SUMMARY
Angiogenesis, the formation of new capillaries from existing vasculature, is a critical process in normal physiology as well as several physiopathologies. A desire to curb the supportive role angiogenesis plays in the development and metastasis of cancers has driven exploration into anti-angiogenic strategies as cancer therapeutics. Key to this, angiogenesis additionally displays an exquisite sensitivity to bioavailable copper. Depletion of copper has been shown to inhibit angiogenesis in a wide variety of cancer cell and xenograft systems. Several clinical trials using copper chelation as either an adjuvant or primary therapy have been conducted. Yet, the biological basis for the sensitivity of angiogenesis remains unclear. Numerous molecules important to angiogenesis regulation have been shown to be either directly or indirectly influenced by copper, yet a clear probative answer to the connection remains elusive.
Measurements of copper in biological systems have historically relied on techniques that, although demonstrably powerful, provide little or no information as to the spatial distribution of metals in a cellular context. Therefore, several new approaches have been developed to image copper in a biological context. One such approach relies on synchrotron-derived X-rays from third-generation synchrotrons and the technique of high resolution X-ray fluorescence microprobe (XFM) analysis.
Recent applications of XFM approaches to the role of copper in regulating angiogenesis have provided unique insight into the connection between copper and cellular behaviour. Using XFM, copper has been shown to be highly spatially regulated, as it is translocated from perinuclear areas of the cell towards the tips of extending filopodia and across the cell membrane into the extracellular space during angiogenic processes. Such findings may explain the heightened sensitivity of this cellular process to this transition metal and set a new paradigm for the kinds of regulatory roles that the spatial dynamics of cellular transition metals may play.
Keywords: angiogenesis, copper, endothelial cells, metallobiology, metalloproteomics, microprobe, synchrotron radiation, tubulogenesis, X-ray fluorescence, X-ray imaging
INTRODUCTION: IMPORTANCE OF ANGIOGENESIS IN CANCER
Blood vessels are formed and remodelled by three separate processes: vasculogenesis, arteriogenesis and angiogenesis.1 Vasculogenesis refers to the embryological formation of new blood vessels, in which progenitor cells migrate to sites of vascularization and differentiate into endothelial cells, forming the vascular plexus.2,3 Arteriogenesis refers to the remodelling of an existing artery to increase its cross-section in response to increased blood flow.4 Finally, angiogenesis refers to the budding of new capillary branches from existing blood vessels.1 Capillaries are formed by endothelial cells creating primitive tubules, which are then further supported and augmented by interactions with vascular pericytes. Angiogenesis is intimately involved not only with normal and developmental physiological processes, but also in the development of a number of pathological conditions, including rheumatoid arthritis, psoriasis, retinopathies and cancer. Angiogenesis is a complex multistep process comprised of endothelial cell proliferation, migration, differentiation and remodelling of the extracellular matrix.3,5 Several lines of evidence have indicated that the growth, persistence and metastasis of solid tumours is dependent upon angiogenesis.5 Furthermore, recent studies have indicated that angiogenesis is also involved in the pathogenesis of haematopoietic malignancies.6,7 Because of this association of angiogenesis with multiple and diverse tumour types, it has long been a goal of clinicians to disrupt angiogenesis as a novel strategy for cancer therapy. Anti-angiogenic therapy relies on stopping the formation of new capillary vessels around a tumour and breaking up the existing network of abnormal capillaries that feed the growing cancerous mass, thereby both starving it of nutrients and cutting off a pathway for primary metastasis through the endogenous circulatory system.8 The promise of such an approach has provided the impetus for a full understanding of angiogenesis and led to the discovery of a wide variety of anti-angiogenic endogenous cytokines, such as interferon (IFN)-α/β, angiostatin, endostatin, vasostatin, tumostatin, platelet factor-4, interleukin (IL)-10, IL-12 and thrombospondin-1. Such molecules are thought to exist in a delicate balance with pro-angiogenic molecules, such as vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF2), platelet-derived endothelial cell growth factor (PD-ECGF), transforming growth factor (TGF)-α/β and tumour necrosis factor (TNF)-α, in order to maintain vascular homeostasis in normal tissues. As tumours progress, tumour cells acquire an angiogenic phenotype characterized by the expression of a large number of pro-angiogenic molecules, which upsets the balance to endogenous anti-angiogenic molecules, and tumour angiogenesis is initiated.8,9 In addition, endothelial cells in nascent solid tumour capillary vessels express surface proteins that are absent or significantly underexpressed in normal vascular endothelium, further exacerbating the growth of these new vessels.10 Therefore, angiogenesis plays an important role in both tumour growth and metastasis.
