) signaling plays a key role in cardioprotection, we hypothesized that PKG activation with tadalafil would limit myocardial ischemia/reperfusion (I/R) injury and dysfunction. Additionally, we contemplated that cardioprotection with tadalafil is mediated by hydrogen sulfide (H2S) signaling in a PKG-dependent fashion.
Methods and Results
After baseline transthoracic echocardiography (TTE), adult ICR mice were injected i.p. with vehicle (10% DMSO) or tadalafil (1 mg/kg) with or without KT5823 (KT, PKG blocker, 1 mg/kg) or dl-propargylglycine [PAG, Cystathionine-γ-lyase (CSE, H2S-producing enzyme) blocker; 50 mg/kg] 1 h prior to coronary artery ligation for 30 min and reperfusion for 24 h, whereas C57BL-wild type and CSE-knockout mice were treated with either vehicle or tadalafil. After reperfusion, TTE was performed and hearts were collected for infarct size (IS) measurement using TTC staining. Survival was increased with tadalafil (95%) compared with control (65%, P<0.05). Infarct size was reduced with tadalafil (13.2±1.7%) compared to vehicle (40.6±2.5%; P<0.05). KT and PAG abolished tadalafil-induced protection (IS: 39.2±1% and 51.2±2.4%, respectively) similar to genetic deletion of CSE (47.2±5.1%). Moreover, tadalafil preserved fractional shortening (FS: 31±1.5%) compared to control (FS: 22±4.8%, P<0.05). Baseline FS was 44±1.7%. KT and PAG abrogated the preservation of LV function with tadalafil by a decline in FS to 17±1% and 23±3%, respectively. Compared to vehicle, myocardial H2S production was significantly increased with tadalafil and was abolished with KT.
Conclusion
PKG activation with tadalafil limits myocardial infarction and preserves LV function through H2S signaling.
Keywords: Phosphodiesterase inhibitors; Ischemia-reperfusion injury; PKG; CSE, H2S
Introduction
Phosphodiesterase-5 (PDE-5) inhibitors including sildenafil (Viagra), vardenafil (Levitra) and tadalafil (Cialis) are FDA-approved for treatment of erectile dysfunction. More recently, sildenafil was indicated for management of pulmonary arterial hypertension (1). There has been a substantial growth in evidence supporting the cardioprotective role of PDE-5 inhibitors against ischemia/reperfusion injury (I/R). Several studies from our laboratory have shown that sildenafil and vardenafil reduce infarct size, apoptosis, and attenuate cardiac dysfunction following I/R or permanent coronary artery ligation (2,3,4). However, relatively little work has been done in identifying the role of tadalafil in cardioprotection against I/R. Tadalafil is a selective PDE-5 inhibitor whose effects can last up to 36 h, whereas the durations of action of sildenafil and vardenafil are generally 4 to 8 h. Tadalafil is also the only PDE-5 inhibitor whose activity is unaffected by food and has a relatively short time to onset of action (16–17 minutes). The pharmacokinetic profile of tadalafil shows maximal plasma concentration within 2.0 h and an elimination half-life of 17.5 h. In this respect, the use of tadalafil is quite attractive for long-term management of cardiovascular disease. Moreover, tadalafil is a highly selective inhibitor of PDE with >10,000-fold selectivity for PDE-5 over PDE-1 to PDE-4 and approximately 700-fold selectivity for PDE-5 over PDE-6 (5).
Sesti et al. have recently shown that tadalafil reduces infarct size in rats following I/R (6), but its impact on myocardial contractility remains uncertain. Also, there is currently no information on the mechanism of protection by tadalafil although sildenafil protects the heart through activation of PKC, expression of endothelial and inducible nitric oxide synthases (eNOS/iNOS), protein kinase G (PKG) and opening of mitochondrial KATP (mitoKATP) channels in the heart (2,3,4,7,8,9).
Hydrogen sulfide (H2S) is a gaseous molecule that is produced enzymatically on a continuous basis at micromolar levels in mammals and exerts a number of physiological actions in the cardiovascular system. Similar to PKG, H2S has been shown to protect the heart via opening of mitoKATP channel (10). The H2S-producing enzyme, cystathionine-γ-lyase (CSE), is expressed in the heart and administration of the H2S donor, sodium hydrosulfide, reduces infarct size following I/R. Because PKG and H2S open mitoKATP channels, similar to sildenafil and vardenafil (2,3), we tested the hypothesis that tadalafil might lead to protection against I/R through generation of H2S from CSE. Interestingly, our results show that tadalafil indeed activates myocardial PKG and also induces cardioprotection against I/R, which was abolished with the PKG inhibitor KT5823 (KT). Moreover, the protective effect of tadalafil was blunted by treatment with a CSE inhibitor, dl-propargylglycine (PAG), as well as in CSE-knockout mice suggesting a definite role of endogenous H2S signaling in cardioprotection with tadalafil.
