Abstract
Extracellular superoxide dismutase (ecSOD) is the major extracellular scavenger of superoxide ( ) and a main regulator of nitric oxide (NO) bioactivity in the blood vessel wall, heart, lungs, kidney, and placenta. Involvement of has been implicated in many pathological processes, and removal of extracellular by ecSOD gene transfer has emerged as a promising experimental technique to treat vascular disorders associated with increased oxidant stress. In addition, recent studies have clarified mechanisms that regulate ecSOD expression, tissue binding, and activity, and they have provided new insight into how ecSOD interacts with other factors that regulate vascular function. Finally, studies of a common gene variant in humans associated with disruption of ecSOD tissue binding suggest that displacement of the enzyme from the blood vessel wall may contribute to vascular diseases. The purpose of this review is to summarize recent research findings related to ecSOD function and gene transfer and to stimulate other investigations into the role of this unique antioxidant enzyme in vascular pathophysiology and therapeutics.
Oxidative stress induced by superoxide anion ( ) produced in vascular cells is involved in the pathogenesis of cardiovascular and metabolic diseases, including atherosclerosis,1 ischemia–reperfusion injury,2 diabetes,3 hyperlipidemia,4 and hypertension.5 Moreover, may also contribute to pulmonary hypertension,5 erectile dysfunction,6 cerebral vasospasm,7 and other disorders associated with vascular dysfunction. Consequently, strategies to reduce levels of have emerged as promising approaches to treating cardiovascular diseases and other conditions associated with enhanced oxidative stress.
Scavenging of is performed by a group of anti-oxidant enzymes called superoxide dismutases (SODs), which catalyze the dismutation of to H2O2 and O2 efficiently and specifically.
In mammalian tissues, 3 isoforms of SODs exist: Cu/Zn SOD (SOD1), Mn SOD (SOD2), and extracellular SOD (ecSOD or SOD3). SOD1 is an abundant copper- and zinc-containing cellular protein that is present in the cytosol, nucleus, peroxisomes, and mitochondrial inner membrane. Its primary function is to lower the intracellular steady-state concentration of . SOD1 mutations are associated with neural diseases such as amyotrophic lateral sclerosis.8 SOD2 is a mitochondrial enzyme that disposes of generated by respiratory chain activity. SOD2 can be induced to protect against prooxidant insults. Conversely, SOD2 activity is decreased in physiologic aging and in diseases such as progeria, cancer, asthma, and transplant rejection.9 ecSOD, another copper- and zinc-containing dismutase, is a primary antioxidant enzyme secreted to the extracellular space. ecSOD is expressed highly in selected tissues, including blood vessels, heart, lungs, kidney, placenta, and extracellular fluids. ecSOD plays an important role in regulating blood pressure and vascular contraction, at least in part, through modulating the endothelial function by controlling the levels of extracellular and nitric oxide bioactivity in the vasculature.10,11 ecSOD has also been proposed to play an important role in neurologic, pulmonary, and arthritic diseases.12,13
The relative expression of SOD isoforms in cells and tissues has been investigated extensively and provides clues as to the sources of in pathophysiologic states. Based on our observation, in most tissues, SOD1 is the isoform that is expressed at the highest level. However, many examples exist in which this general pattern of expression differs among tissues and species. For example, ecSOD is expressed highly in vascular tissues, particularly in the arterial wall, and its activity constitutes almost half of the total SOD activity in the human aorta.14,15 Collectively, these observations suggest that in the extracellular space [released from inflammatory and vascular cells, most likely through nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity] contributes significantly to oxidant stress in the vascular wall.
, ecSOD, AND EXPERIMENTAL GENE TRANSFER
Decreasing -associated oxidative stress by enhancing ecSOD has been applied successfully in various experimental disease models16–34 (Table I). For example, ecSOD has been shown to restore erectile function in streptozotocin-induced diabetes,33 protect against vascular dysfunction with aging,19 blunt ischemia/reperfusion-induced liver injury,32 reduce systemic vascular resistance and arterial pressure in spontaneously hypertensive rats,21 improve endothelial dysfunction in hypertension and in heart failure models,23,35 and ameliorate inflammatory arthritis.28,29 In most of the aforementioned studies, human ecSOD gene transfer was employed to augment ecSOD activity in animal models. Regarding the behavior and fate of the delivered foreign gene in recipient hosts, most studies showed an increase of ecSOD activity, with a corresponding decrease in levels, after gene transfer (Table I). Conversely, in other cases, ecSOD gene therapy failed to protect against cardiovascular diseases (Table I). For example, Laukkanen et al18 reported that short-term overexpression of ecSOD in vivo did not affect atherogenesis in LDL receptor−/− mice. Yamaguchi et al24 showed that human ecSOD gene transfer failed to prevent cerebral vasospasm in a canine model of subarachnoid hemorrhage. Zimmerman et al36 reported that adenoviral-mediated delivery of human ecSOD to the subfornical organ failed to prevent the development of angiotensin II-induced hypertension in mice. The subfornical organ is a region of the brain lying outside the blood– brain barrier and is known to be a primary sensor for blood-borne angiotensin II. The mechanisms associated with the apparent failure of ecSOD gene transfer are still unknown. Note that most successful studies with ecSOD overexpression have been performed in rats, possibly because the level of expression of endogenous vascular ecSOD is lower in rats as compared with many other species of animals.37 Therefore, the relative degree of enhancement of ecSOD activity after gene transfer is typically higher in rats as compared with other species such as dog,24 which makes it easier to demonstrate a therapeutic effect.
Table I.
Studies of experimental gene transfer with ecSOD
| Target tissue + condition | Species | Model | Vector | End points | Reference |
|---|---|---|---|---|---|
| Aorta + heart failure | rat | in vivo IV | Ad | Restored NO levels and responses to acetylcholine; decreased levels of and peroxynitrite | 16 |
| + hypercholesterolemia | mouse | in vivo IV | Ad | Did not affect atherogenesis | 18 |
| + aging | rat | in vivo IV | Ad | Decreased vascular | 19 |
| + spontaneous hypertension | rat | in vivo IV | Ad | Decreased levels of and nitrotyrosine | 20 |
| + LPS treatment | rat | in vivo IV | Ad | Decreased and LPS-induced adhesion of leukocytes to aortic endothelium | 22 |
| + balloon denudation | rabbit | in vivo local delivery | Ad | Decreased , neointimal formation, and macrophage infiltration; IV gene transfer did not decrease restenosis | 17 |
| Carotid artery + spontaneous hypertension | rat | in vivo IV | Ad | Increased basal NO levels and responses to acetylcholine | 20 |
| + spontaneous hypertension | rat | in vivo IV | Ad | Increased sodium balance and relaxation to acetylcholine; decreased mean arterial pressure | 21 |
| + stroke-prone, spontaneous hypertension | rat | in vivo IV | Ad | Increased NO availability; improved endothelial function | 23 |
| Basilar artery + subarachnoid hemorrhage | dog | in vivo cisterna magna | Ad | Failed to prevent delayed cerebral vasospasm | 24 |
| Brain | rabbit | in vivo cisterna magna | Ad | Increased SOD activity in cerebrospinal fluid | 25 |
| Basilar artery + subarachnoid hemorrhage | rabbit | in vivo cisterna magna | Ad | Decreased cerebral vasospasm | 26 |
| Portal vein + acute liver injury | mouse | in vivo IV | ecSOD lipoplexes | Increased serum SOD activity and liver glutathione levels; decreased lipid peroxidation | 27 |
| Knee joint + antigen-induced arthritis | rat | ex vivo | ecSOD-expressing synoviocytes | Decreased joint swelling and gelatinase activity; decreased infiltration of inflammatory cells into the synovial membrane | 28 |
| + collagen-induced arthritis | mouse | ex vivo | ecSOD-expressing fibroblasts | Decreased mononuclear cell infiltration and synovial proliferation; improved histologic abnormalities, including destruction of cartilage and bone | 29 |
| Heart + myocardial infarction | rabbit | in vivo IV | Ad | Markedly decreased infarct size | 30 |
| + myocardial stunning | rabbit | in vivo IV | Ad | Increased ecSOD levels in the liver; improved left ventricular wall motion | 31 |
| Liver + paracetamol-induced damage | mouse | in vivo IV | Ad | Significantly attenuated release of liver enzymes and inhibited necrosis and apoptosis | 18 |
| + ischemia/reperfusion- induced liver injury | mouse | in vivo IV | polylipid nanoparticles | Increased SOD activity and decreased levels and oxidative stress in liver; decreased enzyme release and improved liver histology | 32 |
| Cavernosum + diabetes | rat | in vivo IV | Ad | Decreased levels | 33 |
| Bowel + colitis | mouse | ex vivo | ecSOD-expressing fibroblasts | Increased chance of survival | 34 |
Abbreviations: Ad, adenovirus; IV, intravenous; LPS, lipopolysaccharide.