Copper within adult differentiated tissue has been shown to have a unique role in both the neurological and endothelial cell systems.11 This is not entirely surprising because both neural and endothelial cells have been shown to share many similarities in the signal transduction pathways used for neural and endothelial cell morphogenesis.12 Within neural systems, copper has been shown to be involved in neural post-synaptic transmission and involved in the aetiology of several neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS), prion-related encephalopathies and Parkinson’s and Alzheimer’s disease.13 Angiogenesis has long been associated with a heightened sensitivity to copper. Some of the first clues of this association arose from examining levels of serum ceruloplasmin, the major binder of copper in serum, in rabbits during tumour xenograft development and regression. Ceruloplasmin levels were shown to be increased four- to eightfold of normal during the progression of the malignant process. With tumour regression, ceruloplasmin levels returned to normal; when metastasis developed, the ceruloplasmin levels remained high.14 This association was further strengthened when it was found that copper complexes, and even copper salts themselves, stimulated angiogenesis and endothelial cell migration.15 Later studies then determined that copper levels were increased in rabbit corneas as neovascularization was induced.16 Furthermore, in this model the addition of copper salts themselves was found to be sufficient to induce vascularization.17 When such rabbits were made deficient in copper, the rabbits the induction of neovascularization was consequently inhibited.16,17 These findings were extended to other systems, because it was determined that copper induced the migration of bovine aortic endothelial cells,18 and in vivo.19 In human systems, copper metabolism appears to be altered in tumors20 and serum copper levels themselves have been shown to be elevated in a number of tumour types.21 In breast cancer, increasing serum copper levels have been shown to scale to the severity of disease.22
Because these studies established an association between copper levels and angiogenesis, many researchers explored whether the converse is true; namely, that depletion of copper would inhibit vascular formation. Numerous agents are available for the physiological depletion of copper, several arising from the development of treatments for copper storage diseases, such as Wilson’s and Menke’s diseases. Among those agents that have proven most useful clinically are tetrathiomolybdate, penicillamine and trientine. The first study examining in vivo suppression of angiogenesis by copper chelation was performed using a rat glioblastoma xenograft system.23 Here, penicillamine was shown to be effective in limiting the growth of a significant percecntage of xenograft tumours; those that did grow were characterized by a decreased tumour volume and decreased microvessel density. Similar results were obtained in a second xenograft system.24 Animal studies have since been performed in a wide variety of xenograft and tumour model systems, including head and neck cancer,25 hepatocellular carcinoma,26 breast cancer27 and lung cancer,28 suggesting that the effects of copper chelation on the suppression of angiogenesis are not cell type specific.
Taken together, such findings have led many to believe that systemic removal of copper may be promising in the treatment of human tumours as an anti-angiogenic strategy. Indeed, a number of clinical trials for the treatment of solid tumours by copper chelation therapy have already been initiated. The first such studies to be reported used tetrathiomolybdate in patients with a variety of solid tumours (prostate, colon, lung and breast), with some finding of disease stabilization in a subset of patients able to achieve decreased serum ceruloplasmin levels.29 A second phase II trial against IL-2-unresponsive metastatic renal cancer showed some efficacy as well, finding disease stabilization in 31% of those treated.30 Tetrathiomolybdate is also being used in a trial against mesothelioma31 and new chelators are undergoing phase I trials against both solid and haematopoietic malignancies. The major findings of these studies to date have been disease stabilization rather than eradication, but such results are promising for the translation of copper chelation to the clinic, perhaps adjuvantly in combination with a second chemotherapeutic.
All these in vitro and in vivo experiments have established that angiogenesis displays, either directly or indirectly, a heightened sensitivity towards modulation by copper. But what is the precise molecular context of this copper? Copper is a critical cofactor or activator of a growing number of proteins; however, copper homeostasis must be quite carefully controlled.32 Excess or mismanaged copper can be threatening in a cellular context owing to the adventitious redox reactions catalysed by ‘free’ copper. Copper uptake is largely accomplished through the Ctr1 transporter33 and, once inside the cell, closely guarded by a number of metallochaperones and metal trafficking proteins such as Menkes protein, Wilson disease protein, the Copper Chaperone for Superoxide Dismutase and Antioxidant-1, which control the distribution and delivery of copper to nuclear, mitochondrial and vesicular targets, where, in some cases, further proteins yet escort it to enzymatic sites.34 In the context of copper-binding proteins that may impact angiogenesis, several findings are salient. The activity of VEGF, a primary pro-angiogenic cytokine, is induced by the treatment of cells with copper salts.35 Another cytokine involved in angiogenesis, namely FGF1, has been shown to bind copper in affinity purification assays36 and the cellular secretion of FGF1 is induced by the Cu-dependent formation of a complex consisting of FGF1, S100A13 and p40Syt1.37 Another copper-sensitive pro-angiogenic protein is angiogenin.38 Angiogenin binds copper39 and it has been suggested that copper-activated angiogenin may more effectively interact with endothelial cells, increasing its stimulatory capacity for new vessel formation. In addition to these specific angiogenic targets of copper with strong evidence of direct modulation, there are a number of other targets and pathways that are influenced by the variation of copper levels.40 However, no clear consensus as to the molecular basis, nor any direct causative connection, for the exquisite sensitivity of angiogenesis to copper has been reached. Can an approach that measures levels of copper in situ within a cell provide any new insight into the sensitivity of angiogenesis to modulation by copper?