Materials and Methods
Animals
Adult male out-bred ICR mice were supplied by Harlan Sprague Dawley (Indianapolis, IN). The mean body weight was 32.2 ± 0.4 g. C57BL mice were supplied by the Jackson Laboratories (Bar Harbor, ME). CSE-knockout mice (4 males and 5 females) were kindly provided by Dr. David J. Lefer (Emory University School of Medicine). All animal experiments were conducted under the guidelines on humane use and care of laboratory animals for biomedical research published by National Institutes of Health (No. 85-23, revised 1996).
Drugs and chemicals
KT5823, dl-propargylglycine, triphenyltetrazolium chloride (TTC) and Dimethyl sulfoxide (DMSO; vehicle for tadalafil) were purchased from Sigma-Aldrich (St. Loius, MO). Tadalafil powder was kindly provided by Lilly ICOS, Inc.
Myocardial Infarction protocol
The methodology of myocardial infarction was described previously (4). In brief, the left descending coronary artery was identified and occluded for 30 min by a 7.0 silk ligature that was placed around it and a small piece of polyethylene tubing (PE10) that was positioned on top of it. After coronary artery occlusion for 30 min, reperfusion was established by removing the PE10 tube that was compressing the coronary artery. After reperfusion, the air was expelled from the chest and the animals were extubated.
Experimental Groups
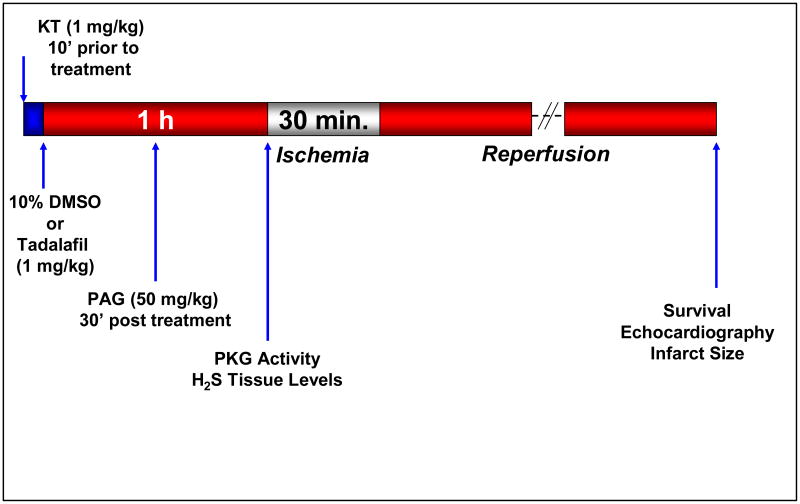
Ten groups were used. 1- Dimethyl sulfoxide (DMSO, vehicle for Tadalafil): Each mouse received 0.2 ml 10% DMSO; ip, 1 h prior to I/R; 2- Tadalafil: Mice received an ip injection of 1 mg/kg (in 0.2 ml 10% DMSO), 1 h prior to I/R; 3- PKG Inhibitor; KT5823 (1 mg/kg; ip) + Tadalafil: KT was given 10 min. before tadalafil which was administered as in group 2; 4- Tadalafil + CSE Inhibitor PAG (50 mg/kg): PAG was given 30 min. after tadalafil treatment as in group 2; 5- KT + DMSO control: KT given 10 min before DMSO as in group 1; 6- DMSO + PAG control: PAG was given 30 min. after DMSO administration as in group 1; 7- Sham: Mice were subjected to a left thoracotomy without coronary artery ligation as a control for the surgical procedure (the animals in this group received no treatment until sampling of the heart); 8- DMSO-treated C57BL wild-type mice (control for CSE-knockout): Each mouse received DMSO as in group 1; 9- Tadalafil-treated C57BL wild-type mice: Mice received tadalafil as in group 2; 10- Tadalafil in CSE-knockout mice: Six mice (4 male and 2 female) received tadalafil as in group 2. In Groups 1 – 6 and 8 – 10, infarct size was measured 24 h after I/R. Prior to sacrifice, LV function was analyzed using echocardiography. Six to eight mice in each group were used for infarct size assessment and for functional analysis using echocardiography. The detailed experimental protocol is shown in Figure 1. Three additional mice in groups 1 and 2 were used for measurement of hemodynamics throughout pre-ischemia, ischemia and reperfusion.
Figure 1.
Experimental protocol. Arrows indicate time points for treatment, performance of surgical procedures and measurement of various parameters.
Survival
Survival rate was determined based on the animals that survived the experimental protocol starting at recovery following surgery until 24 h after infarction.
Infarct size assessment
After 24 h of reperfusion, the heart was quickly removed and mounted on a Langendorff apparatus as described previously (4). The areas of infarcted tissue, risk zone, and whole left ventricle were determined using TTC staining by computer morphometry using a Bioquant imaging software.
