Abstract
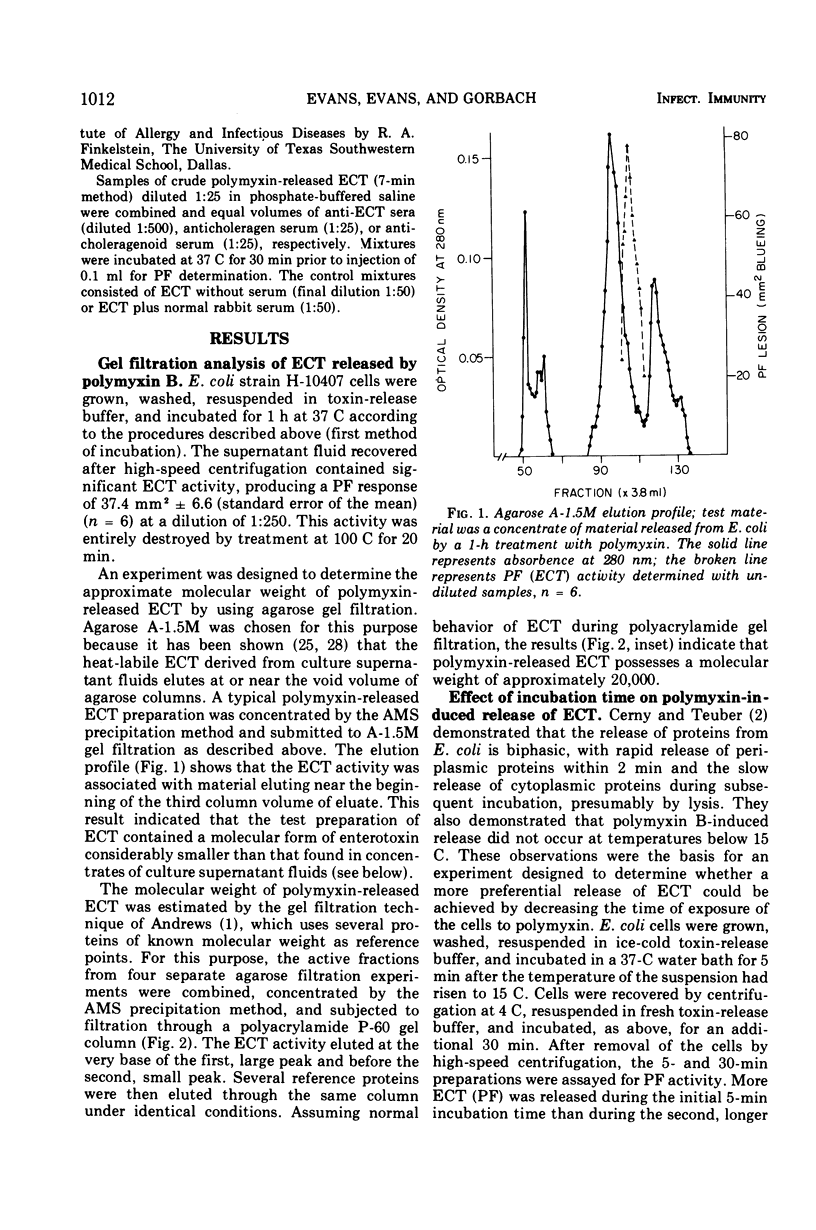
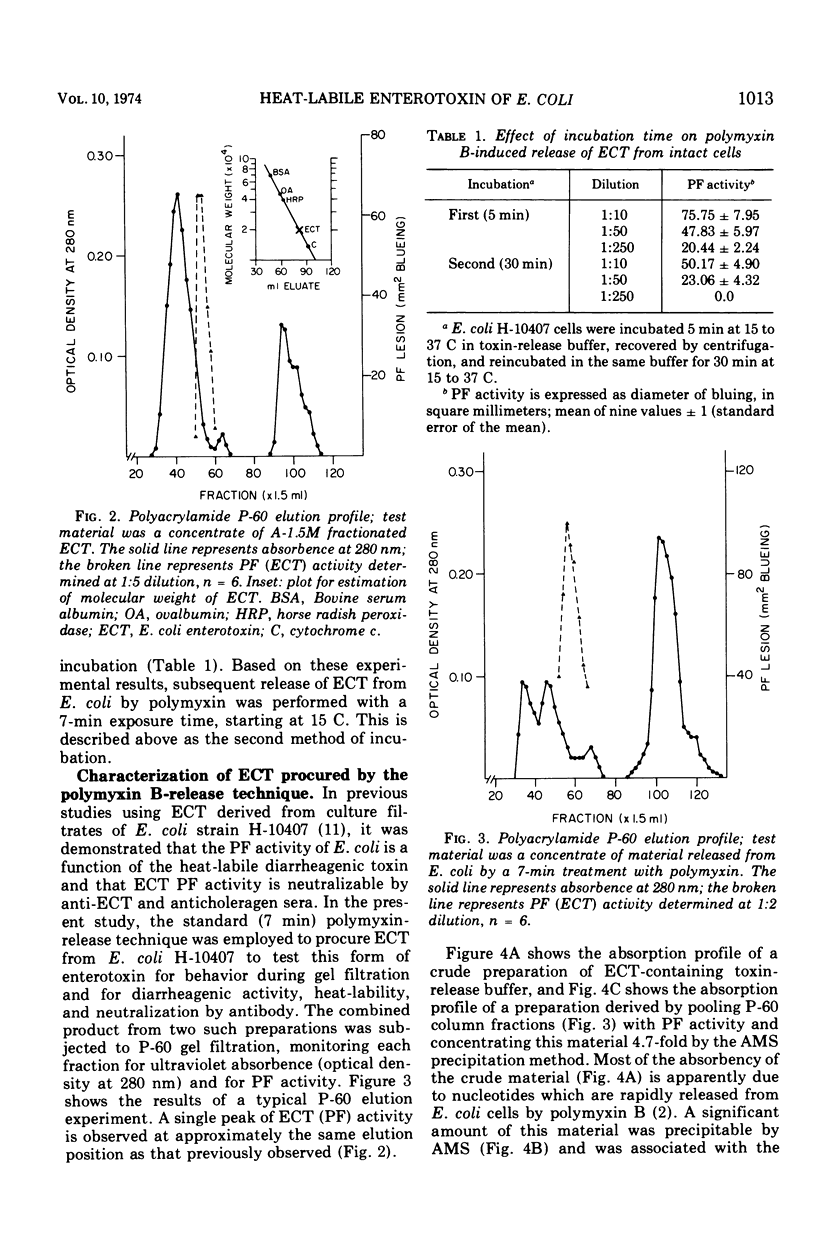
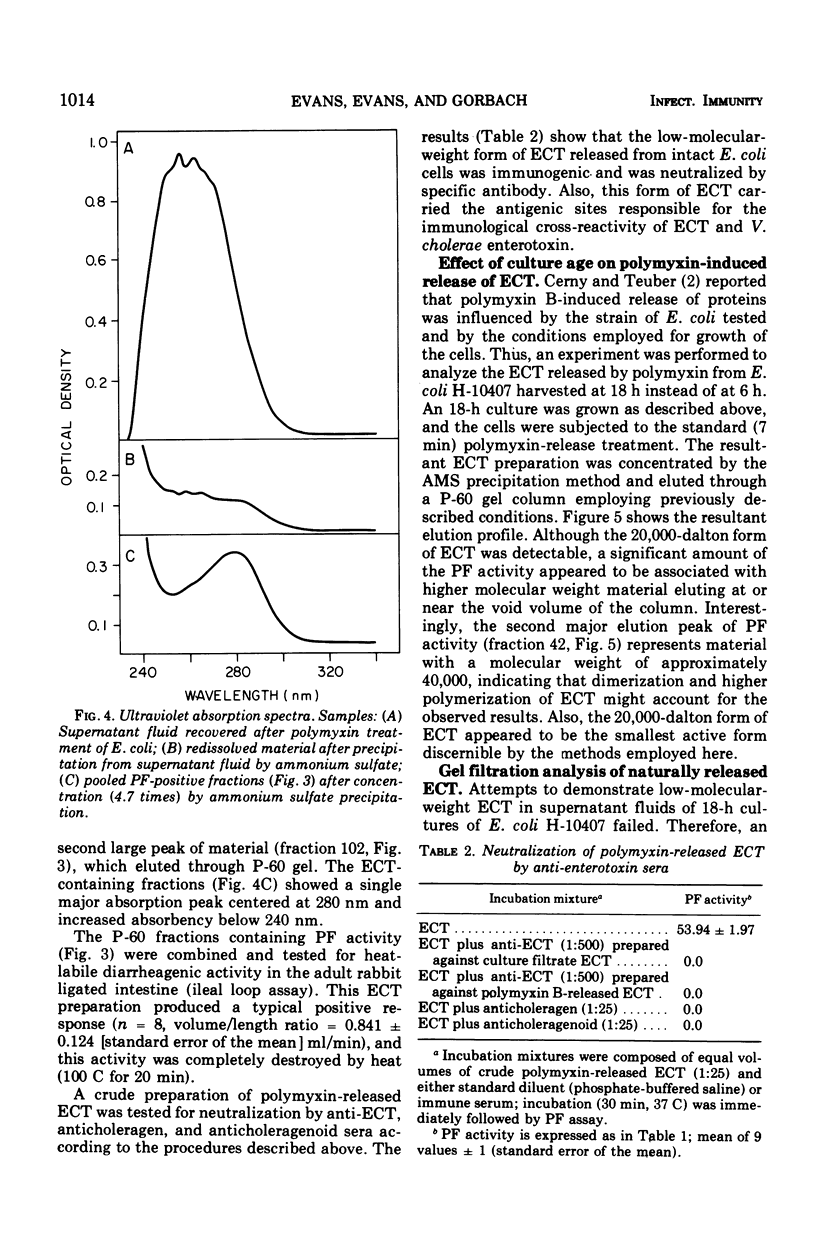
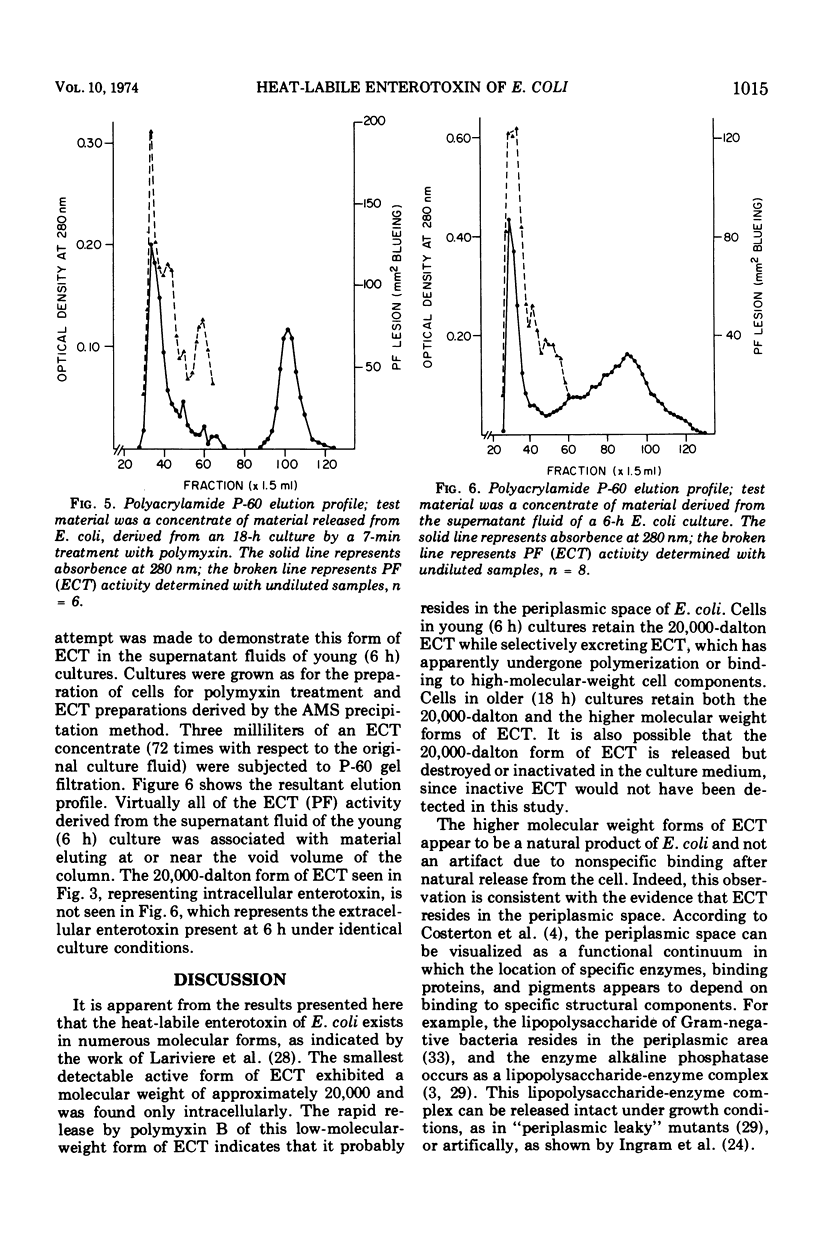
Polymyxin B-induced release of enterotoxin from Escherichia coli strain H-10407 was demonstrated. Incubation of E. coli cells derived from 6-h cultures with polymyxin caused the rapid release of enterotoxin with a molecular weight of approximately 20,000, as estimated by the gel filtration technique. The rapidity of the release of enterotoxin indicates that it probably resides in the periplasmic space of the cell. The low-molecular-weight enterotoxin possessed vascular permeability factor and diarrheagenic activities, both of which were found to be heat-labile. The permeability factor activity of this enterotoxin was neutralized by antisera prepared against crude E. coli enterotoxin, Vibrio cholerae enterotoxin (choleragen), and V. cholerae toxoid (choleragenoid), respectively. Supernatant fluids of 6-h E. coli cultures did not contain this molecular form of enterotoxin but did contain very high-molecular-weight, heat-labile enterotoxin. Incubation of cells derived from older (18 h) cultures with polymyxin caused the release of both low- (20,000) and high-molecular-weight forms of enterotoxin. We concluded that either the 20,000-dalton form of heat-labile enterotoxin is not released by E. coli under in vitro growth conditions or that enterotoxin released in this form is rapidly destroyed or inactivated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny G., Teuber M. Differential release of periplasmic versus cytoplasmic enzymes from Escherichia coli B by polymixin B. Arch Mikrobiol. 1971;78(2):166–179. doi: 10.1007/BF00424873. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Interactions of alkaline phosphatase and the cell wall of Pseudomonas aeruginosa. J Bacteriol. 1971 Jul;107(1):325–336. doi: 10.1128/jb.107.1.325-336.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- DuPont H. L., Formal S. B., Hornick R. B., Snyder M. J., Libonati J. P., Sheahan D. G., LaBrec E. H., Kalas J. P. Pathogenesis of Escherichia coli diarrhea. N Engl J Med. 1971 Jul 1;285(1):1–9. doi: 10.1056/NEJM197107012850101. [DOI] [PubMed] [Google Scholar]
- Etkin S., Gorbach S. L. Studies on enterotoxin from Escherichia coli associated with acute diarrhea in man. J Lab Clin Med. 1971 Jul;78(1):81–87. [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Gorbach S. L. Identification of enterotoxigenic Escherichia coli and serum antitoxin activity by the vascular permeability factor assay. Infect Immun. 1973 Nov;8(5):731–735. doi: 10.1128/iai.8.5.731-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Chen L. C., Curlin G. T., Evans D. G. Stimulation of adenyl cyclase by Escherichia coli enterotoxin. Nat New Biol. 1972 Apr 5;236(66):137–138. doi: 10.1038/newbio236137a0. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Gorbach S. L. Production of vascular permeability factor by enterotoxigenic Escherichia coli isolated from man. Infect Immun. 1973 Nov;8(5):725–730. doi: 10.1128/iai.8.5.725-730.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G. Three characteristics associated with enterotoxigenic Escherichia coli isolated from man. Infect Immun. 1973 Sep;8(3):322–328. doi: 10.1128/iai.8.3.322-328.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Crystalline cholera toxin and toxoid. Science. 1972 Feb 4;175(4021):529–530. doi: 10.1126/science.175.4021.529. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A. Monospecific equine antiserum against cholera exo-enterotoxin. Infect Immun. 