PERSPECTIVE ON COPPER IN CARDIOVASCULAR BIOLOGY: INSIGHTS WITH X-RAY FLUORESCENCE MICROSCOPY
Our ability to quantify and determine the cellular localization of metals has lagged behind our ability to measure other cellular components. Optical microscopy and immunochemical techniques have long allowed for the visualization and localization of proteins and organelles within the cell, with spatial resolution enhanced by the development of electron microscopy and other high-resolution imaging techniques. In contrast, metal ions present a unique challenge to imaging and measurement, because cellular metals, such as copper, are themselves embedded within macromolecules, making their detection challenging, even with sensitive new fluorescent indicating dyes.41,42 Most of what we know about the cell biology of metals has arisen from amplification and purification of their natural ligands. Indeed, metallobiology began in the isolation, purification and spectroscopic analysis of metal–ligand species from biological sources.43 Colourimetric assays44–46 with relatively poor sensitivity formed the basis of metal quantification techniques prior to the advent of analytical atomic spectroscopy.47 The incorporation of both atomic emission spectroscopy (AES) and mass spectrometry (MS) with inductively coupled plasma (ICP) ionization (as in ICP-AES and ICP-MS) has enabled rapid detection of many metals in liquid samples at p.p.m.–p.p.b. concentrations. For example, it has allowed us to measure copper levels in samples of plaques from brains of Alzheimer’s patients,48 liver sections from those suffering from Wilson’s disease49 and in urine samples of cancer patients undergoing chelation therapy.50 Yet, what is lacking in these approaches is information on the spatial distribution of these metals. Elegant experiments using subcellular fractionation in combination with these techniques have worked around this obstacle by separately measuring copper in fractionated components of the cell.51,52 Alternatively, others have cleverly captured the spatial redistribution of copper export through careful experiments using radioactive copper isotopes;53 yet, such approaches rely on indirect evidence rather than direct visualization.
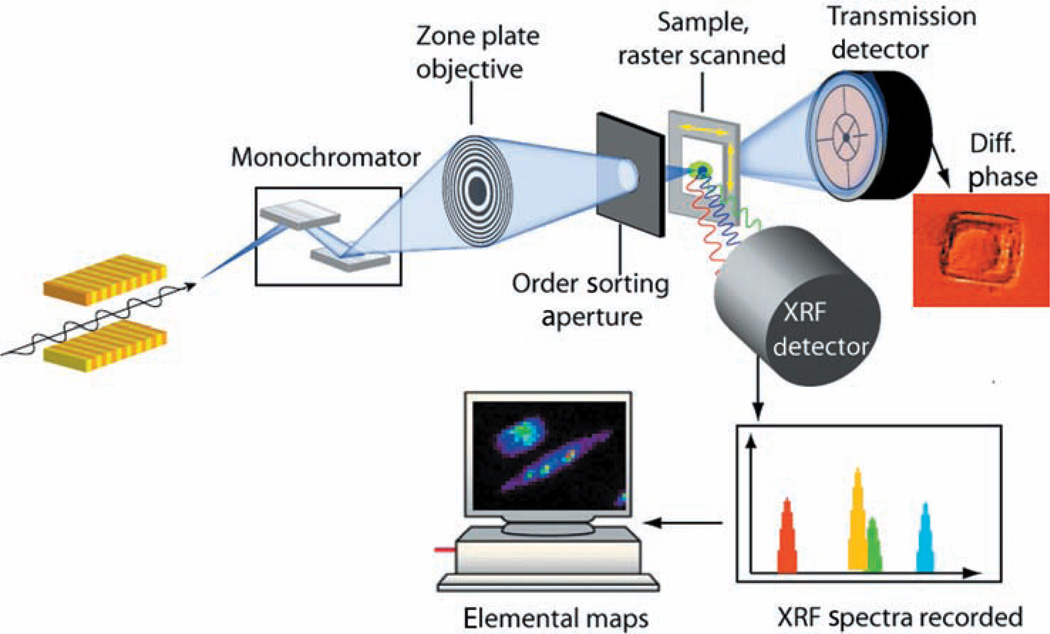
Many approaches have been developed for the visualization and quantitative measurement of cellular metals in situ. Fluorescent dyes (e.g. Fura-2 for Ca2+, Zinquin for Zn2+) that display enhanced emission when bound to metals have been used. Visible light microscopy allows for the visualization of ions, but the selectivity of the dyes and accurate quantification is difficult, detection of tightly bound ions is problematic and the achievable spatial resolution is ultimately limited by the wavelength of the illumination (typically to > 200 nm). A number of probe-based methodologies have proven useful for in situ detection as well. Electron-probe X-ray microanalysis,54 electron energy loss spectroscopy55,56 and proton induced X-ray emission57 can be used to quantify the elemental content of biological specimens. Cryo methods must commonly be used to preserve ionic integrity; specimen sectioning is typically needed and its sensitivity is limited due to the presence of bremsstrahlung background.58 NanoSIMS combines ion bombardment with mass spectrometry detection and, although initially limited in spatial resolution, upgrades in instrumentation have allowed for < 100 nm resolution, making such an approach promising for biological element mapping.59,60 Synchrotron-based approaches, the focus of this issue of Clinical and Experimental Pharmacology and Physiology, have shown great promise in transition-metal detection and quantification. Hard X-ray fluorescence microscopy (XFM) allows for determining and visualizing the distributions of essential cellular metals, such as copper, in situ at high sensitivity and high spatial resolution. Incident hard X-rays excite