Abstract
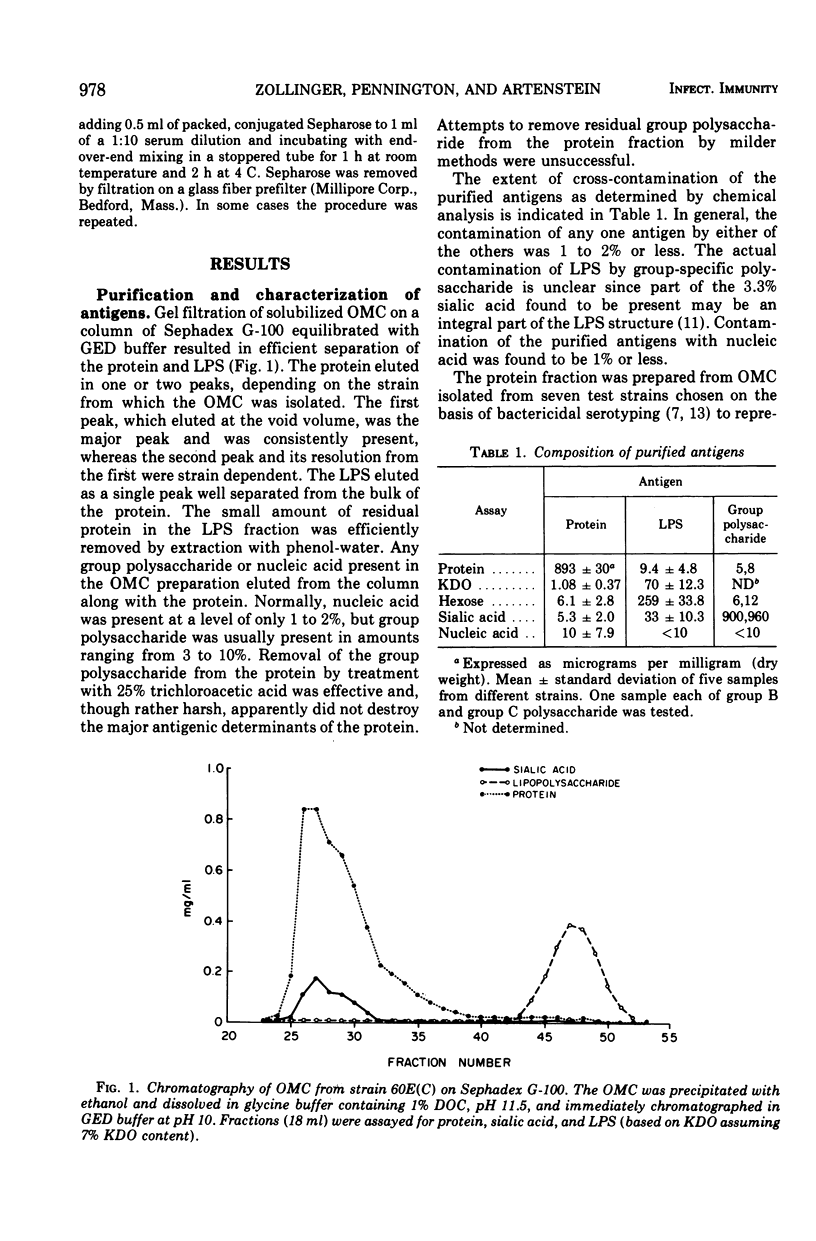
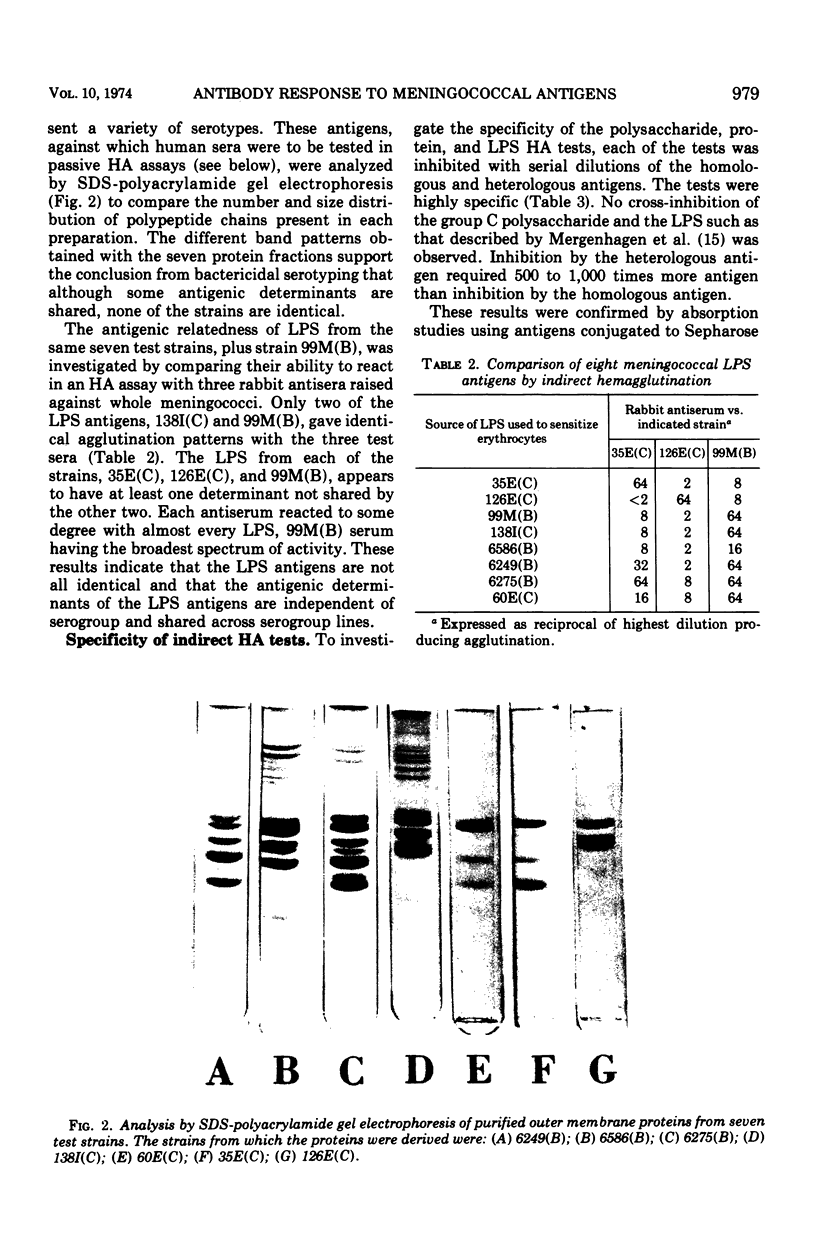
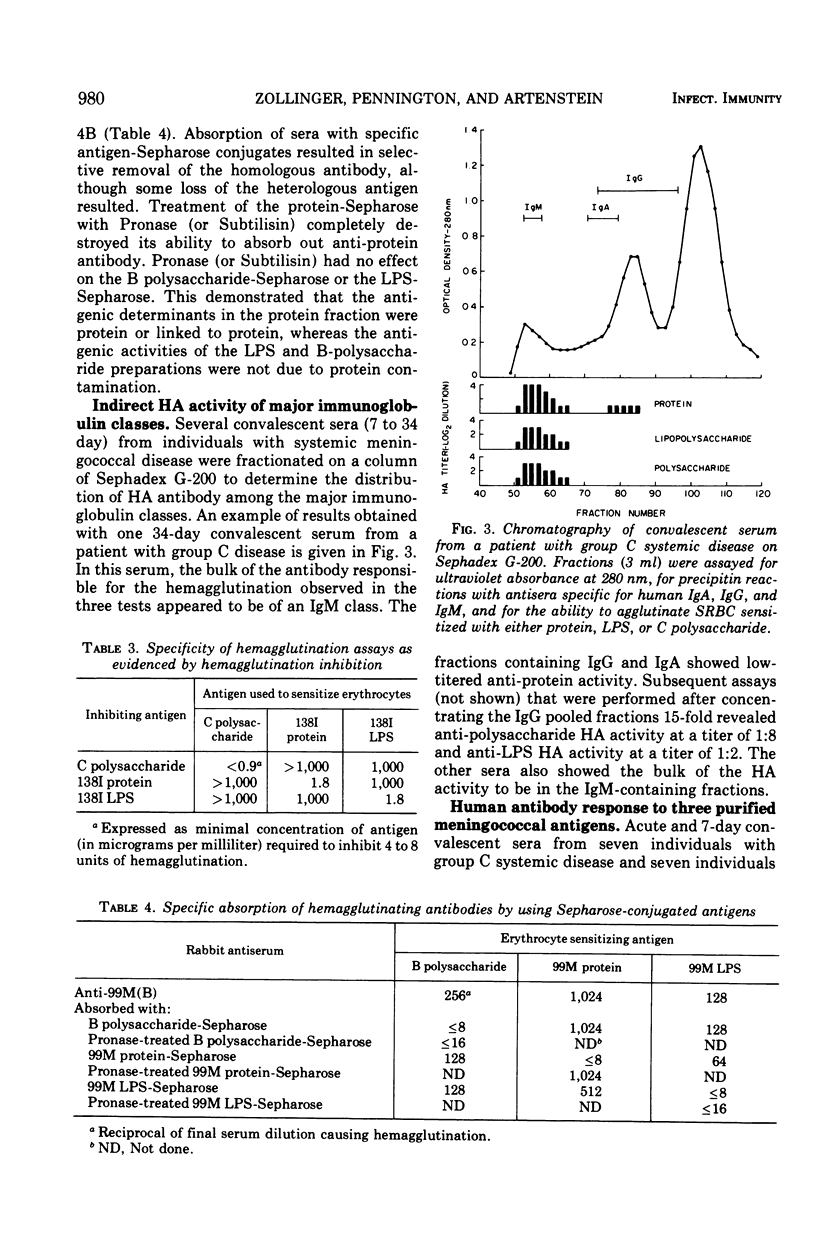
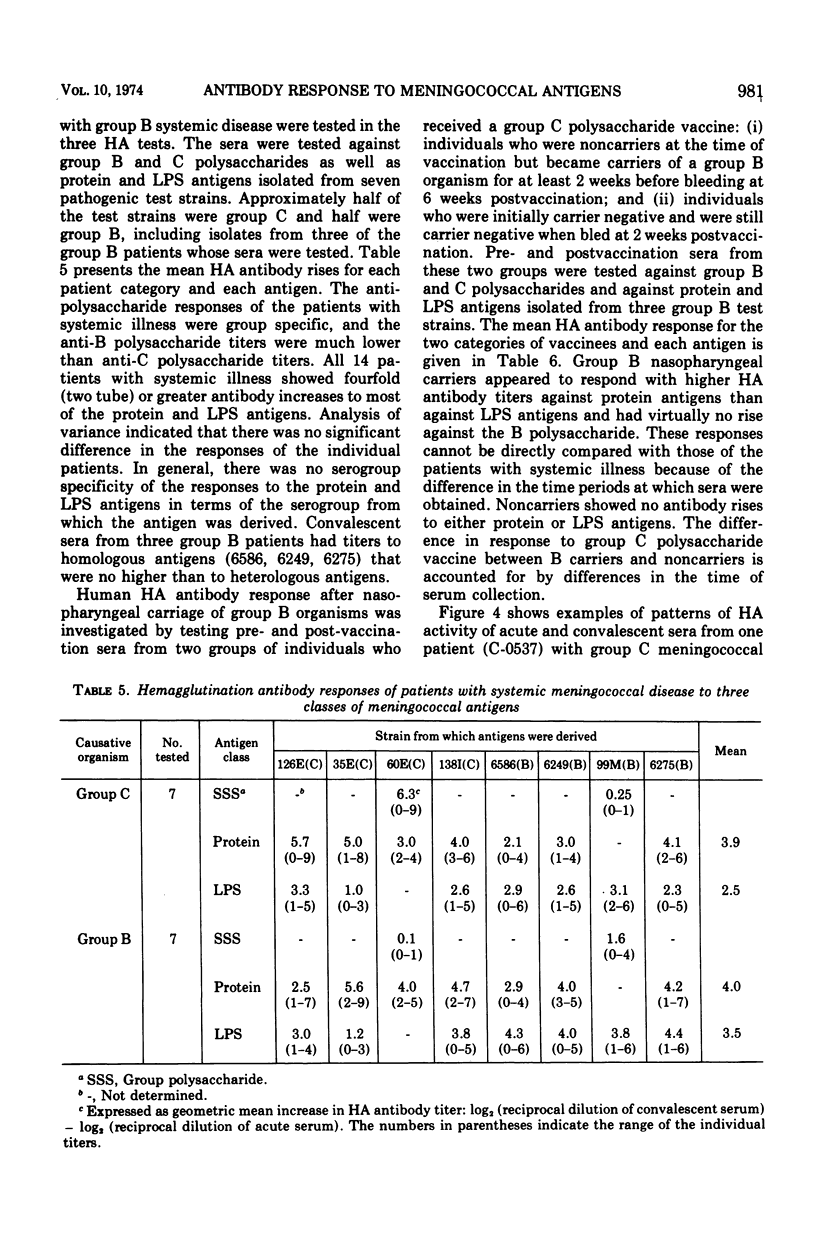
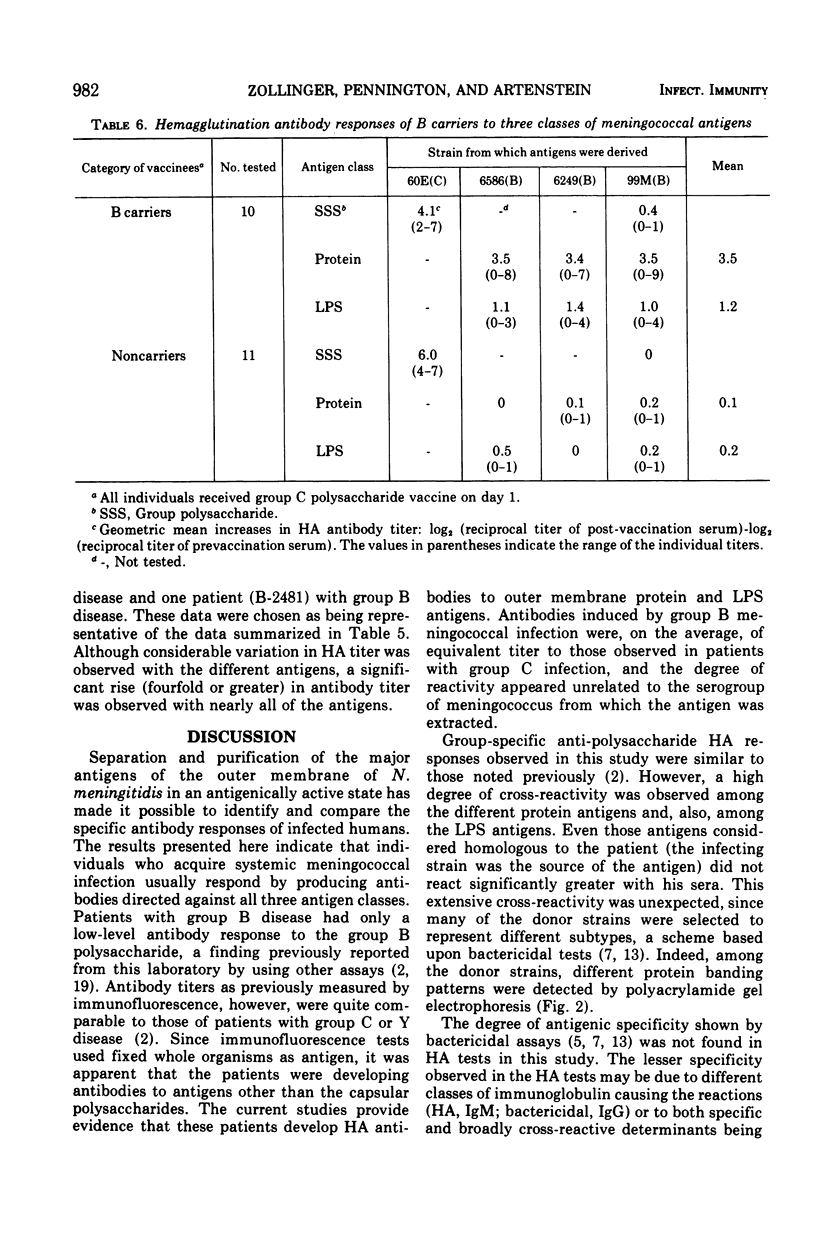
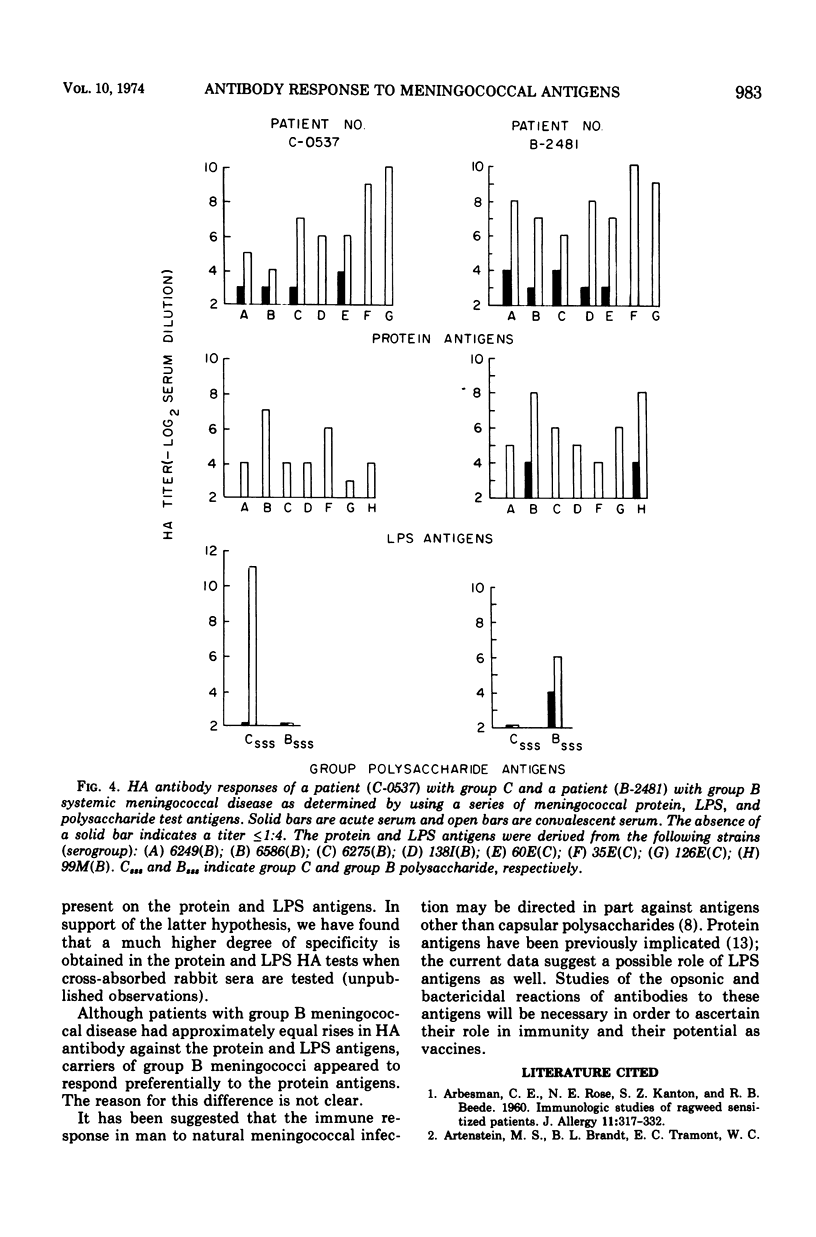
Three cell surface antigens, protein, lipopolysaccharide, and polysaccharide, were purified from group B and group C strains of Neisseria meningitidis representing a variety of serotypes. Chemical analysis indicated that cross-contamination was on the order of 1%. Sensitization of sheep erythrocytes with these antigens resulted in highly specific passive hemagglutination assays for the three kinds of antigens. Paired human sera from several groups of individuals were tested by hemagglutination for antibody against each of the antigens. Patients with group B or C systemic meningococcal disease showed increases in antibody titer against all three kinds of antigens, but the antibody response to B polysaccharide was low compared with the response to C polysaccharide. Nasopharyngeal carriers of group B meningococci showed significant increases in titer only against the protein antigens, and noncarriers who received a C-polysaccharide vaccine had a specific response to the C polysaccharide. A given protein or lipopolysaccharide antigen reacted on the average equally well with either group B or C convalescent sera. These results suggest that all three antigens may play a role in the broad human immunity following natural infection.