1970 Dec;2(6):691–697. doi: 10.1128/iai.2.6.691-697.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Peterson J. W., Lospalluto J. J. Conversion of cholera exo-enterotoxin (choleragen) to natural toxoid (choleragenoid). J Immunol. 1971 Mar;106(3):868–871. [PubMed] [Google Scholar]
- Gorbach S. L., Banwell J. G., Chatterjee B. D., Jacobs B., Sack R. B. Acute undifferentiated human diarrhea in the tropics. I. Alterations in intestinal micrflora. J Clin Invest. 1971 Apr;50(4):881–889. doi: 10.1172/JCI106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach S. L., Khurana C. M. Toxigenic Escherichia coli: a cause of infantile diarrhea in Chicago. N Engl J Med. 1972 Oct 19;287(16):791–795. doi: 10.1056/NEJM197210192871603. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Ganguly U., Casper A. G., Moore E. J., Pierce N. F., Carpenter C. C. Effect of Escherichia coli on fluid transport across canine small bowel. Mechanism and time-course with enterotoxin and whole bacterial cells. J Clin Invest. 1973 Jul;52(7):1707–1714. doi: 10.1172/JCI107352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyles C. L., Barnum D. A. A heat-labile enterotoxin from strains of Eschericha coli enteropathogenic for pigs. J Infect Dis. 1969 Oct;120(4):419–426. doi: 10.1093/infdis/120.4.419. [DOI] [PubMed] [Google Scholar]
- Gyles C. L. Immunological study of the heat-labile enterotoxins of Escherichia coli and Vibrio cholerae. Infect Immun. 1974 Mar;9(3):564–570. doi: 10.1128/iai.9.3.564-570.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Söderlind O., Wadström T. Cross-reactivity between heat labile enterotoxins of Vibrio cholerae and Escherichia coli in neutralization tests in rabbit ileum and skin. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Dec;81(6):757–762. doi: 10.1111/j.1699-0463.1973.tb02272.x. [DOI] [PubMed] [Google Scholar]