inner shell electrons and their relaxation emits X-ray fluorescence photons at a characteristic energy for each excited element. The amount of X-ray fluorescence emitted correlates directly with the amount of element present, making the technique quantitative in a linear fashion.61 Single cells are raster scanned through the microprobe (Fig. 1), generating image data pixel by pixel. Detection and acquisition of full X-ray fluorescence spectra at each scan position enables least squares fitting of modified Gaussians to elemental peaks or, alternatively, multivariate statistical analysis62 to determine elemental content with high precision, further reducing the influence of background, such as multiple inelastic scattering of the incident beam or incomplete charge collection of the X-ray fluorescence (XRF) detector. Comparison of XRF intensities to a standard calibration curve using specialized software63 converts total fluorescence to mass per unit area for each element. Fast fly scanning approaches, in which dwell times at each pixel are reduced to 10–50 msec, facilitate sample orientation and minimize setup time, using a combination of differential phase contrast with measurements of total fluorescence yield64 that allows overview scans several hundreds of microns in size in a matter of minutes. Although other methods exist for imaging at the submicron scale, XFM-based techniques have the unique advantage of combining great elemental sensitivity (due to the absence of a bremsstrahlung background) with the ability to image whole, unsectioned cells, as well as comparatively thick tissue sections, at very high spatial resolution. Only a third-generation synchrotron source for hard X-rays (such as exist in the US (Advanced Photon Source), France (European Synchrotron Radiation Facility) and Japan (Super Photon Ring 8GeV)) provides the high brilliance at the high photon energies required to image biologically relevant (trace) metals at high spatial resolution. This becomes even more critical in imaging copper, which is present, like many other biologically important elements, at very low intracellular concentrations.
Fig. 1.
A schematic depicting the layout of a typical hard X-ray microprobe at Sector 2 of the Advanced Photon Source at Argonne National Laboratory in Argonne (IL, USA). An undulator (depicted at left) generates hard X-rays, which are then mono-chromatized to the desired energy. X-Rays are focused using zone plate optics; in combination with a central stop on the zone plate, an order sorting aperture removes any X-rays not going into first-order focus. The sample is scanned through the focused beam in x and y directions, X-rays excite inner shell electrons and subsequently emitted X-ray fluorescence is captured with an energy dispersive detector. Full fluorescence spectra are stored at each scan position for later detailed analysis. Transmitted X-rays are also detected using a multi-element downstream detector, allowing for differential phase contrast imaging.
ROLE OF COPPER IN ANGIOGENESIS
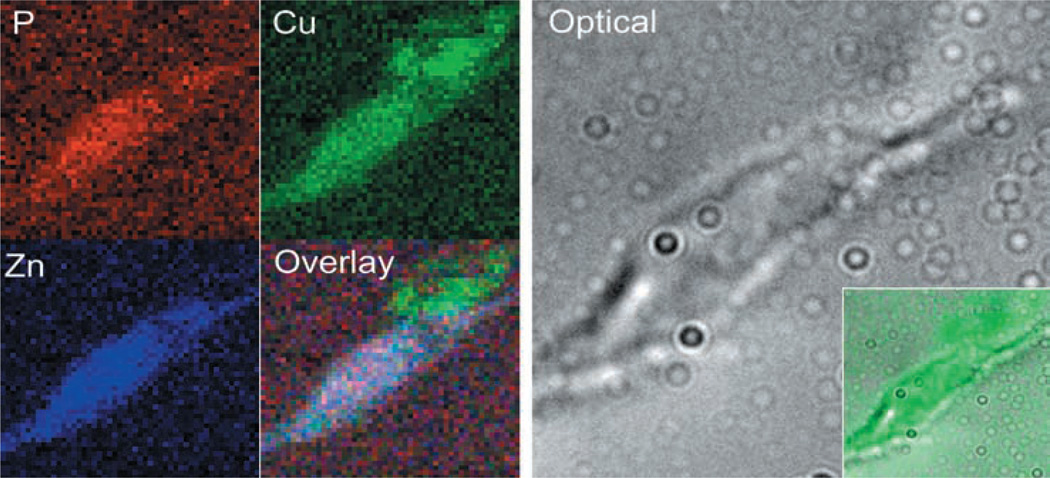
Imaging by X-ray fluorescence microscopy has given us a completely new view of cellular copper.65,66 For example, quantitative and spatial mapping has shown that copper and zinc are specifically colocalized with the β-amyloid plaques in Alzheimer’s disease.67 At the cellular level, pioneering studies on the cellular distribution of copper in fibroblast cells, correlating optical copper probe images with XFM scans, have identified a kinetically labile copper pool localized in the Golgi and the mitochondria.42 We have approached the issue of copper’s role in angiogenesis by using XFM approaches in the well-characterized model system of angiogenesis in which primary human microvascular endothelial cells (HMVEC) are induced to form vascular networks on basement membrane substrates.68 In HMVEC cells plated on gelatin substrates and uninduced, XFM scanning