Please note that in this review, we will not discuss methods or approaches commonly employed to optimize delivery of SOD genes. Such information is provided elegantly in a recent review by Heistad.38 Also, basic information about the biochemical properties and biology of ecSOD will not be included here but is available from several comprehensive reviews.39,40 Instead, we will focus on factors known to regulate expression, activity, and function of ecSOD, with special emphasis placed on those relevant to the cardiovascular system.
It is also necessary to point out that in addition to serving as an injurious radical specie,41 can, under some circumstances, serve as a signal transduction molecule that is important to cellular homeostasis.42,43 Thus, therapies that reduce levels below a certain threshold may actually be harmful. In addition, the balance between extracellular and intracellular levels may be another important factor. Considering that it is not possible to measure levels in vivo with sufficient special and temporal resolution, it may be difficult to deliver precisely the appropriate amount of ecSOD to the proper location at the correct time. Moreover, we do not have complete information about how this dismutase is regulated in vivo and in vitro. Such challenges underlie the field of free radical biology in general and complicate interpretation of basic and clinical research data regarding the role of oxidative stress in vascular diseases.
FACTORS INVOLVED IN THE REGULATION OF ecSOD EXPRESSION AND ACTIVITY
Copper and copper transport pathways
Copper, which is a redox active metal, is an essential element that is required for normal cellular function. During the past decade, our understanding of the factors that regulate cellular copper distribution and transport has improved greatly. First, intracellular copper availability is extraordinarily restricted, as the intracellular milieu has a great capacity to chelate copper.44 Second, in the cytoplasm, copper is distributed among several proteins, known as copper chaperones, which include antioxidant protein 1 (Atox1) synthesis of cytochrome c oxidase 2, and copper chaperone for superoxide dismutase 1. Copper chaperones compete with chelators for copper and directly insert the cofactor into the target apo-cuproenzymes, such as cytochrome C oxidase, SOD1, and Menkes ATPase, thus converting the latter from an inactive to an active state (holo-cuproenzymes).45 Third, the metallochaper-one Atox1 directly interacts with the Menkes ATPase and plays a critical role in perinatal copper homeostasis.46 Menkes ATPase serves as a copper efflux pump that regulates the amount of copper leaving the cell and supplies copper to secreted cuproenzymes, including ecSOD.47 These findings provide a basis for understanding how ecSOD biosynthesis could be modulated by copper transport pathways.
The central region of human ecSOD contains the essential amino acid residues involved in the coordination of Cu(II) and Zn(II) ions, which is termed the copper, zinc binding domain. Fukai and colleagues48,49 recently determined that copper delivery to ecSOD modulates its enzymatic activity. The investigators found that ecSOD-specific activity in the conditioned medium from cultured fibroblasts isolated from Atox1−/− mice or Menkes ATPase mutant mice was decreased markedly and was restored partially by the addition of copper to the conditioned medium.48,49 Co-immunoprecipitation and in vitro pull-down assays demonstrated a direct interaction between ecSOD and Menkes ATPase, and confocal immunoflurescence microscopy showed co-localization of ecSOD with Menkes ATPase in the trans-Golgi network.49 These observations suggest that Menkes ATPase transports copper to ecSOD in the trans-Golgi network through a direct physical interaction.49 In keeping with these in vitro observations, the aortas of Menkes ATPase mutant mice revealed a decrease in activity of ecSOD in association with a robust increase in levels in vivo.49
Zinc
The essentiality of zinc for human health is well established, and the consequences of severe zinc deficiency have been documented in population studies worldwide.50,51 Although zinc is not redox active, zinc supplementation has been shown to reduce oxidative damage.52–54 Zinc has also been observed to have an antiatherosclerotic effect.55–58 Epidemiologic studies revealed that zinc was associated inversely with cardiovascular disease.59–61 Olin et al62 observed that juvenile rats fed zinc-deficient diets exhibited low plasma zinc levels and low ecSOD activity. The enzymatic activity of ecSOD, however, was not restored by in vitro addition of zinc to the plasma samples. Furthermore, adolescent rhesus macaques fed diets that contained a marginal amount of zinc also had low plasma zinc levels and low ecSOD activity compared with control animals fed diets containing sufficient zinc.62 In another study in rats fed a zinc-deficient diet, a trend toward decreased ecSOD activity was also observed.63,64
Although positive correlations between zinc levels and ecSOD activities were observed in the animal studies, such correlations have not been observed consistently in humans. Paik et al65 reported that in healthy adult Koreans, ecSOD activity correlated positively with increasing serum zinc concentrations. Interestingly, SOD1 activity correlated negatively with zinc, which suggests that SOD1 and ecSOD are regulated differentially by this cation. The same group also conducted a clinical trial to evaluate the effects of prenatal zinc supplementation on pregnancy outcome in African-American women.66 Although a positive effect of zinc supplementation on birth weight was observed, plasma ecSOD activities in these subjects were lower than reported previously for healthy adults,65 which suggests that plasma ecSOD activity is not a sensitive marker for marginal zinc deficiency. These results are not surprising given the complex interactions of dietary zinc with other factors that regulate ecSOD in vivo. For example, dietary zinc deficiency can cause tissue iron accumulation,67 which in turn may regulate ecSOD function (as discussed in the next section). In future study, the effect of zinc transport pathway on ecSOD function may also need to be evaluated.
Iron
Like copper, iron is a redox active metal that is critical to cellular homeostasis.68 Stralin et al69 reported that the addition of FeCl2 to smooth muscle cell (SMC) cultures induced a marked dose-dependent increase in ecSOD expression. In addition, low concentrations of iron increased secretion of ecSOD to the medium, whereas higher concentrations inhibited both ecSOD expression and secretion. The mechanisms by which iron may modulate ecSOD are unknown currently. One possibility is that iron-dependent production may modulate directly ecSOD secretion in the vasculature. However, removal of external iron by desferal and bathophenanthroline disulfonate did not affect ecSOD expression in smooth muscle cells, and the addition of SOD1 or catalase to suppress Haber–Weiss chemistry did not influence the effects of iron on ecSOD expression.69 A second possibility is that iron interferes with the proper functioning of the Golgi apparatus and the endoplasmic reticulum involved in secretion of ecSOD. It has been reported that the subcellular distribution (cytosolic vs microsomal) of some proteins is affected by cellular iron status.70 The third possibility is that iron interferes with the proper functioning of copper and modulates indirectly the ecSOD secretion, because the metabolic fates of copper and iron are linked intimately. For example, systemic copper deficiency is associated with cellular iron deficiency.71
TISSUE BINDING OF ecSOD: DISPLACEMENT BY HEPARIN
Heparin is a highly sulfated glycosaminoglycan that is best known for its antithrombotic effects. Heparin also inhibits proliferation and migration of SMCs and regulates the synthesis of proteins in SMCs72 and fibroblasts.73,74 Moreover, heparin displaces lipoprotein lipase bound to the endothelial surface, which thus affects lipoprotein metabolism.72 ecSOD also has a high affinity to heparan sulfate proteoglycans located on endothelial cell surfaces and in the connective tissue matrix.