- Ingram J. M., Cheng K. J., Costerton J. W. Alkaline phosphatase of Pseudomonas aeruginosa: the mechanism of secretion and release of the enzyme from whole cells. Can J Microbiol. 1973 Nov;19(11):1407–1415. doi: 10.1139/m73-227. [DOI] [PubMed] [Google Scholar]
- Jacks T. M., Wu B. J., Braemer A. C., Bidlack D. E. Properties of the enterotoxic component in Escherichia coli enteropathogenic for swine. Infect Immun. 1973 Feb;7(2):178–189. doi: 10.1128/iai.7.2.178-189.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor H. S., Tao P., Gorbach S. L. Stimulation of intestinal adenyl cyclase by Escherichia coli enterotoxin: comparison of strains from an infant and an adult with diarrhea. J Infect Dis. 1974 Jan;129(1):1–9. doi: 10.1093/infdis/129.1.1. [DOI] [PubMed] [Google Scholar]
- Larivière S., Gyles C. L., Barnum D. A. A comparative study of the rabbit and pig gut loop systems for the assay of Escherichia coli enterotoxin. Can J Comp Med. 1972 Oct;36(4):319–328. [PMC free article] [PubMed] [Google Scholar]
- Lariviére S., Gyles C. L., Barnum D. A. Preliminary characterization of the heat-labile enterotoxin of Escherichia coli F11(P155). J Infect Dis. 1973 Sep;128(3):312–320. doi: 10.1093/infdis/128.3.312. [DOI] [PubMed] [Google Scholar]
- Lindsay S. S., Wheeler B., Sanderson K. E., Costerton J. W., Cheng K. J. The release of alkaline phosphatase and of lipopolysaccharide during the growth of rough and smooth strains of Salmonella typhimurium. Can J Microbiol. 1973 Mar;19(3):335–343. doi: 10.1139/m73-056. [DOI] [PubMed] [Google Scholar]