Hemodynamics
Systemic hemodynamics including systolic, diastolic and mean blood pressures as well as heart rate were measured using a non-invasive tail cuff volume pressure recording system (CODA-2, Kent Scientific, Torrington, CT) in the anesthetized mouse. Tail cuff inflation was performed on twenty occasions before measurements were taken. The mean of a minimum of 30 recordings on each occasion was taken and the mean data were compared between the groups.
PKG Activity
Mice were divided into three groups: 1- No treatment; 2- 10% DMSO; and 3- Tadalafil 1 h prior to heart explantation. PKG activity was measured according to the manufacturer's instructions (Cyclex, Nagano, Japan). Spectrophotometric absorbance was measured at 450 nm and the results were normalized per mg of protein.
Cardiac Tissue Levels of H2S
A subset of 3 mice per group was utilized for H2S measurement. Group 1 was treated with 10% DMSO as control; Group 2 was treated with tadalafil 1 h prior to heart collection; and Group 3 was given KT, 10 min. prior to tadalafil as in group 2. The tissue concentration of H2S was measured by homogenizing snap-frozen hearts in 1 mL of 100 mM potassium phosphate buffer (pH 7.4). To trap H2S, 250 μL of zinc acetate (1% wt/vol) was added to the tissue homogenate followed by 30 min incubation at 37°C. The reaction was stopped by adding 250 μL of trichloroacetic acid (10% wt/vol) to the assay mixture and incubated for 60 min at 37°C before centrifugation at 14,000 g for 10 min. H2S concentration of the supernatants was measured using a highly specific H2S sensor connected to a single channel analyzer (Apollo 1000, WPI, Sarasota, FL) and was calculated using a calibration curve of NaHS standards. Protein concentration was measured spectrophotometrically at 595 nm. The results are expressed as μM/mg of protein (11).
Echocardiography
Echocardiography was performed using the Vevo770™ imaging system (VisualSonics Inc., Toronto, Canada) prior to surgery (baseline) and 24 h after surgery prior to sacrificing the animal. Pentobarbital (30 mg/kg; ip) was used for anesthesia and the procedure was carried out as previously described (4) to measure LV end-diastolic diameter (LVEDD) and end-systolic diameter (LVESD). LV fractional shortening (FS) was calculated as (LVEDD-LVESD)/LVEDD*100.
Statistics
All measurements of infarct size and risk areas are expressed as group means ± SE. Changes in echocardiography parameters and infarct size were analyzed using the random effects ANOVA for repeated-measures to determine the main effect of time, group, time-by-group interaction, and the post-hoc two-sided Dunnett's test to compare 2 groups at a time. Statistical differences were considered significant if the P value was <0.05. Discrete variables were presented as absolute and percent value. The Chi-square test (or the Fisher's exact test when appropriate) was used to compare discrete variable in different groups. The Bonferroni correction for post-hoc analysis was used when comparing 2 groups from 3 or more groups.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Survival
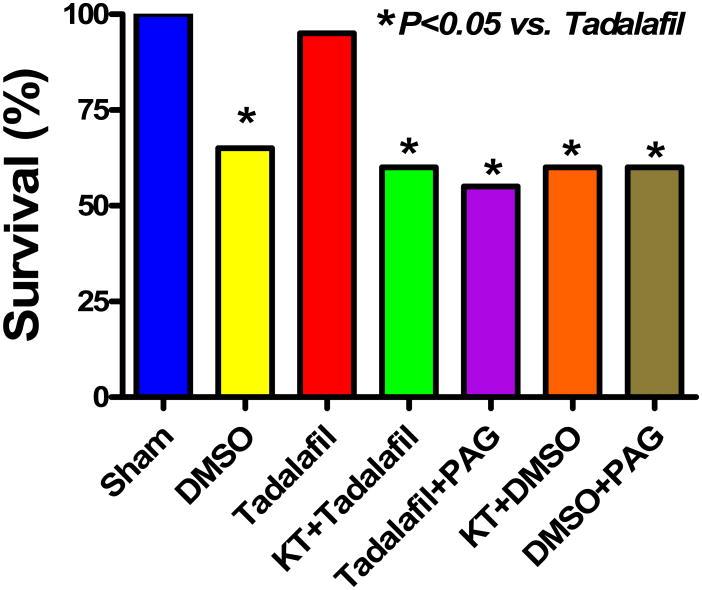
A total of 135 mice were used in this study. 19 out of 20 mice survived with tadalafil (95%) as compared to 13 out of 20 with DMSO (65%, P<0.05, Figure 2). PKG and CSE inhibition with KT and PAG in addition to tadalafil reduced survival rate to 60% and 55%, respectively, P<0.05 vs. tadalafil. KT and PAG alone had no adverse effects on survival as compared with DMSO (P>0.05). The survival rate was 100 % in sham-operated mice. Six out of 7 C57BL mice (the controls for CSE-knockout mice) survived in the tadalafil group (86%) and 6 out of 10 survived in the DMSO group (60%). All 4 males and only 2 females survived out of a total of 9 mice treated with tadalafil (67%).