showed copper to be localized to a perinuclear area. As cells were induced to undergo angiogenesis, we found a remarkable redistribution of cellular copper. As cells begin to extend filopodia towards each other (0.5–2 h), copper redistributed from the perinuclear area to the tips of filopodia and across the cell membrane (2 h).68 When quantified across several cells at each time-point examined this relocalization was shown to be statistically significant, with some 80–90% of cellular copper being redistributed from perinuclear areas to growing filopodia away from the centre of the cell.68 By examining the leading edge of filopodia at the resolution limit of our current optics, it became clear that some of this copper was being transported across the cell membrane and into the extracellular space (Fig. 2).68 Such translocations were seen only in endothelial cells during tubulogenesis and not in neural process extensions. To extend these findings to an in vivo setting, we also examined highly vascularized ductal breast carcinoma samples. In these samples, copper was distributed roughly perinuclearly in mature capillary cross-sections, as in control cells.68 However, cells in areas of nascent lymphatic invasion displayed markedly altered distributions, with copper localized in a punctuate pattern at the leading edge of the cells.68
Fig. 2.
X-Ray fluorescence microscopy imaging depicting copper being translocated across the cell membrane. Human microvascular endothelial cells (HMVEC) were induced to undergo angiogenesis and X-ray fluorescence microprobe imaging of areas of the cells at the tips of nascent filopodia was performed. Elemental maps for phosphorous (P), zinc (Zn) and copper (Cu) were generated, overlaid and compared with an image obtained by differential interference contrast light microscopy. Overlay of copper maps with the optical image (inset, lower right) demonstrate copper to be concentrated at the tip of HMVEC filopodia, with transfer to the extracellular space evident.
Such observations have provided new insight into the sensitivity of angiogenesis to copper chelation. One mechanism that has been proposed for the action of these chelators has been that they are reducing cellular copper levels in endothelial cells by reducing circulating serum copper content and that this is affecting some copper-sensitive aspect of these cells.69,70 By using XFM imaging, we were able to show that cellular copper is actually being translocated across the cell membrane, leading us to believe that this exposure of copper to the extracellular milieu may impart the sensitivity of this process to chelation.68 In fact, in this same work we examined the effect of a variety of copper chelators on angiogenesis, some of which are known to be highly membrane impermeant, and found that all chelators acted similarly in inhibiting not process formation, but, instead, its directionality in the formation of a mature network.68 The timing of this cellular event is coincidental to the time at which copper is being seen by XFM to translocate across the cellular membrane.68 We believe these observations support a mechanism in which cellular copper activates an extracellular target, perhaps a known pro-angiogenic cytokine or, alternatively, an unknown target and copper chelation interferes with this interaction, inhibiting the pro-angiogenic stimulus required for endothelial cell communication. As X-ray imaging resolution continues to improve and complementary techniques at the interface of X-ray science and biochemistry develop, new activators of angiogenesis will emerge and the nature of this target of copper will be identified. The power of imaging techniques like XFM at third-generation synchrotrons has only just begun to shed light onto long-standing biological questions. There is no doubt they will continue to provide novel insights into the emerging study of cellular metals as we move into the future.