Intravenous injection of heparin displaces ecSOD from proteoglycans and leads to a prompt increase in plasma ecSOD activity in humans75 and other mammals.76,77 For example, 1000 IU of heparin/kg body weight produces a maximal release of ecSOD in pigs. Injection of 50-IU heparin/kg body weight into healthy human volunteers led to an immediate 2.4–2.8-fold increase in serum ecSOD levels. Thus, heparin is highly potent at displacing ecSOD into the circulation. The ecSOD was released from endothelial cells, not from the blood cells.75 The half-life of ecSOD release into the serum after heparin injection was about 90 min.78 Furthermore, in vivo and in vitro experiments suggested that the ecSOD released into the plasma by heparin reestablished its binding to glycocalyx on the vascular endothelial cell surface in proportion to the elimination of heparin from the vascular system. In addition, heparin was shown to induce both ecSOD mRNA and protein expression in cultured skin fibroblasts.79 The extent of ecSOD induction seems to be dependent on the level of glycosaminoglycan sulfation and may involve either receptor binding or a direct effect on promoter elements.79 Only a portion of the tissue-bound ecSOD is displaced by heparin injection,75 which suggests that other ligands for ecSOD such as collagen type I80 or fibulin-581 may also play an important role in regulating levels of tissue bound ecSOD.
The pathophysiologic significance of heparin-induced ecSOD release has been investigated in a clinical trial, which showed that the levels of ecSOD released by heparin were significantly lower in patients with atherosclerosis as compared with controls. Moreover, the coronary artery disease score was correlated inversely with heparin-induced ecSOD release. As for factors affecting the level of heparin-induced ecSOD release, high-density lipoprotein cholesterol levels and age were correlated positively.82 Because heparin is a common therapeutic drug for pulmonary embolism and deep vein thrombosis, an interesting question is whether heparin-induced ecSOD release also facilitates the therapeutic effect of heparin.
POTENTIAL INTERACTIONS OF ecSOD WITH OTHER FACTORS THAT MODULATE CARDIOVASCULAR FUNCTION
Estrogen and progesterone
Estrogen and progester-one are 2 steroid hormones that regulate several aspects of cardiovascular function. For example, estrogen acts as an antioxidant83 by enhancing nitric oxide (NO) bioavailability and by inhibiting the reactive oxygen species (ROS)-generating NADPH oxidase.84,85 The impact of progesterone on oxidative stress is not as well established as that of estrogens. Strelow et al86 and Wassman et al87 studied the effects of estrogen and progesterone on ecSOD expression and activity.86,87 They reported that in SMCs, estrogen upregulated ec-SOD expression (mRNA and protein level) and enzymatic activity.86 Conversely, progesterone downregulated basal ecSOD expression and activity and reversed ecSOD induction by estrogen.87 Moreover, ovariectomy led to a downregulation of ecSOD expression in mice, which was associated with increased levels of vascular ROS. These effects were blunted by estrogen replacement or treatment with pegylated-SOD.86 Conversely, administration of progesterone to ovariectomized mice abrogated the antioxidant effects of estrogen replacement, including the enhancement of ecSOD expression.87 In humans, increased estrogen levels correlated with enhanced ecSOD expression in circulating monocytes.86
Angiotensin II
Angiotensin II is the major effector hormone of the renin-angiotensin system (RAS), which regulates blood volume, arterial pressure, and cardiac and vascular function. Angiotensin II binds to 2 distinct receptors, angiotensin type 1 (AT1) and angiotensin type 2 (AT2). AT1 receptors are distributed widely and mediate most biologic responses of angiotensin II, whereas AT2 receptors antagonize several AT1 receptor-mediated responses; together, these 2 subtypes seem to coregulate blood pressure homeostasis and sodium excretion. Many hypertensive effects of angio-tensin II have been attributed to increased oxidative stress in blood vessels and to the central nervous system. Recently, angiotensin II has been found to upregulate ecSOD expression acutely in murine blood vessels in vivo,88 in cultured human aortic SMCs,88 and in human uterine arterial SMCs.89 The upregulation of vascular ecSOD may counterbalance the increased vascular production and the decreased total SOD activity90 stimulated by angiotensin II.
Although angiotensin II upregulated ecSOD expression in vascular tissues in vivo, the peptide produced opposite effects on ecSOD expression in the kidney. Chabrashvili et al91 and Welch et al92 reported that angiotensin II infusion in rats decreased ecSOD mRNA levels in the kidneys, and this effect was reversed by candesartan, which is an AT1 receptor antagonist,91 or by Tempol, which is an SOD mimetic.92 These results suggest that ecSOD may contribute to the protective effects of AT1 receptor blockade on oxidative stress in the kidneys.93 The reason for the differences between vascular and renal expression of ecSOD in response to angiotensin II is unclear but may relate to differences in redox signaling, vasoactive effects, and/or function of AT2 receptors in kidneys versus blood vessels (for review, see Johren et al94).
Modulation of the RAS by angiotensin converting enzyme (ACE) inhibitors and AT1 receptor antagonists in humans produces many beneficial effects on the cardiovascular system. Hornig et al95 randomized patients with coronary artery disease to 4 weeks of ramipril, which is an ACE inhibitor, or losartan, which is an AT1 receptor antagonist. Flow-dependent, endothelium-mediated vasodilation (FDD) of the radial artery was increased significantly after ramipril or losartan; in particular, the portion of FDD mediated by NO was increased by greater than 75%. Concomitantly, therapy with ramipril or losartan increased ecSOD activity by greater than 200%. These findings suggest that long-term inhibition of the RAS in humans may increase ecSOD activity, thereby reducing oxidative stress in the arterial wall.
Homocysteine
Homocysteine is an intermediate sulfur-containing amino acid that is formed during the metabolism of methionine. An increased level of homocysteine in the plasma has been implicated as a risk factor for cardiovascular disease.96,97 Autoxidation of homocysteine can generate and can lead to peroxynitrite formation, thereby promoting oxidative stress.98,99 The resultant increase in levels may in part be counterbalanced by homocysteine-dependent increases in ecSOD.100 For example, Wilcken et al101 reported a positive correlation between plasma levels of homocysteine and ecSOD in patients with coronary artery disease, and therapy that lowered plasma homocysteine also decreased ecSOD levels.101 ecSOD levels were correlated inversely with the incidence of cardiovascular disease in these patients, and risk factors for coronary artery disease, such as male gender and smoking, were associated with decreased levels of ecSOD in a previous study.102 The mechanisms by which homocysteine may modulate plasma ecSOD levels are unknown currently. One possibility is that homocysteine modulates the binding of ecSOD to endothelial cells through direct modification of heparan sulfate proteoglycans on cell surfaces. This mechanism is supported by the work of Yamamoto et al,103 who reported that the binding of recombinant ecSOD to immobilized heparin was decreased markedly by pretreatment of the heparin-bound surface with homocysteine. Also, Nihei et al104 showed that in patients with coronary artery disease, hyperhomocysteinemia is associated with increased release of ecSOD from the endothelium. Another potential mechanism is that homocysteine may perturb endoplasmic reticulum function, thereby disrupting disulfide bond formation or glycosylation of ecSOD, which is required for normal protein assembly and association with cell membranes.105
Other factors
Increased oxidative stress is thought to underlie the cardiovascular complications associated with diabetes mellitus. In Japanese patients with diabetes, serum ecSOD levels were correlated positively with the severity of microvascular complications, which may reflect decreased binding of the enzyme to the endothelium and enhanced susceptibility to vascular oxidative stress.106 Navab et al107 demonstrated that levels of ecSOD in aorta were decreased in a rat model of diabetes and that treatment with D-4F, which is an apolipoprotein A1 mimetic with anti-inflammatory properties,107 restored aortic ecSOD protein levels without affecting either Cu, ZnSOD, or MnSOD.108 The mechanism was suggested to be related indirectly to upregulation of the antioxidant enzyme heme oxygenase.108 Furthermore, prooxidants such as xanthine oxidase, paraquat, and tert-butyl hydroperoxide decreased ecSOD expression in fibroblasts in a dose-dependent manner.109 In vascular SMCs and lung alveolar type 2 cells, ecSOD expression was upregulated by IFN-γ and IL-4 and downregulated by TNF-α.110 Finally, growth factors such as TGF-β, PDGF, and FGF depressed ecSOD mRNA levels in fibroblasts and SMCs.111 Moreover, ecSOD activity is modulated by hydrogen peroxide because of its peroxidase activity.112,113 The patho-physiologic relevance of in vitro alterations in ecSOD expression induced by oxidants, cytokines, and growth factors remains to be determined.