Figure 2.
Survival of mice following I/R in the various treatment groups at 24 h post-MI. Note that tadalafil-treated mice exhibited a significant increase in survival compared with animals treated with vehicle or PKG and CSE blockers (P<0.05).
Infarct Size
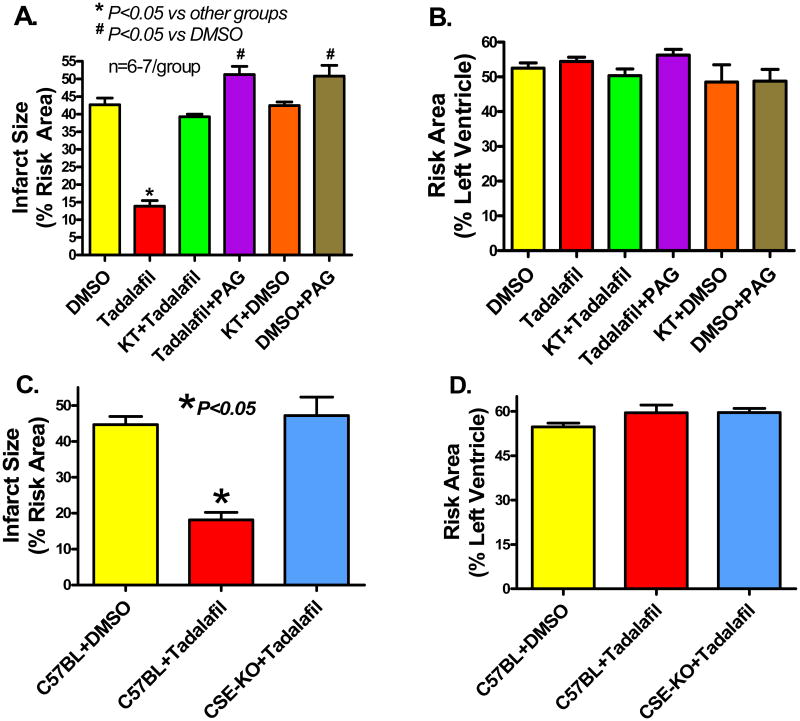
Infarct size (% of risk area) was reduced from 40.6±2.5 with DMSO to 13.2±1.7 with tadalafil 24 h after infarction (P<0.05, Figure 3A). The infarct-sparing effect of tadalafil was abolished with KT as shown by an increase in infarct size to 39.2±1.0 (P<0.05). Animals treated with KT alone had an infarct size of 40.9±0.9, which was not different from the DMSO group (P>0.05). PAG not only blunted the cardioprotection exerted by tadalafil, but exacerbated I/R injury as shown by an increase in infarct size to 51.2±2.4 (P<0.05 vs. DMSO). Control animals treated with PAG alone had a similar increase in infarct size to 51.5±4.7 (P<0.05 vs. DMSO). The risk areas (% LV) were not statistically different between the groups (Figure 3B). Sham operated mice did not exhibit any infarction. Genetic deletion of CSE abrogated the infarct-sparing effect of tadalafil (47.2±5.1%) compared to wild-type mice treated with tadalafil (18.1±2.1%, P<0.05, Figure 3C).
Figure 3.
A. Myocardial infarct size (% of RA) measured 24 h post-MI in the various groups. Note that infarct size was significantly reduced with tadalafil, which was blocked by KT and PAG. B. The area-at-risk, expressed as percent of the left ventricle, was similar in all groups. C. Myocardial infarct size measured 24 h post-MI in C57BL wild-type mice, mice treated with DMSO or tadalafil, and in CSE-knockout mice. Note that genetic deletion of CSE abrogated the infarct-sparing effect of tadalafil. D. The area-at-risk was similar in C57BL wild-type mice, mice treated with DMSO or tadalafil, and in CSE-knockout mice.
Hemodynamics
The systemic mean arterial pressure decreased immediately after opening of the chest (data not shown) and during ischemia, but was restored to baseline after reperfusion and closing of the chest. Administration of tadalafil did not cause marked changes in hemodynamic parameters at 1 h following treatment. Except at the indicated time points, the mean values were not significantly different between the groups (Table 1).
Table 1. Hemodynamics.
| Group | DMSO | Tadalafil | ||||
|---|---|---|---|---|---|---|
| Baseline | Ischemia | Reperfusion | Baseline | Ischemia | Reperfusion | |
| SBP | 98±6 | 78±3a | 93±5 | 94±4 | 81±4 | 94±3 |
| DBP | 70±4 | 56±6 | 72±4 | 71±3 | 58±4 | 73±3 |
| MAP | 79±3 | 63±5a | 79±2b | 77±2c | 65±3 | 80±2c |
| HR | 524±21 | 496±38 | 502±16 | 517±41 | 526±42 | 558±28 |
Values are means±SEM. SBP-Systolic Blood Pressure (mmHg); DBP-Diastolic Blood Pressure (mmHg); MAP-Mean Arterial Blood Pressure (mmHg); HR-Heart Rate (bpm).