REFERENCES
- 1.Carmeliet P. Manipulating angiogenesis in medicine. J. Intern. Med. 2004;255:538–561. doi: 10.1111/j.1365-2796.2003.01297.x. [DOI] [PubMed] [Google Scholar]
- 2.Risau W, Flamme I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 4.Heil M, Eitenmüller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: Similarities and differences. J. Cell Mol. Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 6.Hussong J, Rodgers G, Shami P. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood. 2000;95:309–313. [PubMed] [Google Scholar]
- 7.Xu JL, Lai R, Kinoshita T, Nakashima N, Nagasaka T. Proliferation, apoptosis, and intratumoral vascularity in multiple myeloma. Correlation with the clinical stage and cytological grade. J. Clin. Pathol. 2002;55:530–534. doi: 10.1136/jcp.55.7.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan TP, Jaggar R, Bicknell R. Controlling the vasculature: Angiogenesis, anti-angiogenesis and vascular targeting of gene therapy. Trends Pharmacol. Sci. 1995;16:57–66. doi: 10.1016/s0165-6147(00)88979-8. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J. Angiogenesis. Annu. Rev. Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Alitalo K. Clinical application of angiogenic growth factors and their inhibitors. Nat. Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 11.Leary SC, Winge DR. The Janus face of copper: Its expanding roles in biology and the pathophysiology of disease. Meeting on Copper and Related Metals in Biology. EMBO Rep. 2007;8:224–227. doi: 10.1038/sj.embor.7400915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glesne DA, Zhang W, Mandava S, et al. Subtractive transcriptomics: Establishing polarity drives in vitro human endothelial morphogenesis. Cancer Res. 2006;66:4030–4040. doi: 10.1158/0008-5472.CAN-05-3294. [DOI] [PubMed] [Google Scholar]
- 13.Gaggelli E, Kozlowski H, Valensin D, Valensin G. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis) Chem. Rev. 2006;106:1995–2044. doi: 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]
- 14.Ungar-Waron H, Gluckman A, Spira E, Waron M, Trainin Z. Ceruloplasmin as a marker of neoplastic activity in rabbits bearing the VX-2 carcinoma. Cancer Res. 1978;38:1296–1299. [PubMed] [Google Scholar]
- 15.McAuslan BR, Reilly W. Endothelial cell phagokinesis in response to specific metal ions. Exp. Cell Res. 1980;130:147–157. doi: 10.1016/0014-4827(80)90051-8. [DOI] [PubMed] [Google Scholar]
- 16.Ziche M, Jones J, Gullino PM. Role of prostaglandin E1 and copper in angiogenesis. J. Natl Cancer Inst. 1982;69:475–482. [PubMed] [Google Scholar]
- 17.Raju KS, Alessandri G, Ziche M, Gullino PM. Ceruloplasmin, copper ions, and angiogenesis. J. Natl Cancer Inst. 1982;69:1183–1188. [PubMed] [Google Scholar]
- 18.McAuslan BR, Reilly WG, Hannan GN, Gole GA. Angiogenic factors and their assay: Activity of formyl methionyl leucyl phenylalanine, adenosine diphosphate, heparin, copper, and bovine endothelium stimulating factor. Microvasc. Res. 1983;26:323–338. doi: 10.1016/0026-2862(83)90080-8. [DOI] [PubMed] [Google Scholar]
- 19.Alessandri G, Raju K, Gullino PM. Angiogenesis in vivo and selective mobilization of capillary endothelium in vitro by heparin–copper complex. Microcirc. Endothelium Lymphatics. 1984;1:329–346. [PubMed] [Google Scholar]
- 20.Apelgot S, Coppey J, Fromentin A, Guille E, Poupon MF, Roussel A. Altered distribution of copper (64Cu) in tumor-bearing mice and rats. Anticancer Res. 1986;6:159–164. [PubMed] [Google Scholar]
- 21.Coates RJ, Weiss NS, Daling JR, Rettmer RL, Warnick GR. Cancer risk in relation to serum copper levels. Cancer Res. 1989;49:4353–4356. [PubMed] [Google Scholar]
- 22.Gupta SK, Shukla VK, Vaidya MP, Roy SK, Gupta S. Serum trace elements and Cu/Zn ratio in breast cancer patients. J. Surg. Oncol. 1991;46:178–181. doi: 10.1002/jso.2930460311. [DOI] [PubMed] [Google Scholar]
- 23.Brem S, Tsanaclis AM, Zagzag D. Anticopper treatment inhibits pseudopodial protrusion and the invasive spread of 9L gliosarcoma cells in the rat brain. Neurosurgery. 1990;26:391–396. doi: 10.1097/00006123-199003000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Brem SS, Zagzag D, Tsanaclis AM, Gately S, Elkouby MP, Brien SE. Inhibition of angiogenesis and tumor growth in the brain. Suppression of endothelial cell turnover by penicillamine and the depletion of copper, an angiogenic cofactor. Am. J. Pathol. 1990;137:1121–1142. [PMC free article] [PubMed] [Google Scholar]
- 25.Cox C, Teknos TN, Barrios M, Brewer GJ, Dick RD, Merajver SD. The role of copper suppression as an antiangiogenic strategy in head and neck squamous cell carcinoma. Laryngoscope. 2001;111:696–701. doi: 10.1097/00005537-200104000-00024. [DOI] [PubMed] [Google Scholar]
