Abstract
Defective control of Epstein–Barr virus (EBV) infection by cytotoxic CD8+ T cells might predispose to multiple sclerosis (MS) by allowing EBV-infected autoreactive B cells to accumulate in the central nervous system. We have treated a patient with secondary progressive MS with in vitro-expanded autologous EBV-specific CD8+ T cells directed against viral latent proteins. This adoptive immunotherapy had no adverse effects and the patient showed clinical improvement with reduced disease activity on magnetic resonance imaging and decreased intrathecal immunoglobulin production. This is the first report of the use of EBV-specific adoptive immunotherapy to treat MS or any other autoimmune disease.
Keywords: Adoptive immunotherapy, B cell, CD8+ T cell, Epstein–Barr virus, multiple sclerosis, treatment
Introduction
A large body of evidence indicates that infection with Epstein–Barr virus (EBV) has a role in the pathogenesis of multiple sclerosis (MS).1,2 It has been hypothesized that defective elimination of EBV-infected B cells by cytotoxic CD8+ T cells predisposes to MS by allowing EBV-infected autoreactive B cells to accumulate in the central nervous system (CNS).3 This hypothesis predicts that adoptive immunotherapy to boost EBV-specific CD8+ T-cell immunity might be an effective treatment for MS.3 Consistent with the hypothesis, patients with MS have a decreased frequency of CD8+ T cells reactive to their own EBV-infected B cells4 which accumulate in the CNS.5,6
AdE1-LMPpoly is a novel recombinant adenovirus vector encoding multiple CD8+ T-cell epitopes from three EBV latent proteins, namely EBV nuclear antigen-1 (EBNA1), latent membrane protein 1 (LMP1) and LMP2A.7 Adoptive immunotherapy with autologous T cells expanded in vitro with AdE1-LMPpoly increases survival in patients with metastatic nasopharyngeal carcinoma, where the EBV-infected carcinoma cells express EBNA1, LMP1 and LMP2A.7 Because EBV-infected B cells in the brain in MS express the same three EBV proteins,5,8 adoptive immunotherapy with AdE1-LMPpoly might be an effective way to increase the number of CD8+ T cells available to eliminate EBV-infected B cells from the CNS. Here we report the use of adoptive immunotherapy with AdE1-LMPpoly to treat a patient with secondary progressive MS, for which currently there is no effective treatment.
Case report
The patient was a 42-year-old man whose first attack of MS occurred in 1994. The diagnosis of MS was confirmed by magnetic resonance imaging (MRI) of the brain and by the presence of oligoclonal immunoglobulin G (IgG) bands restricted to the cerebrospinal fluid (CSF). At first presentation he was IgG seropositive for EBNA and EBV viral capsid antigen (VCA) but IgM seronegative for VCA, indicating past infection with EBV. The course of his MS was relapsing–remitting until 2004 when it became secondary progressive. From 2000–2008 he was treated with interferon β-1b. Since 2008 he had been unable to walk or transfer himself. His MS had been complicated by: a bleeding duodenal ulcer requiring blood transfusion and resulting from the use of ibuprofen for flu-like symptoms of interferon β therapy; a decubitus ulcer requiring plastic surgery; painful hamstring spasms treated with baclofen and intramuscular botulinum toxin; urinary incontinence requiring a permanent indwelling urinary catheter leading to a severe urinary tract infection; and trigeminal neuralgia. By 2012, intention tremor was progressively limiting the use of his hands and he had a flexion contracture of the right knee. He was still working full-time from home as a manager. His Expanded Disability Status Scale Score was 8.0. He was a good candidate for T-cell therapy with AdE1-LMPpoly because the proportion of EBV-specific CD8+ T cells in his blood was below the 10th percentile in healthy EBV carriers and he carried HLA-A2 and HLA-B7, which are restricting elements for several of the EBNA1, LMP1 and LMP2A epitopes in AdE1-LMPpoly. He also had the general CD8+ T-cell deficiency (8.0% of peripheral blood mononuclear cells compared with a median of 18.6% and interquartile range of 15.7–23.0% in healthy subjects) and increased CD4:CD8 ratio (5.61 compared with a median of 2.27 and interquartile range of 1.76–3.07 in healthy subjects) typical of MS.9
This treatment was approved by the Royal Brisbane and Women’s Hospital Clinical Ethical Review Group and through the Special Access Scheme (Category B) of the Australian Government Therapeutic Goods Administration. With informed consent, we collected 400 ml of blood and expanded his EBV-specific T cells by in vitro stimulation with AdE1-LMPpoly and interleukin-2.7 After expansion, 38.46% of CD8+ T cells but only 0.22% of CD4+ T cells reacted to the LMP peptides. The EBV-specific T cells were returned to the patient intravenously (IV) at fortnightly intervals. To reduce the risk of aggravating CNS inflammation, we chose an initial dose of 5 × 106 T cells, which was only 25% of the median dose used for nasopharyngeal carcinoma,7 and escalated the dose gradually over the following three infusions to 1 × 107, 1.5 × 107, and 2 × 107 cells.