P<0.05 vs.Baseline;
P<0.05 vs. Ischemia DMSO;
P<0.05 vs. Ischemia Tadalafil.
PKG Activity
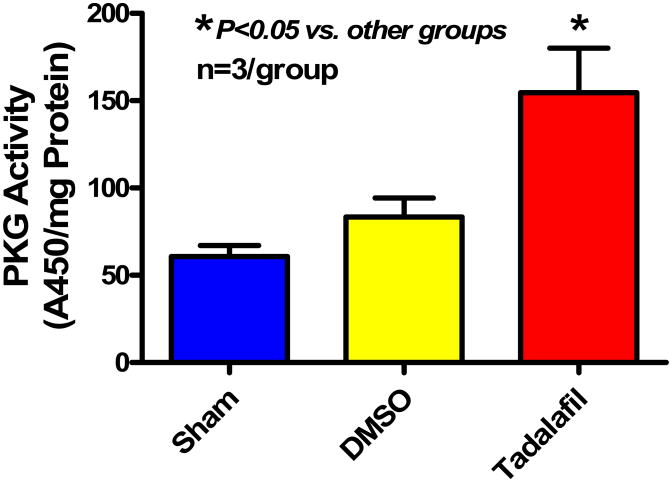
Treatment with tadalafil caused increase in cardiac PKG activity (A450/mg protein) to 154.6±25.5 (P<0.05) as compared with sham (60.7±6.4) and DMSO-treated mice (83.5±10.8) (Figure 4).
Figure 4.
PKG activity 1 h following treatment with tadalafil or 10% DMSO compared with sham hearts. Note that tadalafil increases PKG activity compared with DMSO (P<0.05).
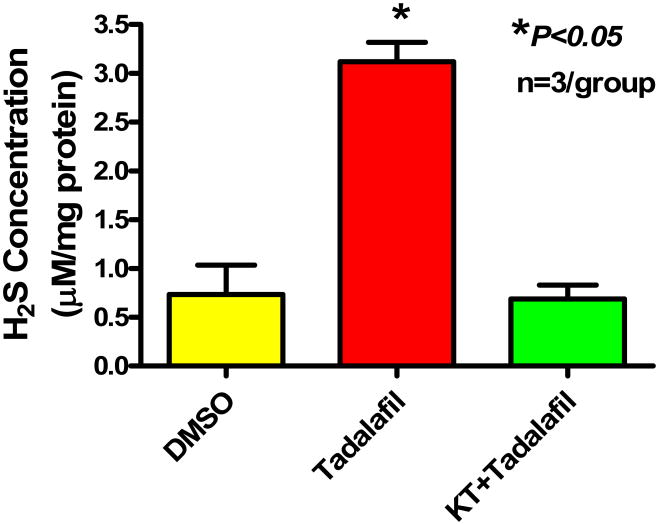
Cardiac Tissue Levels of H2S
Figure 5 illustrates a significant increase in H2S levels with tadalafil treatment (3.1±0.2 μM/mg, P<0.05) as compared to DMSO (0.7±0.3 μM/mg). Treatment with KT prior to tadalafil abrogated the increase in H2S seen with tadalafil alone to 0.7±0.1 μM/mg, which is not different from DMSO control (P>0.05).
Figure 5.
H2S tissue levels assessed in hearts harvested 1 h after treatment. Note that tadalafil caused an increase in cardiac H2S compared with DMSO (P<0.05). Interestingly, KT abrogated the increase in H2S caused by tadalafil (P<0.05).
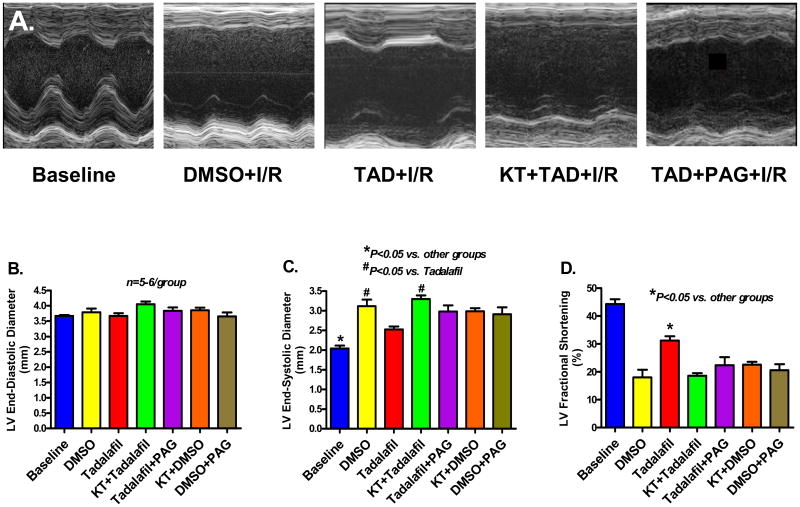
Left Ventricular Remodeling and Function
Figure 6A shows representative M-mode images from sham operated, DMSO (vehicle)-treated, tadalafil-treated, KT+tadalafil-treated, and tadalafil+PAG-treated mice at 24 h post MI. None of the groups presented with significant LV dilatation at 24 h post infarction (Figure 6B), however, tadalafil decreased LV end-systolic diameter (LVESD: 2.5±0.1 mm) and preserved fractional shortening (FS: 31±1.5%) as compared to DMSO (LVESD: 3.1±0.2 mm and FS: 22±4.8%, respectively; P<0.05). Baseline LVESD and FS were 2.0 ± 0.1 mm and 44 ± 1.7%, respectively, and KT administration prior to tadalafil abrogated the preservation of LV function as demonstrated by marked increase in LVESD to 3.3±0.1 mm and a decline in FS to 17±1%, P<0.05 vs. tadalafil. Similarly, PAG treatment blocked the functional improvement in tadalafil-treated mice as shown by an increase in LVESD to 3.0±0.2 mm and a deterioration in FS to 24±3%, P<0.05 (Figure 6C and 6D).