- Lospalluto J. J., Finkelstein R. A. Chemical and physical properties of cholera exo-enterotoxin (choleragen) and its spontaneously formed toxoid (choleragenoid). Biochim Biophys Acta. 1972 Jan 26;257(1):158–166. doi: 10.1016/0005-2795(72)90265-6. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Greenough W. B., 3rd, Carpenter C. C., Jr Vibrio cholerae enterotoxin and its mode of action. Bacteriol Rev. 1971 Mar;35(1):1–13. doi: 10.1128/br.35.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B. Immunization with Escherichia coli enterotoxin protects against homologous enterotoxin challenge. Infect Immun. 1973 Oct;8(4):641–644. doi: 10.1128/iai.8.4.641-644.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shands J. W. Localization of somatic antigen on gram-negative bacteria using ferritin antibody conjugates. Ann N Y Acad Sci. 1966 Jun 30;133(2):292–298. doi: 10.1111/j.1749-6632.1966.tb52372.x. [DOI] [PubMed] [Google Scholar]
- Skerman F. J., Formal S. B., Falkow S. Plasmid-associated enterotoxin production in a strain of Escherichia coli isolated from humans. Infect Immun. 1972 Apr;5(4):622–624. doi: 10.1128/iai.5.4.622-624.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Gyles C. L. The relationship between two apparently different enterotoxins produced by enteropathogenic strains of Escherichia coli of porcine origin. J Med Microbiol. 1970 Aug;3(3):387–401. doi: 10.1099/00222615-3-3-387. [DOI] [PubMed] [Google Scholar]
- Smith H. W. The production of diarrhoea in baby rabbits by the oral administration of cell-free preparations of enteropathogenic Escherichia coli and Vibrio cholerae: the effect of antisera. J Med Microbiol. 1972 Aug;5(3):299–303. doi: 10.1099/00222615-5-3-299. [DOI] [PubMed] [Google Scholar]
- Smith N. W., Sack R. B. Immunologic cross-reactions of enterotoxins from Escherichia coli and Vibrio cholerae. J Infect Dis. 1973 Feb;127(2):164–170. doi: 10.1093/infdis/127.2.164. [DOI] [PubMed] [Google Scholar]