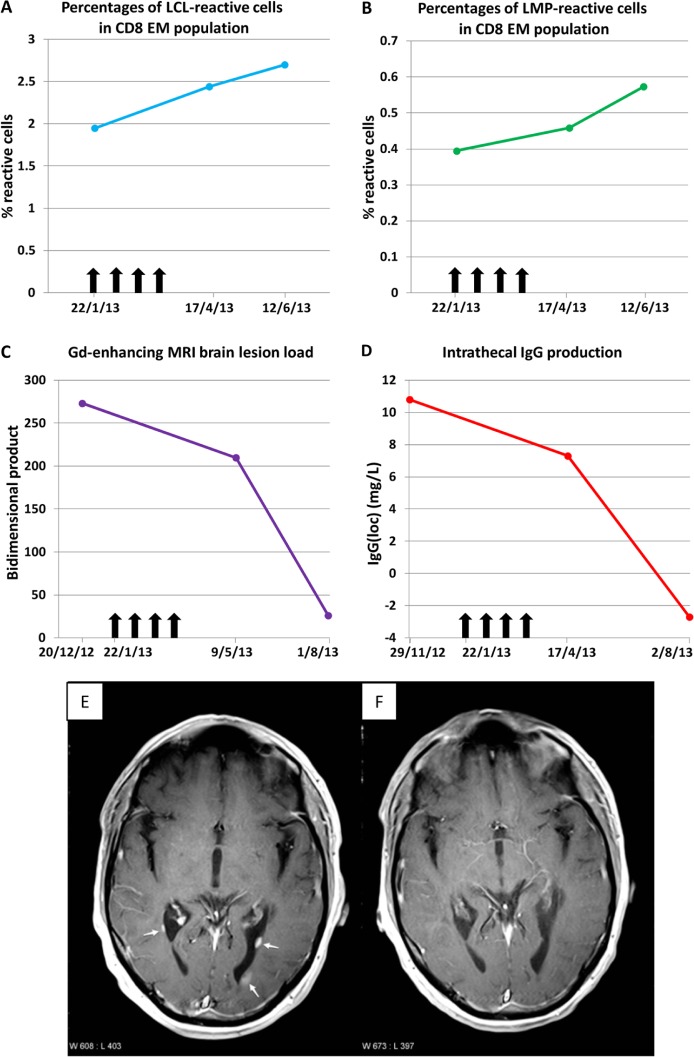
The treatment was successfully completed without significant adverse effects. In particular there were no fevers, flu-like symptoms or malaise. Two to three days after the second and third infusions the patient had tingling and numbness of the lips and tongue for 3–6 h, but in the previous year there had been two attacks of these symptoms, each lasting a week. Following the treatment he experienced a reduction in fatigue and painful lower limb spasms, an improvement in cognition and hand function, and increased productivity at work. These improvements were sustained up to the time of the latest review, 21 weeks after the final T-cell infusion, when neurological examination demonstrated increased voluntary movement of his lower limbs, particularly of the right knee flexors and left knee flexors and extensors to a Medical Research Council grade of 3/5, compared with 1/5 prior to the T-cell therapy. Following treatment the frequency of circulating EBV-specific CD8+ T cells increased (Figure 1(a) and (b)), and there were decreases in the number and size of gadolinium-enhancing MRI brain lesions and in intrathecal IgG production (Figure 1(c–f)). The CSF IgG index decreased from 0.79 (normal <0.70) before treatment to 0.74, 6 weeks after completion of treatment, and then decreased further to 0.63, 21 weeks after completion.
Figure 1.