ecSOD GENE VARIANTS
Substitution of arginine-213 with glycine (R213G), which is located in the center of the carboxyl-terminal cluster of positively charged amino acid residues of the heparin binding domain, is a common human gene variant of ecSOD.114–117 Although this variant does not affect ecSOD enzymatic activity,115,116 plasma concentrations of ecSOD are increased dramatically in the 2% to 5% of the population that carries the gene variant. The increased concentration of ecSOD in the plasma of R213G carriers is in part caused by impaired heparin and collagen binding affinities, which leads to a 50-fold decrease in binding of the variant gene product to endothelial cells in vitro.115,116,118 In addition, the R213G gene product is resistant to proteolysis by trypsin and neutrophil-derived proteases.119 In spontaneously hypertensive rats, overexpression of the R213G variant of ecSOD did not improve blood pressure, vascular function, or vascular oxidant stress, which suggests that disruption of the heparin binding domain negates the protective effects of ecSOD in the vasculature.20
The clinical significance of ecSOD(R213G) has been investigated in several association studies. Patients with diabetes and end-stage renal disease carrying ecSOD(R213G) had an increased 5-year mortality rate, with significantly higher death rates from ischemic heart disease and cerebrovascular disease than did those of noncarriers.120 Also, the R213G gene variant was suggested to accelerate the progression of renal failure and atherosclerosis in uremic patients.121 Finally, a study in Denmark detected a 2.3-fold increase in risk of ischemic heart disease in heterozygotes carrying ecSOD(R213G), with a 9-fold increase for plasma levels of ecSOD,122 presumably because of increased in the vasculature of ecSOD(R213G) carriers.
Several other variants of the ecSOD gene have also been described in humans, including a threonine-to-alanine substitution at position 40 in the amino terminus and a silent substitution at amino acid 280. Table II summarizes newly identified variants in the human ecSOD gene.123,124 The functional significance of these variants, however, remains unknown.125
Table II.
Newly identified variants of the human ecSOD gene
| Mutation | Type | Country | Findings | Reference |
|---|---|---|---|---|
| Ala40Thr | Missense mutation | Japan | Frequency of Thr allele, and the number of subjects with Thr allele (Ala/Thr + Thr/Thr), was higher in type 2 diabetic patients; patients with Thr allele also exhibited earlier age of onset of diabetes and decreased insulin sensitivity | 123 |
| Leu53Leu | Silent mutation | Japan | No differences in allele frequencies were found between diabetic and nondiabetic subjects | 123 |
| T40A | Missense mutation | Italy | Allele frequency 43% | 124 |
| F131C | Missense mutation | Italy | Allele frequency 5% | 124 |
| V160L | Missense mutation | Italy | Allele frequency 0.25% | 124 |
| R202L | Missense mutation | Italy | Allele frequency 0.84% | 124 |
| 32 (GCG > GCT)* | Silent mutation | Italy | Allele frequency 0.34% | 124 |
Based on codon position.
CONCLUSIONS AND FUTURE DIRECTIONS
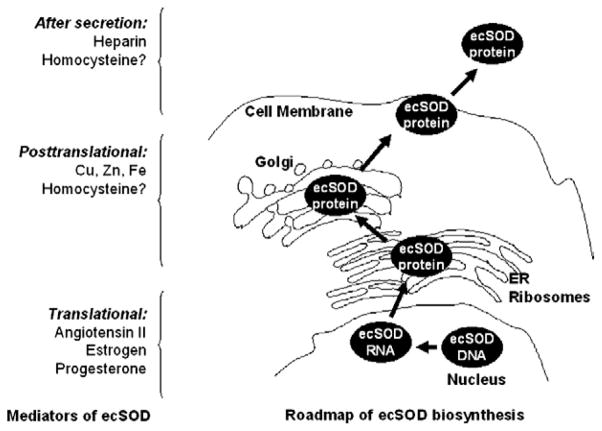
ecSOD has emerged as an important endogenous regulator of oxidative stress. Moreover, ecSOD gene transfer represents a promising approach to reduce extracellular and to protect against oxidative stress, particularly in the cardiovascular system. In this review, we have discussed possible factors affecting endogenous ecSOD expression and activity, tissue-binding, as well as interaction with other factors. Figure 1 illustrates the proposed mechanisms of modulation of ecSOD by endogenous mediators. Understanding the potential therapeutic role of ecSOD in cardiovascular diseases, however, requires fundamental knowledge of how the enzyme is regulated under normal and pathophysiologic conditions. In this regard, several important questions remain to be answered before modulation of ecSOD can be translated into human therapeutics:
Fig 1.
Proposed mechanisms of modulation of ecSOD by endogenous mediators. Angiotensin II, estrogen, and progesterone modulate ecSOD mRNA levels. Cu and Zn incorporate into the de novo protein in the Golgi secretion pathway and are necessary to maintain enzymatic activity. Heparin modulates binding of the secreted ecSOD enzyme. ER, endoplasmic reticulum.
How do alterations in cellular copper, zinc, and iron levels modulate the efficacy of ecSOD gene transfer?
Do endogenous angiotensin II, estrogen, progesterone, and/or homocysteine influence the activity of ecSOD expressed by gene transfer?
Is endogenous SOD activity altered by ecSOD gene transfer?
To what extent does upregulation of ecSOD by pharmacologic agents (ie, estrogens, ACE inhibitors) contribute to the cardiovascular effects of these agents?
Is displacement of ecSOD from the vasculature by heparin of clinical importance, particularly in the setting of acute coronary syndromes?
Acknowledgments
Supported by NIH grants HL070860, HL076684, and HL62984 (to N.L.W.), and HL70187-01 (to T.F.), and by University of Cincinnati URC faculty development grant (to Z.Q.).