Full text
PDF



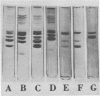
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBESMAN C. E., ROSE N. R., KANTOR S. Z., BEEDE R. B. Immunologic studies of ragweed-sensitive patients. I. Specificity and sensitivity of hemagglutination reactions. J Allergy. 1960 Jul-Aug;31:317–322. doi: 10.1016/0021-8707(60)90068-x. [DOI] [PubMed] [Google Scholar]
- Artenstein M. S., Brandt B. L., Tramont E. C., Branche W. C., Jr, Fleet H. D., Cohen R. L. Serologic studies of meningococcal infection and polysaccharide vaccination. J Infect Dis. 1971 Sep;124(3):277–288. doi: 10.1093/infdis/124.3.277. [DOI] [PubMed] [Google Scholar]
- Brandt B. L., Wyle F. A., Artenstein M. S. A radioactive antigen-binding assay for Neisseria meningitidis polysaccharide antibody. J Immunol. 1972 Apr;108(4):913–920. [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Frasch C. E., Chapman S. S. Classification of Neisseria meningitidis group B into distinct serotypes. I. Serological typing by a microbactericidal method. Infect Immun. 1972 Jan;5(1):98–102. doi: 10.1128/iai.5.1.98-102.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Gold R., Wyle F. A. New Classification of Neisseria meningitidis by Means of Bactericidal Reactions. Infect Immun. 1970 May;1(5):479–484. doi: 10.1128/iai.1.5.479-484.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969 Jun 1;129(6):1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969 Jun 1;129(6):1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y., Artenstein M. S. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J Exp Med. 1969 Jun 1;129(6):1349–1365. doi: 10.1084/jem.129.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenthal E. Distribution of keto-deoxyoctonic acid and sialic acid in N. meningitidis. J Immunol. 1969 Apr;102(4):1099–1102. [PubMed] [Google Scholar]
- Himmelspach K., Wrede J. Use of 2-[(4-aminophenyl)-sulfonyl]-ethyl hydrogen sulfate for the preparation of a dextran-specific immunogen. FEBS Lett. 1971 Oct 15;18(1):118–120. doi: 10.1016/0014-5793(71)80423-4. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Winkelhake J. L., Brandt B. L., Artenstein M. S. Antigenic specificity of bactericidal antibodies in antisera to Neisseria meningitidis. J Infect Dis. 1973 Apr;127(4):378–387. doi: 10.1093/infdis/127.4.378. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MERGENHAGEN S. E., MARTIN G. R., SCHIFFMANN E. Studies on an endotoxin of a group C Neisseria meningitidis. J Immunol. 1963 Feb;90:312–317. [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROE J. H. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1955 Jan;212(1):335–343. [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Tramont E. C., Artenstein M. S. Latex agglutination test for measurement of antibodies to meningococcal polysaccharides. Infect Immun. 1972 Mar;5(3):346–351. doi: 10.1128/iai.5.3.346-351.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- Zollinger W. D., Kasper D. L., Veltri B. J., Artenstein M. S. Isolation and characterization of a native cell wall complex from Neisseria meningitidis. Infect Immun. 1972 Nov;6(5):835–851. doi: 10.1128/iai.6.5.835-851.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]