Laboratory and medical imaging findings before and after EBV-specific adoptive immunotherapy. Panels A and B show the frequencies of T cells in the peripheral blood reactive to an EBV-infected autologous B cell lymphoblastoid cell line (LCL) (Panel A) and to a pool of the LMP1 and LMP2A (LMP) peptides contained within AdE1-LMPpoly (Panel B). T-cell reactivity to EBV was measured by flow cytometry and intracellular interferon-γ staining and is shown as the percentages of reactive cells within the CD8+ effector memory (EM; CD45RA–CD62L–) T-cell population. Vertical arrows indicate successive T-cell infusions of 5 × 106, 1 × 107, 1.5 × 107 and 2 × 107 cells. Panel C shows the total gadolinium(Gd)-enhancing brain lesion load, as measured by the bidimensional product, which was calculated as the sum of the product (maximum diameter of a lesion multiplied by the largest diameter perpendicular to this maximum diameter) of each lesion. Panel D shows the quantity of intrathecal IgG production (IgG(loc)) which was calculated by the formula of Reiber and Felgenhauer:10 IgG(loc) (mg/L) = {(CSF IgG ÷ serum IgG) − [0.8 × (√((CSF albumin ÷ serum albumin)2 + 15))] + 1.8} × serum IgG. Panels E and F show axial plane T1-weighted images at the level of the posterior horns of the lateral ventricles 5 minutes after the IV injection of gadolinium. Panel E demonstrates three periventricular gadolinium-enhancing lesions (arrows) 5 weeks before the commencement of therapy whereas Panel F shows no enhancing lesions 9 weeks after the completion of therapy.
Discussion
This study provides proof of principle that adoptive immunotherapy with autologous EBV-specific T cells can be safely administered to the patient with MS. The improvements in the patient’s symptoms and signs indicate a beneficial effect, which is supported by the reduction in disease activity on MRI and by the decrease in intrathecal IgG production. These effects can be explained by the killing of EBV-infected B cells in the CNS by the adoptively transferred CD8+ T cells. A clinical trial is needed to determine therapeutic efficacy across the clinical spectrum of MS. This study has implications for the treatment of other chronic autoimmune diseases where EBV also has a pathogenic role.3
Acknowledgments
We are grateful to the staff of Ward 7B North and the Internal Medicine Day Treatment Unit at the Royal Brisbane and Women’s Hospital for excellent care of the patient.
Footnotes
Conflict of interest: Michael Pender, Peter Csurhes, Corey Smith, Leonie Beagley, Kaye Hooper, Meenakshi Raj and Alan Coulthard have nothing to disclose. Scott Burrows and Rajiv Khanna hold a patent on the EBV epitopes included in the AdE1-LMPpoly construct.
Funding: This study was supported by Multiple Sclerosis Research Australia, the Trish Multiple Sclerosis Research Foundation and the QIMR Rio-Tinto Flagship Program on Immunotherapy.
References
- 1. Ascherio A, Munger KL. Epstein-Barr virus infection and multiple sclerosis: A review. J Neuroimmune Pharmacol 2010; 5: 271–277. [DOI] [PubMed] [Google Scholar]
- 2. Pender MP. The essential role of Epstein-Barr virus in the pathogenesis of multiple sclerosis. Neuroscientist 2011; 17: 351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pender MP. Infection of autoreactive B lymphocytes with EBV, causing chronic autoimmune diseases. Trends Immunol 2003; 24: 584–588. [DOI] [PubMed] [Google Scholar]
- 4. Pender MP, Csurhes PA, Lenarczyk A, et al. Decreased T cell reactivity to Epstein-Barr virus infected lymphoblastoid cell lines in multiple sclerosis. J Neurol Neurosurg Psychiatry 2009; 80: 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Serafini B, Rosicarelli B, Franciotta D, et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J Exp Med 2007; 204: 2899–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tzartos JS, Khan G, Vossenkamper A, et al. Association of innate immune activation with latent Epstein-Barr virus in active MS lesions. Neurology 2012; 78: 15–23. [DOI] [PubMed] [Google Scholar]
- 7. Smith C, Tsang J, Beagley L, et al. Effective treatment of metastatic forms of Epstein-Barr virus-associated nasopharyngeal carcinoma with a novel adenovirus-based adoptive immunotherapy. Cancer Res 2012; 72: 1116–1125. [DOI] [PubMed] [Google Scholar]
- 8. Serafini B, Severa M, Columba-Cabezas S, et al. Epstein-Barr virus latent infection and BAFF expression in B cells in the multiple sclerosis brain: Implications for viral persistence and intrathecal B-cell activation. J Neuropathol Exp Neurol 2010; 69: 677–693. [DOI] [PubMed] [Google Scholar]
- 9. Pender MP, Csurhes PA, Pfluger CMM, et al. Decreased CD8+ T cell response to Epstein-Barr virus infected B cells in multiple sclerosis is not due to decreased HLA class I expression on B cells or monocytes. BMC Neurology 2011; 11: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reiber H, Felgenhauer K. Protein transfer at the blood cerebrospinal fluid barrier and the quantitation of the humoral immune response within the central nervous system. Clin Chim Acta 1987; 163: 319–328. [DOI] [PubMed] [Google Scholar]