Abbreviations
- ACE
angiotensin converting enzyme
- AT1
angiotensin type 1
- AT2
angiotensin type 2
- Atox1
antioxidant protein 1
- ecSOD
extracellular superoxide dismutase
- FDD
flow-dependent, endothelium-mediated vasodilation
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
superoxide anion
- RAS
renin–angiotensin system
- ROS
reactive oxygen species
- R213G
arginine-213 with glycine
- SMC
smooth muscle cell
- SOD
superoxide dismutase
References
- 1.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 2.Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53:135–59. [PubMed] [Google Scholar]
- 3.Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radic Biol Med. 2006;40:183–92. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Warnholtz A, Mollnau H, Oelze M, Wendt M, Munzel T. Antioxidants and endothelial dysfunction in hyperlipidemia. Curr Hypertens Rep. 2001;3:53–60. doi: 10.1007/s11906-001-0081-z. [DOI] [PubMed] [Google Scholar]
- 5.Lassegue B, Griendling KK. Reactive oxygen species in hypertension: an update. Am J Hypertens. 2004;17:852–60. doi: 10.1016/j.amjhyper.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal A, Nandipati KC, Sharma RK, Zippe CD, Raina R. Role of oxidative stress in the pathophysiological mechanism of erectile dysfunction. J Androl. 2006;27:335–47. doi: 10.2164/jandrol.05136. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald RL, Weir BK. Cerebral vasospasm and free radicals. Free Radic Biol Med. 1994;16:633–43. doi: 10.1016/0891-5849(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 8.Selverstone Valentine J, Doucette PA, Zittin Potter S. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu Rev Biochem. 2005;74:563–93. doi: 10.1146/annurev.biochem.72.121801.161647. [DOI] [PubMed] [Google Scholar]
- 9.Macmillan-Crow LA, Cruthirds DL. Invited review: manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–36. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- 10.Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res. 2003;93:622–9. doi: 10.1161/01.RES.0000092140.81594.A8. [DOI] [PubMed] [Google Scholar]
- 11.Gongora MC, Qin Z, Laude K, et al. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48:473–81. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 12.Bowler RP, Crapo JD. Oxidative stress in airways: is there a role for extracellular superoxide dismutase? Am J Respir Crit Care Med. 2002;66:S38–43. doi: 10.1164/rccm.2206014. [DOI] [PubMed] [Google Scholar]
- 13.Nozik-Grayck E, Suliman HB, Piantadosi CA. Extracellular superoxide dismutase. Int J Biochem Cell Biol. 2005;37:2466–71. doi: 10.1016/j.biocel.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismutase in vessels and airways of humans and baboons. Free Radic Biol Med. 1996;20:957–65. doi: 10.1016/0891-5849(95)02222-8. [DOI] [PubMed] [Google Scholar]
- 15.Stralin P, Karlsson K, Johansson BO, Marklund SL. The inter-stitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler Thromb Vasc Biol. 1995;15:2032–6. doi: 10.1161/01.atv.15.11.2032. [DOI] [PubMed] [Google Scholar]
- 16.Iida S, Chu Y, Weiss RM, Kang YM, Faraci FM, Heistad DD. Vascular effects of a common gene variant of extracellular superoxide dismutase in heart failure. Am J Physiol Heart Circ Physiol. 2006;291:H914–20. doi: 10.1152/ajpheart.00080.2006. [DOI] [PubMed] [Google Scholar]
- 17.Laukkanen MO, Kivela A, Rissanen T, et al. Adenovirus-mediated extracellular superoxide dismutase gene therapy reduces neointima formation in balloon-denuded rabbit aorta. Circulation. 2002;106:1999–2003. doi: 10.1161/01.cir.0000031331.05368.9d. [DOI] [PubMed] [Google Scholar]
- 18.Laukkanen MO, Leppanen P, Turunen P, Porkkala-Sarataho E, Salonen JT, Yla-Herttuala S. Gene transfer of extracellular superoxide dismutase to atherosclerotic mice. Antioxid Redox Signal. 2001;3:397–402. doi: 10.1089/15230860152409040. [DOI] [PubMed] [Google Scholar]
- 19.Brown KA, Chu Y, Lund DD, Heistad DD, Faraci FM. Gene transfer of extracellular superoxide dismutase protects against vascular dysfunction with aging. Am J Physiol Heart Circ Physiol. 2006;290:H2600–5. doi: 10.1152/ajpheart.00676.2005. [DOI] [PubMed] [Google Scholar]
- 20.Chu Y, Alwahdani A, Iida S, Lund DD, Faraci FM, Heistad DD. Vascular effects of the human extracellular superoxide dismutase R213G variant. Circulation. 2005;112:1047–53. doi: 10.1161/CIRCULATIONAHA.104.531251. [DOI] [PubMed] [Google Scholar]
- 21.Chu Y, Iida S, Lund DD, et al. Gene transfer of extracellular superoxide dismutase reduces arterial pressure in spontaneously hypertensive rats: role of heparin-binding domain. Circ Res. 2003;92:461–8. doi: 10.1161/01.RES.0000057755.02845.F9. [DOI] [PubMed] [Google Scholar]
- 22.Lund DD, Gunnett CA, Chu Y, Brooks RM, Faraci FM, Heistad DD. Gene transfer of extracellular superoxide dismutase improves relaxation of aorta after treatment with endotoxin. Am J Physiol Heart Circ Physiol. 2004;287:H805–11. doi: 10.1152/ajpheart.00907.2003. [DOI] [PubMed] [Google Scholar]
- 23.Fennell JP, Brosnan MJ, Frater AJ, et al. Adenovirus-mediated overexpression of extracellular superoxide dismutase improves endothelial dysfunction in a rat model of hypertension. Gene Ther. 2002;9:110–7. doi: 10.1038/sj.gt.3301633. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi M, Zhou C, Heistad DD, Watanabe Y, Zhang JH. Gene transfer of extracellular superoxide dismutase failed to prevent cerebral vasospasm after experimental subarachnoid hemorrhage. Stroke. 2004;35:2512–7. doi: 10.1161/01.STR.0000145198.07723.8e. [DOI] [PubMed] [Google Scholar]
- 25.Nakane H, Chu Y, Faraci FM, Oberley LW, Heistad DD. Gene transfer of extracellular superoxide dismutase increases super-oxide dismutase activity in cerebrospinal fluid. Stroke. 2001;32:184–9. doi: 10.1161/01.str.32.1.184. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe Y, Chu Y, Andresen JJ, Nakane H, Faraci FM, Heistad DD. Gene transfer of extracellular superoxide dismutase reduces cerebral vasospasm after subarachnoid hemorrhage. Stroke. 2003;34:434–40. doi: 10.1161/01.str.0000051586.96022.37. [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Liu L, Yen RD, Catana A, Nantz MH, Zern MA. Liposome-mediated extracellular superoxide dismutase gene delivery protects against acute liver injury in mice. Hepatology. 2004;40:195–204. doi: 10.1002/hep.20288. [DOI] [PubMed] [Google Scholar]
- 28.Dai L, Claxson A, Marklund SL, et al. Amelioration of antigen-induced arthritis in rats by transfer of extracellular superoxide dismutase and catalase genes. Gene Ther. 2003;10:550–8. doi: 10.1038/sj.gt.3301916. [DOI] [PubMed] [Google Scholar]
- 29.Iyama S, Okamoto T, Sato T, et al. Treatment of murine collagen-induced arthritis by ex vivo extracellular superoxide dismutase gene transfer. Arthritis Rheum. 2001;44:2160–7. doi: 10.1002/1529-0131(200109)44:9<2160::aid-art369>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Bolli R, Qiu Y, Tang XL, Guo Y, French BA. Gene therapy with extracellular superoxide dismutase protects conscious rabbits against myocardial infarction. Circulation. 2001;103:1893–8. doi: 10.1161/01.cir.103.14.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Bolli R, Qiu Y, Tang XL, Murphree SS, French BA. Gene therapy with extracellular superoxide dismutase attenuates myocardial stunning in conscious rabbits. Circulation. 1998;98:1438–48. doi: 10.1161/01.cir.98.14.1438. [DOI] [PubMed] [Google Scholar]