- 26.Yoshii J, Yoshiji H, Kuriyama S, et al. The copper-chelating agent, trientine, suppresses tumor development and angiogenesis in the murine hepatocellular carcinoma cells. Int. J. Cancer. 2001;94:768–773. doi: 10.1002/ijc.1537. [DOI] [PubMed] [Google Scholar]
- 27.Pan Q, Kleer CG, van Golen KL, et al. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 2002;62:4854–4859. [PubMed] [Google Scholar]
- 28.Khan MK, Miller MW, Taylor J, et al. Radiotherapy and antiangiogenic TM in lung cancer. Neoplasia. 2002;4:164–170. doi: 10.1038/sj.neo.7900218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brewer GJ, Dick RD, Grover DK, et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin. Cancer Res. 2000;6:1–10. [PubMed] [Google Scholar]
- 30.Redman BG, Esper P, Pan Q, et al. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin. Cancer Res. 2003;9:1666–1672. [PubMed] [Google Scholar]
- 31.Brewer GJ. Copper lowering therapy with tetrathiomolybdate as an antiangiogenic strategy in cancer. Curr. Cancer Drug Targets. 2005;5:195–202. doi: 10.2174/1568009053765807. [DOI] [PubMed] [Google Scholar]
- 32.Balamurugan K, Schaffner W. Copper homeostasis in eukaryotes: Teetering on a tightrope. Biochim. Biophys. Acta. 2006;1763:737–746. doi: 10.1016/j.bbamcr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Maryon EB, Molloy SA, Zimnicka AM, Kaplan JH. Copper entry into human cells: Progress and unanswered questions. Biometals. 2007;20:355–364. doi: 10.1007/s10534-006-9066-3. [DOI] [PubMed] [Google Scholar]
- 34.O’Halloran TV, Culotta VC. Metallochaperones, an intracellular shuttle service for metal ions. J. Biol. Chem. 2000;275:25 057–25 060. doi: 10.1074/jbc.R000006200. [DOI] [PubMed] [Google Scholar]
- 35.Sen CK, Khanna S, Venojarvi M, et al. Copper-induced vascular endothelial growth factor expression and wound healing. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1821–H1827. doi: 10.1152/ajpheart.01015.2001. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe T, Seno M, Sasada R, Igarashi K. Molecular characterization of recombinant human acidic fibroblast growth factor produced in E. coli: Comparative studies with human basic fibroblast growth factor. Mol. Endocrinol. 1990;4:869–879. doi: 10.1210/mend-4-6-869. [DOI] [PubMed] [Google Scholar]
- 37.Landriscina M, Bagalá C, Mandinova A, et al. Copper induces the assembly of a multiprotein aggregate implicated in the release of fibroblast growth factor 1 in response to stress. J. Biol. Chem. 2001;276:25 549–25 557. doi: 10.1074/jbc.M102925200. [DOI] [PubMed] [Google Scholar]
- 38.Fett JW, Strydom DJ, Lobb RR, et al. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985;24:5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- 39.Soncin F, Guitton JD, Cartwright T, Badet J. Interaction of human angiogenin with copper modulates angiogenin binding to endothelial cells. Biochem. Biophys. Res. Commun. 1997;236:604–610. doi: 10.1006/bbrc.1997.7018. [DOI] [PubMed] [Google Scholar]
- 40.Lowndes SA, Harris AL. The role of copper in tumor angiogenesis. J. Mammary Gland Biol. Neoplaasia. 2005;10:299–310. doi: 10.1007/s10911-006-9003-7. [DOI] [PubMed] [Google Scholar]
- 41.Zeng L, Miller EW, Pralle A, Isacoff EY, Chang CJ. A selective turn-on fluorescent sensor for imaging copper in living cells. J. Am. Chem. Soc. 2006;128:10–11. doi: 10.1021/ja055064u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L, McRae R, Henary MM, et al. Imaging of the intracellular topography of copper with a fluorescent sensor and by synchrotron X-ray fluorescence microscopy. Proc. Natl Acad. Sci. USA. 2005;102:11 179–11 184. doi: 10.1073/pnas.0406547102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertini I, Gray HB, Stiefel EI, Valentine JS, editors. Biological Inorganic Chemistry: Structure and Reactivity. Sausalito, CA: University Science Books; 2007. [Google Scholar]
- 44.Brenner AJ, Harris ED. A quantitative test for copper using bicinchoninic acid. Anal. Biochem. 1995;226:80–84. doi: 10.1006/abio.1995.1194. [DOI] [PubMed] [Google Scholar]
- 45.Mustafin IS, Sivanova OV. Indicators with inner light filters. Mercurimetric indicator. Hydrone III. Zh. Anal. Khim. 1964;19:163–167. [Google Scholar]
- 46.Tikhonov VN, Mustatin IS. Photometric determination of copper in magnesium metal and magnesium alloys with bicinchoninic acid. Zh. Anal. Khim. 1965;20:390–392. [Google Scholar]
- 47.Szpunar J. Bio-inorganic speciation analysis by hyphenated techniques. Analyst. 2000;125:963–988. doi: 10.1039/a909137h. [DOI] [PubMed] [Google Scholar]