Figure 6.
A. Representative M-mode images demonstrating the preservation of LV contractility with tadalafil compared with vehicle. KT and PAG abolished this preservation in LV function. B. LV End-Diastolic Diameter, End-Systolic Diameter and Fractional Shortening measured in the various treatment groups.
Discussion
The cytoprotective effects of the gaseous molecules, NO and carbon monoxide (CO), against I/R have been well documented in the literature (12,13). H2S is well known as a toxic gas often used to describe the smell of rotten eggs (14). The production of H2S in mammalian systems has been attributed to two key enzymes, CSE and cystathionine β-synthase (CBS). CSE and CBS are heme-containing enzymes whose activities are dependent on the cofactor pyridoxal 5′-phosphate. CBS is capable of catalyzing the reaction of cysteine with other free thiols to generate H2S; and likewise thiocysteine generated by CSE can interact with thiols to generate H2S (15). CSE is physiologically activated by calcium-calmodulin, a mechanism for H2S formation in response to vascular activation (16). It has been shown that genetic deletion of this enzyme in mice markedly reduces H2S levels in the serum, heart, aorta, and other tissues and these animals display pronounced hypertension and diminished endothelium-dependent vasorelaxation. In the present study, we investigated the potential role of H2S generated from CSE in tadalafil-induced protection against I/R injury. Our results show that tadalafil significantly reduced infarct size and attenuated LV dysfunction at 24 h post infarction as shown by preservation of fractional shortening and improvement in survival of mice. Moreover, tadalafil-induced cardioprotection was associated with increased activity of PKG and enhanced generation of endogenous H2S through a PKG-dependent pathway. The infarct-sparing effect of tadalafil was abolished with the PKG inhibitor KT, the CSE inhibitor PAG as well as in CSE-knockout mice. To our knowledge, this is the first investigation that has (1) implicated H2S as a mediator of cardioprotection with tadalafil, and (2) uncovered a potentially novel role of PKG in generation of H2S by tadalafil.
PDE-5 inhibition prevents the breakdown of cGMP into 5′GMP which leads to cGMP accumulation. In addition to its presence in the cavernosal tissue and penile arteries, PDE-5 also exists in vascular smooth muscle cells and murine ventricular cardiomyocytes (17). We have shown that PDE-5 inhibition increases cGMP levels and activate PKG, which plays an essential role in sildenafil-induced cardioprotection. We also demonstrated that direct adenoviral overexpression of PKG Iα (the isozyme expressed in the heart) in cardiomyocytes induced both eNOS/iNOS and protected them against simulated I/R (18), and reduced infarct size following I/R in vivo (19). The increases in PKG activity and expression by sildenafil were responsible for enhanced Bcl-2/Bax ratio, phosphorylation of Akt, ERK1/2, and inactivation of GSK-3β that eventually resulted in attenuation of necrosis and apoptosis following simulated ischemia/reoxygenation (10). The evidence that PKG is involved in tadalafil-induced protection is illustrated by a complete loss of the cardioprotective effect with the PKG inhibitor KT in terms of infarct size reduction, prevention of LV systolic dysfunction, and increased survival rate. Tadalafil caused a significant increase in PKG activity at 1 h after treatment, which is consistent with the induction of ischemia in mice that underwent I/R.