- 32.He SQ, Zhang YH, Venugopal SK, et al. Delivery of antioxi-dative enzyme genes protects against ischemia/reperfusion-induced liver injury in mice. Liver Transpl. 2006;12:1869–79. doi: 10.1002/lt.21001. [DOI] [PubMed] [Google Scholar]
- 33.Bivalacqua TJ, Usta MF, Kendirci M, et al. Superoxide anion production in the rat penis impairs erectile function in diabetes: influence of in vivo extracellular superoxide dismutase gene therapy. J Sex Med. 2005;2:187–97. doi: 10.1111/j.1743-6109.2005.20228_1.x. discussion 197–8. [DOI] [PubMed] [Google Scholar]
- 34.Oku T, Iyama S, Sato T, et al. Amelioration of murine dextran sulfate sodium-induced colitis by ex vivo extracellular super-oxide dismutase gene transfer. Inflamm Bowel Dis. 2006;12:630–40. doi: 10.1097/01.MIB.0000225335.68614.73. [DOI] [PubMed] [Google Scholar]
- 35.Iida S, Chu Y, Francis J, et al. Gene transfer of extracellular superoxide dismutase improves endothelial function in rats with heart failure. Am J Physiol Heart Circ Physiol. 2005;289:H525–32. doi: 10.1152/ajpheart.00108.2005. [DOI] [PubMed] [Google Scholar]
- 36.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–6. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 37.Carlsson LM, Marklund SL, Edlund T. The rat extracellular superoxide dismutase dimer is converted to a tetramer by the exchange of a single amino acid. Proc Natl Acad Sci USA. 1996;93:5219–22. doi: 10.1073/pnas.93.11.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heistad DD. Gene therapy for vascular disease. Vascul Pharmacol. 2006;45:331–3. doi: 10.1016/j.vph.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med. 2003;35:236–56. doi: 10.1016/s0891-5849(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 40.Petersen SV, Enghild JJ. Extracellular superoxide dismutase: structural and functional considerations of a protein shaped by two different disulfide bridge patterns. Biomed Pharmacother. 2005;59:175–82. doi: 10.1016/j.biopha.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Kim HW, Lin A, Guldberg RE, Ushio-Fukai M, Fukai T. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circ Res. 2007;101:409–19. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 42.Buetler TM, Krauskopf A, Ruegg UT. Role of superoxide as a signaling molecule. News Physiol Sci. 2004;19:120–3. doi: 10.1152/nips.01514.2003. [DOI] [PubMed] [Google Scholar]
- 43.Werner E. GTPases and reactive oxygen species: switches for killing and signaling. J Cell Sci. 2004;117:143–53. doi: 10.1242/jcs.00937. [DOI] [PubMed] [Google Scholar]
- 44.Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–8. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 45.Prohaska JR, Gybina AA. Intracellular copper transport in mammals. J Nutr. 2004;134:1003–6. doi: 10.1093/jn/134.5.1003. [DOI] [PubMed] [Google Scholar]
- 46.Hamza I, Faisst A, Prohaska J, Chen J, Gruss P, Gitlin JD. The metallochaperone Atox1 plays a critical role in perinatal copper homeostasis. Proc Natl Acad Sci USA. 2001;98:6848–52. doi: 10.1073/pnas.111058498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mercer JF, Llanos RM. Molecular and cellular aspects of copper transport in developing mammals. J Nutr. 2003;133:1481S– 4S. doi: 10.1093/jn/133.5.1481S. [DOI] [PubMed] [Google Scholar]
- 48.Jeney V, Itoh S, Wendt M, et al. Role of antioxidant-1 in extracellular superoxide dismutase function and expression. Circ Res. 2005;96:723–9. doi: 10.1161/01.RES.0000162001.57896.66. [DOI] [PubMed] [Google Scholar]
- 49.Qin Z, Itoh S, Jeney V, Ushio-Fukai M, Fukai T. Essential role for the Menkes ATPase in activation of extracellular superoxide dismutase: implication for vascular oxidative stress. FASEB J. 2006;20:334–6. doi: 10.1096/fj.05-4564fje. [DOI] [PubMed] [Google Scholar]
- 50.Sandstead HH. Requirement of zinc in human subjects. J Am Coll Nutr. 1985;4:73–82. doi: 10.1080/07315724.1985.10720068. [DOI] [PubMed] [Google Scholar]
- 51.Prasad AS. Clinical manifestations of zinc deficiency. Annu Rev Nutr. 1985;5:341–63. doi: 10.1146/annurev.nu.05.070185.002013. [DOI] [PubMed] [Google Scholar]
- 52.Jenner A, Ren M, Rajendran R, et al. Zinc supplementation inhibits lipid peroxidation and the development of atherosclerosis in rabbits fed a high cholesterol diet. Free Radic Biol Med. 2007;42:559–66. doi: 10.1016/j.freeradbiomed.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 53.Girotti AW, Thomas JP, Jordan JE. Inhibitory effect of zinc(II) on free radical lipid peroxidation in erythrocyte membranes. J Free Radic Biol Med. 1985;1:395–401. doi: 10.1016/0748-5514(85)90152-7. [DOI] [PubMed] [Google Scholar]
- 54.Bray TM, Bettger WJ. The physiological role of zinc as an antioxidant. Free Radic Biol Med. 1990;8:281–91. doi: 10.1016/0891-5849(90)90076-u. [DOI] [PubMed] [Google Scholar]
- 55.Ren M, Rajendran R, Ning P, et al. Zinc supplementation decreases the development of atherosclerosis in rabbits. Free Radic Biol Med. 2006;41:222–5. doi: 10.1016/j.freeradbiomed.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 56.Alissa EM, Bahijri SM, Lamb DJ, Ferns GA. The effects of coadministration of dietary copper and zinc supplements on atherosclerosis, antioxidant enzymes and indices of lipid peroxidation in the cholesterol-fed rabbit. Int J Exp Pathol. 2004;85:265–75. doi: 10.1111/j.0959-9673.2004.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reiterer G, MacDonald R, Browning JD, et al. Zinc deficiency increases plasma lipids and atherosclerotic markers in LDL-receptor-deficient mice. J Nutr. 2005;135:2114–8. doi: 10.1093/jn/135.9.2114. [DOI] [PubMed] [Google Scholar]
- 58.Beattie JH, Kwun IS. Is zinc deficiency a risk factor for atherosclerosis? Br J Nutr. 2004;91:177–81. doi: 10.1079/BJN20031072. [DOI] [PubMed] [Google Scholar]
- 59.Lee DH, Folsom AR, Jacobs DR., Jr Iron, zinc, and alcohol consumption and mortality from cardiovascular diseases: the Iowa Women’s Health Study. Am J Clin Nutr. 2005;81:787–91. doi: 10.1093/ajcn/81.4.787. [DOI] [PubMed] [Google Scholar]
- 60.Reunanen A, Knekt P, Marniemi J, Maki J, Maatela J, Aromaa A. Serum calcium, magnesium, copper and z risk of cardiovascular death. Eur J Clin Nutr. 1996;50:431–7. [PubMed] [Google Scholar]
- 61.Kok FJ, Van Duijn CM, Hofman A, et al. Serum copper and zinc and the risk of death from cancer and cardiovascular disease. Am J Epidemiol. 1988;128:352–9. doi: 10.1093/oxfordjournals.aje.a114975. [DOI] [PubMed] [Google Scholar]
- 62.Olin KL, Golub MS, Gershwin ME, Hendrickx AG, Lonnerdal B, Keen CL. Extracellular superoxide dismutase activity is affected by dietary zinc intake in nonhuman primate and rodent models. Am J Clin Nutr. 1995;61:1263–7. doi: 10.1093/ajcn/61.6.1263. [DOI] [PubMed] [Google Scholar]
- 63.Oldereid NB, Thomassen Y, Attramadal A, Olaisen B, Purvis K. Concentrations of lead, cadmium and zinc in the tissues of reproductive organs of men. J Reprod Fertil. 1993;99:421–5. doi: 10.1530/jrf.0.0990421. [DOI] [PubMed] [Google Scholar]
- 64.Oteiza PI, Adonaylo VN, Keen CL. Cadmium-induced testes oxidative damage in rats can be influenced by dietary zinc intake. Toxicology. 1999;137:13–22. doi: 10.1016/s0300-483x(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 65.Paik HY, Joung H, Lee JY, Lee HK, King JC, Keen CL. Serum extracellular superoxide dismutase activity as an indicator of zinc status in humans. Biol Trace Elem Res. 1999;69:45–57. doi: 10.1007/BF02783914. [DOI] [PubMed] [Google Scholar]