- 48.Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 49.Ferenci P, Steindl-Munda P, Vogel W, et al. Diagnostic value of quantitative hepatic copper determination in patients with Wilson’s disease. Clin. Gastroenterol. Hepatol. 2005;3:811–818. doi: 10.1016/s1542-3565(05)00181-3. [DOI] [PubMed] [Google Scholar]
- 50.Brem S, Grossman SA, Carson KA, et al. Phase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastoma. Neuro-oncol. 2005;7:246–253. doi: 10.1215/S1152851704000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huster D, Finegold MJ, Morgan CT, et al. Consequences of copper accumulation in the livers of the Atp7b−/− (Wilson disease gene) knockout mice. Am. J. Pathol. 2006;168:423–434. doi: 10.2353/ajpath.2006.050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cobine PA, Ojeda LD, Rigby KM, Winge DR. Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J. Biol. Chem. 2004;279:14 447–14 455. doi: 10.1074/jbc.M312693200. [DOI] [PubMed] [Google Scholar]
- 53.Schlief ML, Craig AM, Gitlin JD. NMDA receptor activation mediates copper homeostasis in hippocampal neurons. J. Neurosci. 2005;25:239–246. doi: 10.1523/JNEUROSCI.3699-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roomans GM, Dragomir A. X-Ray microanalysis in the scanning electron microscope. Methods Mol. Biol. 2007;369:507–528. doi: 10.1007/978-1-59745-294-6_25. [DOI] [PubMed] [Google Scholar]
- 55.Jeanguillaume C, Tencé M, Zhang L, Ballongue P. Practical aspects of electron energy loss spectroscopy (EELS) in biology. Cell Mol. Biol. 1996;42:439–450. [PubMed] [Google Scholar]
- 56.Leapman RD. Detecting single atoms of calcium and iron in biological structures by electron energy-loss spectrum-imaging. J. Microsc. 2003;210:5–15. doi: 10.1046/j.1365-2818.2003.01173.x. [DOI] [PubMed] [Google Scholar]
- 57.Tylko G, Mesjasz-Przybyowicz J, Przybyowicz WJ. X-Ray microanalysis of biological material in the frozen–hydrated state by PIXE. Microsc. Res. Techn. 2007;70:55–68. doi: 10.1002/jemt.20387. [DOI] [PubMed] [Google Scholar]
- 58.Ingram P, Shelburne J, Roggli V, LeFurgey A. Biomedical Applications of Microprobe Analysis. San Diego, CA: Academic Press; 1999. [Google Scholar]
- 59.Clode PL, Stern RA, Marshall AT. Subcellular imaging of isotopically labeled carbon compounds in a biological sample by ion microprobe (NanoSIMS) Microsc. Res. Techn. 2007;70:220–229. doi: 10.1002/jemt.20409. [DOI] [PubMed] [Google Scholar]
- 60.Guerquin-Kern JL, Wu TD, Quintana C, Croisy A. Progress in analytical imaging of the cell by dynamic secondary ion mass spectrometry (SIMS microscopy) Biochim. Biophys. Acta. 2005;1724:228–238. doi: 10.1016/j.bbagen.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 61.Twining BS, Baines SB, Fisher NS, et al. Quantifying trace elements in individual aquatic protist cells with a synchrotron X-ray fluorescence microprobe. Anal. Chem. 2003;75:3806–3816. doi: 10.1021/ac034227z. [DOI] [PubMed] [Google Scholar]
- 62.Vogt S, Maser J, Jacobsen C. Data analysis for X-ray fluorescence imaging. J. Phys. 2003;104:617–622. [Google Scholar]
- 63.Vogt S. MAPS: A set of software tools for analysis and visualization of 3D x-ray fluorescence data sets. J. Phys. 2003;104:635–638. [Google Scholar]
- 64.Vogt S, Feser A, Legnini D, Kirz J, Maser J. Fast differential phase-constrast imaging and total fluorescence yield mapping in a hard X-ray fluorescence microprobe. Synchrotron Radiat. Instrum. 2004;705:1348–1351. [Google Scholar]
- 65.Paunesku T, Vogt S, Maser J, Lai B, Woloschak G. X-Ray fluorescence microprobe imaging in biology and medicine. J. Cell Biochem. 2006;99:1489–1502. doi: 10.1002/jcb.21047. [DOI] [PubMed] [Google Scholar]
- 66.Fahrni CJ. Biological applications of X-ray fluorescence microscopy: Exploring the subcellular topography and speciation of transition metals. Curr. Opin. Chem. Biol. 2007;11:121–127. doi: 10.1016/j.cbpa.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 67.Miller LM, Wang Q, Telivala TP, Smith RJ, Lanzirotti A, Miklossy J. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with beta-amyloid deposits in Alzheimer’s disease. J. Struct. Biol. 2006;155:30–37. doi: 10.1016/j.jsb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 68.Finney L, Mandava S, Ursos L, et al. X-Ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis. Proc. Natl Acad. Sci. USA. 2007;104:2247–2252. doi: 10.1073/pnas.0607238104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brem S. Angiogenesis and cancer control: From concept to therapeutic trial. Cancer Control. 1999;6:436–458. [PubMed] [Google Scholar]
- 70.Lowndes SA, Harris AL. The role of copper in tumour angiogenesis. J. Mammary Gland Biol. Neoplasia. 2005;10:299–310. doi: 10.1007/s10911-006-9003-7. [DOI] [PubMed] [Google Scholar]