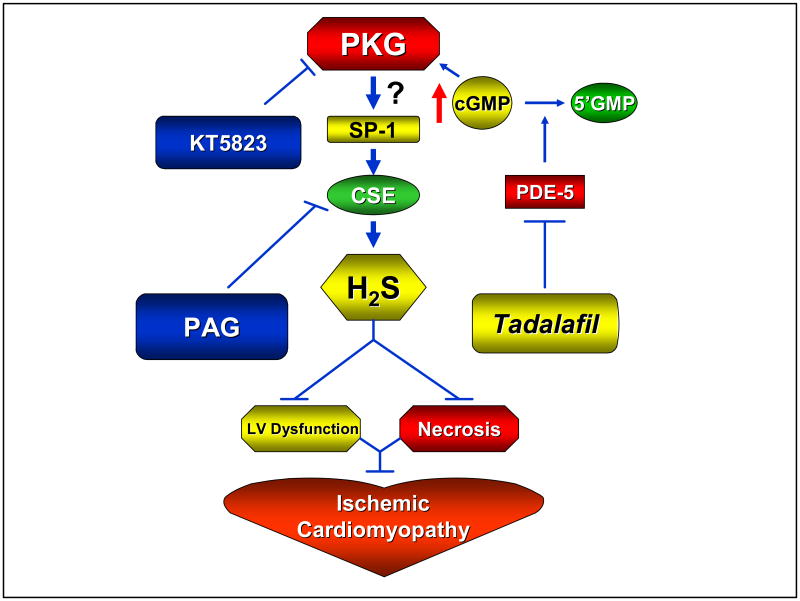
PKG is a serine/threonine protein kinase and is one of the major intracellular receptors for cGMP. There are two types of PKG present in eukaryotic cells: type I and type II. PKG is present in high concentrations in smooth muscle, platelets, cerebellum, hippocampus, dorsal root ganglia, neuromuscular end plate, and the kidney vasculature (20). Activation of PKG phosphorylates many intracellular proteins and regulates important physiological functions such as relaxation of vascular smooth muscle, inhibition of cell differentiation and proliferation, and inhibition of platelet aggregation and apoptosis (21). Exactly how PKG activation is associated with increased H2S generation by tadalafil is not clear from the present study although it may be related to PKG-dependent enhancement of CSE activity in the heart. There is evidence that specificity protein 1 (SP-1; also known as Sp1 transcription factor) plays an important role in the basal transcriptional activity of CSE enzyme (22). PKG can phosphorylate Sp1 on serine residue(s) which results in transcriptional activation of Sp1 in human SW480 colon cancer cells (23) with consequent increase in CSE activity and possibly generation of H2S. It is not known whether PKG-dependent transcriptional activation of Sp1 also occurs in the heart. The validity of this proposed mechanism will be addressed in future studies (Figure 7).
Figure 7.
Proposed scheme outlining the pathway by which tadalafil may lead to PKG-dependent generation of H2S and protection against ischemia/reperfusion injury, LV dysfunction as well as ischemic cardiomyopathy.
H2S has been suggested to regulate cardiovascular homeostasis and promote cellular signals that modulate metabolism, cardiac function, and cell survival. In fact, moderate use of H2S balneotherapy (treatment by bathing) has been shown to raise the tolerance to exercise and reduce the daily need for short-acting nitrates in patients with coronary artery disease (CAD) (24). Clinically, H2S plasma levels in CAD patients are reported to be lower than in angiographically normal control subjects (25). Therefore, the use of pharmacological agents, particularly PDE-5 inhibitors, to augment endogenous H2S synthesis for combating CAD is quite attractive because of the overall safety margin of this class of drugs. Moreover, the implication of H2S in tadalafil-induced cardioprotection suggests a potential role of this gaseous molecule in the therapeutic effect of tadalafil for erectile dysfunction as well. In fact, a pilot study suggested that endogenous H2S plays a role in erectile physiopharmacology (26). In these studies, the administration of PAG resulted in significant reduction in cavernous nerve stimulation-evoked perfusion pressure.
In conclusion, the present study has provided evidence for a novel mechanism by which the long acting PDE-5 inhibitor, tadalafil, exerts cardioprotective effects in mice. We believe that other PDE-5 inhibitors including sildenafil and vardenafil might share a similar pathway of PKG-dependent generation for H2S in protection against I/R injury and possibly post MI-induced heart failure.