- 66.Tamura T, Olin KL, Goldenberg RL, Johnston KE, Dubard MB, Keen CL. Plasma extracellular superoxide dismutase activity in healthy pregnant women is not influenced by zinc supplementation. Biol Trace Elem Res. 2001;80:107–13. doi: 10.1385/BTER:80:2:107. [DOI] [PubMed] [Google Scholar]
- 67.Rogers JM, Lonnerdal B, Hurley LS, Keen CL. Iron and zinc concentrations and 59Fe retention in developing fetuses of zinc-deficient rats. J Nutr. 1987;117:1875–82. doi: 10.1093/jn/117.11.1875. [DOI] [PubMed] [Google Scholar]
- 68.Dunn LL, Rahmanto YS, Richardson DR. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2007;17:93–100. doi: 10.1016/j.tcb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 69.Stralin P, Jacobsson H, Marklund SL. Oxidative stress, NO* and smooth muscle cell extracellular superoxide dismutase expression. Biochim Biophys Acta. 2003;1619:1–8. doi: 10.1016/s0304-4165(02)00419-1. [DOI] [PubMed] [Google Scholar]
- 70.Patton SM, Pinero DJ, Surguladze N, Beard J, Connor JR. Subcellular localization of iron regulatory proteins to Golgi and ER membranes. J Cell Sci. 2005;118:4365–73. doi: 10.1242/jcs.02570. [DOI] [PubMed] [Google Scholar]
- 71.Arredondo M, Nunez MT. Iron and copper metabolism. Mol Aspects Med. 2005;26:313–27. doi: 10.1016/j.mam.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 72.Patel MK, Refson JS, Schachter M, Hughes AD. Characterization of [3H]-heparin binding in human vascular smooth muscle cells and its relationship to the inhibition of DNA synthesis. Br J Pharmacol. 1999;127:361–8. doi: 10.1038/sj.bjp.0702559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferrao AV, Mason RM. The effect of heparin on cell proliferation and type-I collagen synthesis by adult human dermal fibroblasts. Biochim Biophys Acta. 1993;1180:225–30. doi: 10.1016/0925-4439(93)90042-y. [DOI] [PubMed] [Google Scholar]
- 74.Tyagi SC, Kumar S, Katwa L. Differential regulation of extracellular matrix metalloproteinase and tissue inhibitor by heparin and cholesterol in fibroblast cells. J Mol Cell Cardiol. 1997;29:391–404. doi: 10.1006/jmcc.1996.0283. [DOI] [PubMed] [Google Scholar]
- 75.Karlsson K, Marklund SL. Heparin-induced release of extracellular superoxide dismutase to human blood plasma. Biochem J. 1987;242:55–9. doi: 10.1042/bj2420055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karlsson K, Marklund SL. Heparin-, dextran sulfate- and protamine-induced release of extracellular-superoxide dismutase to plasma in pigs. Biochim Biophys Acta. 1988;967:110–4. doi: 10.1016/0304-4165(88)90195-x. [DOI] [PubMed] [Google Scholar]
- 77.Oyanagui Y, Sato S. Heparin, a potent releasing agent of extracellular superoxide dismutase (EC-SOD C), suppresses ischaemic paw oedema in mice. Free Radic Res Commun. 1990;9:87–99. doi: 10.3109/10715769009148576. [DOI] [PubMed] [Google Scholar]
- 78.Adachi T, Yamada H, Futenma A, Kato K, Hirano K. Heparin-induced release of extracellular-superoxide dismutase form (V) to plasma. J Biochem (Tokyo) 1995;117:586–90. doi: 10.1093/oxfordjournals.jbchem.a124748. [DOI] [PubMed] [Google Scholar]
- 79.Adachi T, Hara H, Yamada H, et al. Heparin-stimulated expression of extracellular-superoxide dismutase in human fibroblasts. Atherosclerosis. 2001;159:307–12. doi: 10.1016/s0021-9150(01)00512-3. [DOI] [PubMed] [Google Scholar]
- 80.Petersen SV, Oury TD, Ostergaard L, et al. Extracellular superoxide dismutase (EC-SOD) binds to type i collagen and protects against oxidative fragmentation. J Biol Chem. 2004;279:13705–10. doi: 10.1074/jbc.M310217200. [DOI] [PubMed] [Google Scholar]
- 81.Nguyen AD, Itoh S, Jeney V, et al. Fibulin-5 is a novel binding protein for extracellular superoxide dismutase. Circ Res. 2004;95:1067–74. doi: 10.1161/01.RES.0000149568.85071.FB. [DOI] [PubMed] [Google Scholar]
- 82.Tasaki H, Yamashita K, Tsutsui M, et al. Heparin-released extracellular superoxide dismutase is reduced in patients with coronary artery atherosclerosis. Atherosclerosis. 2006;187:131–8. doi: 10.1016/j.atherosclerosis.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 83.Tiidus PM. Estrogen and gender effects on muscle damage, inflammation, and oxidative stress. Can J Appl Physiol. 2000;25:274–87. doi: 10.1139/h00-022. [DOI] [PubMed] [Google Scholar]
- 84.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23:665–86. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 85.Gragasin FS, Xu Y, Arenas IA, Kainth N, Davidge ST. Estrogen reduces angiotensin II-induced nitric oxide synthase and NAD(P)H oxidase expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:38–44. doi: 10.1161/01.atv.0000047868.93732.b7. [DOI] [PubMed] [Google Scholar]
- 86.Strehlow K, Rotter S, Wassmann S, et al. Modulation of anti-oxidant enzyme expression and function by estrogen. Circ Res. 2003;93:170–7. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]
- 87.Wassmann K, Wassmann S, Nickenig G. Progesterone antagonizes the vasoprotective effect of estrogen on antioxidant enzyme expression and function. Circ Res. 2005;97:1046–54. doi: 10.1161/01.RES.0000188212.57180.55. [DOI] [PubMed] [Google Scholar]
- 88.Fukai T, Siegfried MR, Ushio-Fukai M, Griendling KK, Harrison DG. Modulation of extracellular superoxide dismutase expression by angiotensin II and hypertension. Circ Res. 1999;85:23–8. doi: 10.1161/01.res.85.1.23. [DOI] [PubMed] [Google Scholar]
- 89.Stralin P, Marklund SL. Vasoactive factors and growth factors alter vascular smooth muscle cell EC-SOD expression. Am J Physiol Heart Circ Physiol. 2001;281:H1621–9. doi: 10.1152/ajpheart.2001.281.4.H1621. [DOI] [PubMed] [Google Scholar]
- 90.Welch WJ, Chabrashvili T, Solis G, et al. Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension. 2006;48:934–41. doi: 10.1161/01.HYP.0000242928.57344.92. [DOI] [PubMed] [Google Scholar]
- 91.Chabrashvili T, Kitiyakara C, Blau J, et al. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R117–24. doi: 10.1152/ajpregu.00476.2002. [DOI] [PubMed] [Google Scholar]
- 92.Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS. Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol. 2005;288:H22–8. doi: 10.1152/ajpheart.00626.2004. [DOI] [PubMed] [Google Scholar]
- 93.Welch WJ, Wilcox CS. AT1 receptor antagonist combats oxidative stress and restores nitric oxide signaling in the SHR. Kidney Int. 2001;59:1257–63. doi: 10.1046/j.1523-1755.2001.0590041257.x. [DOI] [PubMed] [Google Scholar]
- 94.Johren O, Dendorfer A, Dominiak P. Cardiovascular and renal function of angiotensin II type-2 receptors. Cardiovasc Res. 2004;62:460–7. doi: 10.1016/j.cardiores.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 95.Hornig B, Landmesser U, Kohler C, et al. Comparative effect of ace inhibition and angiotensin II type 1 receptor antagonism on bioavailability of nitric oxide in patients with coronary artery disease: role of superoxide dismutase. Circulation. 2001;103:799–805. doi: 10.1161/01.cir.103.6.799. [DOI] [PubMed] [Google Scholar]
- 96.Guthikonda S, Haynes WG. Homocysteine: role and implications in atherosclerosis. Curr Atheroscler Rep. 2006;8:100–6. doi: 10.1007/s11883-006-0046-4. [DOI] [PubMed] [Google Scholar]
- 97.Herrmann M, Taban-Shomal O, Hubner U, Bohm M, Herrmann W. A review of homocysteine and heart failure. Eur J Heart Fail. 2006;8:571–6. doi: 10.1016/j.ejheart.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 98.Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337:230–6. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 99.Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest. 1996;98:5–7. doi: 10.1172/JCI118776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weiss N, Heydrick SJ, Postea O, Keller C, Keaney JF, Jr, Loscalzo J. Influence of hyperhomocysteinemia on the cellular redox state–impact on homocysteine-induced endothelial dysfunction. Clin Chem Lab Med. 2003;41:1455–61. doi: 10.1515/CCLM.2003.223. [DOI] [PubMed] [Google Scholar]
- 101.Wilcken DE, Wang XL, Adachi T, et al. Relationship between homocysteine and superoxide dismutase in homocystinuria: possible relevance to cardiovascular risk. Arterioscler Thromb Vasc Biol. 2000;20:1199–202. doi: 10.1161/01.atv.20.5.1199. [DOI] [PubMed] [Google Scholar]
- 102.Wang XL, Adachi T, Sim AS, Wilcken DE. Plasma extracellular superoxide dismutase levels in an Australian population with coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;18:1915–21. doi: 10.1161/01.atv.18.12.1915. [DOI] [PubMed] [Google Scholar]
- 103.Yamamoto M, Hara H, Adachi T. Effects of homocysteine on the binding of extracellular-superoxide dismutase to the endothelial cell surface. FEBS Lett. 2000;486:159–62. doi: 10.1016/s0014-5793(00)02260-2. [DOI] [PubMed] [Google Scholar]
- 104.Nihei S, Tasaki H, Yamashita K, et al. Hyperhomocysteinemia is associated with human coronary atherosclerosis through the reduction of the ratio of endothelium-bound to basal extracellular superoxide dismutase. Circ J. 2004;68:822–8. doi: 10.1253/circj.68.822. [DOI] [PubMed] [Google Scholar]
- 105.Nonaka H, Tsujino T, Watari Y, Emoto N, Yokoyama M. Taurine prevents the decrease in expression and secretion of extracellular superoxide dismutase induced by homocysteine: amelioration of homocysteine-induced endoplasmic reticulum stress by taurine. Circulation. 2001;104:1165–70. doi: 10.1161/hc3601.093976. [DOI] [PubMed] [Google Scholar]
- 106.Kimura F, Hasegawa G, Obayashi H, et al. Serum extracellular superoxide dismutase in patients with type 2 diabetes: relationship to the development of micro- and macrovascular complications. Diabetes Care. 2003;26:1246–50. doi: 10.2337/diacare.26.4.1246. [DOI] [PubMed] [Google Scholar]
- 107.Navab M, Anantharamaiah GM, Reddy ST, et al. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 2004;109:3215–20. doi: 10.1161/01.CIR.0000134275.90823.87. [DOI] [PubMed] [Google Scholar]
- 108.Kruger AL, Peterson S, Turkseven S, et al. D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation. 2005;111:3126–34. doi: 10.1161/CIRCULATIONAHA.104.517102. [DOI] [PubMed] [Google Scholar]
- 109.Stralin P, Marklund SL. Effects of oxidative stress on expression of extracellular superoxide dismutase, CuZn-superoxide dismutase and Mn-superoxide dismutase in human dermal fibroblasts. Biochem J. 1994;298:347–52. doi: 10.1042/bj2980347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stralin P, Marklund SL. Multiple cytokines regulate the expression of extracellular superoxide dismutase in human vascular smooth muscle cells. Atherosclerosis. 2000;151:433–41. doi: 10.1016/s0021-9150(99)00427-x. [DOI] [PubMed] [Google Scholar]
- 111.Marklund SL. Regulation by cytokines of extracellular superoxide dismutase and other superoxide dismutase isoenzymes in fibroblasts. J Biol Chem. 1992;267:6696–701. [PubMed] [Google Scholar]
- 112.Hink HU, Santanam N, Dikalov S, et al. Peroxidase properties of extracellular superoxide dismutase: role of uric acid in modulating in vivo activity. Arterioscler Thromb Vasc Biol. 2002;22:1402–8. doi: 10.1161/01.atv.0000027524.86752.02. [DOI] [PubMed] [Google Scholar]
- 113.Jung O, Marklund SL, Xia N, Busse R, Brandes RP. Inactivation of extracellular superoxide dismutase contributes to the development of high-volume hypertension. Arterioscler Thromb Vasc Biol. 2007;27:470–7. doi: 10.1161/01.ATV.0000254823.15843.1f. [DOI] [PubMed] [Google Scholar]
- 114.Adachi T, Ohta H, Yamada H, Futenma A, Kato K, Hirano K. Quantitative analysis of extracellular-superoxide dismutase in serum and urine by ELISA with monoclonal antibody. Clin Chim Acta. 1992;212:89–102. doi: 10.1016/0009-8981(92)90176-q. [DOI] [PubMed] [Google Scholar]
- 115.Sandstrom J, Nilsson P, Karlsson K, Marklund SL. 10-fold increase in human plasma extracellular superoxide dismutase content caused by a mutation in heparin-binding domain. J Biol Chem. 1994;269:19163–6. [PubMed] [Google Scholar]
- 116.Adachi T, Yamada H, Yamada Y, et al. Substitution of glycine for arginine-213 in extracellular-superoxide dismutase impairs affinity for heparin and endothelial cell surface. Biochem J. 1996;313:235–9. doi: 10.1042/bj3130235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Folz RJ, Peno-Green L, Crapo JD. Identification of a homozygous missense mutation (Arg to Gly) in the critical binding region of the human EC-SOD gene (SOD3) and its association with dramatically increased serum enzyme levels. Hum Mol Genet. 1994;3:2251–4. doi: 10.1093/hmg/3.12.2251. [DOI] [PubMed] [Google Scholar]
- 118.Petersen SV, Olsen DA, Kenney JM, et al. The high concentration of Arg213—>Gly extracellular superoxide dismutase (EC-SOD) in plasma is caused by a reduction of both heparin and collagen affinities. Biochem J. 2005;385:427–32. doi: 10.1042/BJ20041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Adachi T, Morihara N, Yamazaki N, et al. An arginine-213 to glycine mutation in human extracellular-superoxide dismutase reduces susceptibility to trypsin-like proteinases. J Biochem (Tokyo) 1996;120:184–8. doi: 10.1093/oxfordjournals.jbchem.a021383. [DOI] [PubMed] [Google Scholar]
- 120.Yamada H, Yamada Y, Adachi T, et al. Protective role of extracellular superoxide dismutase in hemodialysis patients. Nephron. 2000;84:218–23. doi: 10.1159/000045580. [DOI] [PubMed] [Google Scholar]
- 121.Nakamura M, Ando Y, Sasada K, et al. Role of extracellular superoxide dismutase in patients under maintenance hemodialysis. Nephron Clin Pract. 2005;101:109–15. doi: 10.1159/000086644. [DOI] [PubMed] [Google Scholar]
- 122.Juul K, Tybjaerg-Hansen A, Marklund S, et al. Genetically reduced antioxidative protection and increased ischemic heart disease risk: The Copenhagen City Heart Study. Circulation. 2004;109:59–65. doi: 10.1161/01.CIR.0000105720.28086.6C. [DOI] [PubMed] [Google Scholar]
- 123.Tamai M, Furuta H, Kawashima H, et al. Extracellular super-oxide dismutase gene polymorphism is associated with insulin resistance and the susceptibility to type 2 diabetes. Diabetes Res Clin Pract. 2006;71:140–5. doi: 10.1016/j.diabres.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 124.Campo S, Sardo AM, Campo GM, et al. Extracellular superoxide dismutase (EC-SOD) gene mutations screening in a sample of Mediterranean population. Mutat Res. 2005;578:143–8. doi: 10.1016/j.mrfmmm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 125.Yamada H, Yamada Y, Adachi T, et al. Polymorphism of extracellular superoxide dismutase (EC-SOD) gene: relation to the mutation responsible for high EC-SOD level in serum. Jpn J Hum Genet. 1997;42:353–6. doi: 10.1007/BF02766958. [DOI] [PubMed] [Google Scholar]