Acknowledgments
Funding Sources: This study was supported in part by grants from National Institutes of Health (HL51045, HL59469, and HL79424) to RCK.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Kane LB, Klings ES. Present and future treatment strategies for pulmonary arterial hypertension: focus on phosphodiesterase-5 inhibitors. Treat Respir Med. 2006;5:271–82. doi: 10.2165/00151829-200605040-00005. [DOI] [PubMed] [Google Scholar]
- 2.Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial KATP channels in rabbits. Am J Physiol Heart Circ Physiol. 2002;283:H1263–H1269. doi: 10.1152/ajpheart.00324.2002. [DOI] [PubMed] [Google Scholar]
- 3.Salloum FN, Ockaili RA, Wittkamp M, Marwaha VR, Kukreja RC. Vardenafil: a novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia/reperfusion injury via opening of mitochondrial KATP channels in rabbits. J Mol Cell Cardiol. 2006;40:405–11. doi: 10.1016/j.yjmcc.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Salloum FN, Abbate A, Das A, Houser J, Mudrick CA, Qureshi IZ, Hoke NN, Roy SK, Brown WR, Prabhakar S, Kukreja RC. Sildenafil (Viagra) Attenuates Ischemic Cardiomyopathy and Improves Left Ventricular Function in Mice. Am J Physiol Heart Circ Physiol. 2008;294:H1398–406. doi: 10.1152/ajpheart.91438.2007. [DOI] [PubMed] [Google Scholar]
- 5.Padma-Nathan H. Efficacy and tolerability of tadalafil, a novel phosphodiesterase 5 inhibitor, in treatment of erectile dysfunction. Am J Cardiol. 2003;92:19M–25M. doi: 10.1016/s0002-9149(03)00828-2. [DOI] [PubMed] [Google Scholar]
- 6.Sesti C, Florio V, Johnson EG, Kloner RA. The phosphodiesterase-5 inhibitor tadalafil reduces myocardial infarct size. Int J Impot Res. 2007;19:55–61. doi: 10.1038/sj.ijir.3901497. [DOI] [PubMed] [Google Scholar]
- 7.Das A, Ockaili R, Salloum F, Kukreja RC. Protein kinase C plays an essential role in sildenafil-induced cardioprotection in rabbits. Am J Physiol Heart Circ Physiol. 2003;286:H1455–60. doi: 10.1152/ajpheart.01040.2003. [DOI] [PubMed] [Google Scholar]
- 8.Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res. 2003;92:595–597. doi: 10.1161/01.RES.0000066853.09821.98. [DOI] [PubMed] [Google Scholar]
- 9.Das A, Xi L, Kukreja RC. Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem. 2008;283:29572–85. doi: 10.1074/jbc.M801547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA. 2007;104:15560–5. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su YW, Liang C, Jin HF, Tang XY, Han W, Chai LJ, Zhang CY, Geng B, Tang CS, Du JB. Hydrogen sulfide regulates cardiac function and structure in adriamycin-induced cardiomyopathy. Circ J. 2009;73:741–9. doi: 10.1253/circj.cj-08-0636. [DOI] [PubMed] [Google Scholar]
- 12.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y, Stein AB, Wu WJ, Tan W, Zhu X, Li QH, Dawn B, Motterlini R, Bolli R. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H1649–53. doi: 10.1152/ajpheart.00971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiffenstein R, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol. 1992;2:109–34. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- 15.Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJ. Free radical biology and medicine: it's a gas, man! Am J Physiol Regul Integr Comp Physiol. 2006;291:R491–511. doi: 10.1152/ajpregu.00614.2005. [DOI] [PubMed] [Google Scholar]
- 16.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;22:587–90. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis: Essential role of NO signaling. J Biol Chem. 2005;280:12944–55. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- 18.Das A, Smolenski A, Lohmann SM, Kukreja RC. Cyclic GMP-dependent protein kinase Ialpha attenuates necrosis and apoptosis following ischemia/reoxygenation in adult cardiomyocyte. J Biol Chem. 2006;281:38644–52. doi: 10.1074/jbc.M606142200. [DOI] [PubMed] [Google Scholar]
- 19.Salloum FN, Das A, Ownby ED, Kukreja RC. Gene Therapy with PKG1-α Reduces Myocardial Infarction Following Ischemia/Reperfusion in Mice. Circ Res. 2006;99:1281–1281. [Google Scholar]
- 20.Hofmann F, Ammendola A, Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J Cell Sci. 2000;113:1671–1676. doi: 10.1242/jcs.113.10.1671. [DOI] [PubMed] [Google Scholar]
- 21.Lincoln TM, Dey N, Sellak H. cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol. 2001;91:1421–1430. doi: 10.1152/jappl.2001.91.3.1421. [DOI] [PubMed] [Google Scholar]
- 22.Ishii I, et al. Murine cystathionine γ-lyase: complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem J. 2004;381:113–123. doi: 10.1042/BJ20040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cen B, Deguchi A, Weinstein IB. Activation of protein kinase G Increases the expression of p21CIP1, p27KIP1, and histidine triad protein 1 through Sp1. Cancer Res. 2008;68:5355–62. doi: 10.1158/0008-5472.CAN-07-6869. [DOI] [PubMed] [Google Scholar]
- 24.Zunnunov ZR. Efficacy and safety of hydrogen sulfide balneotherapy in ischemic heart disease the arid zone. Ter Arkh. 2004;76:15–8. [PubMed] [Google Scholar]
- 25.Jiang HL, Wu HC, Li ZL, Geng B, Tang CS. Changes of the new gaseous transmitter H2S in patients with coronary heart disease. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:951–4. [PubMed] [Google Scholar]
- 26.Srilatha B, Adaikan PG, Moore PK. Possible role for the novel gasotransmitter hydrogen sulphide in erectile dysfunction--a pilot study. Eur J Pharmacol. 2006;535:280–2. doi: 10.1016/j.ejphar.2006.02.001. [DOI] [PubMed] [Google Scholar]