Abstract
Heterotypic and homotypic cellular interactions are essential for biological function, and co-culture models are versatile tools for investigating these cellular interactions in vitro. Physiologically relevant co-culture models have been used to elucidate the effects of cell-cell physical contact and/or secreted factors, as well as the influence of substrate geometry and interaction scale on cell response. Identifying the relative contribution of each cell population to co-culture is often experimentally challenging for these cellular interactions studies. In this issue of Biotechnology Journal, Hamilton et al. [1] report on a hydrogel-based co-culture system, that enables paracrine interactions. A simple and elegant method for enzymatic separation of cell populations post co-culture is introduced, thereby enhancing the ease for post-culture analysis of the effects of co-culture on individual cell populations.
The formation, maintenance, and repair of biological tissues rely on the interaction of cells with other cell types and with their extracellular matrix [2, 3]. These cellular communications can be bi-directional or multi-dimensional and may occur at both macro-and micro-scales. Depending on the hypothesis of interest, co-culture models are used to discern the individual and collective effects of physical contact and soluble factors via paracrine signaling [4].
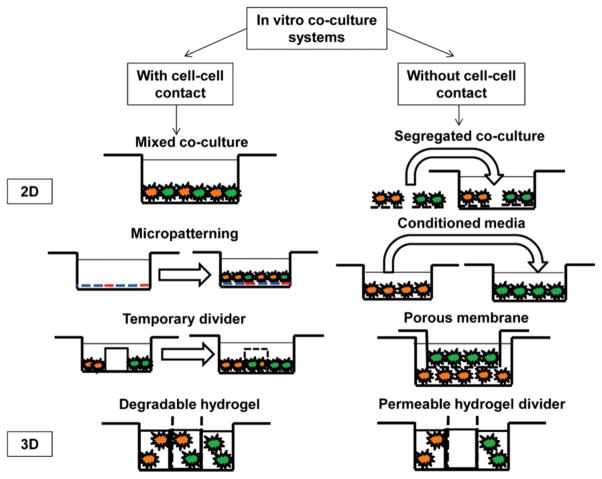
The simplest co-culture system that permits physical contact between cells consists of a mixed monolayer of the cell types of interest [5, 6]. This is achieved by combining the different cell suspensions at the desired co-culture ratio prior to seeding [7] (Fig. 1). The mixed monolayer model maximizes local heterotypic interactions, and can be used to control the relative levels of heterotypic and homotypic communication by altering the seeding densities of each cell type. Interpretation of mixed monolayer co-culture results must take into account a possible dilution effect due to mixed culture, as well as any metabolic differences between cell types. Moreover, the relative contribution of each cell population to any observed effects is not readily discernible in this model. Cell-cell contact can also be controlled by establishing physical barriers, which are used to regulate spatial and temporal cell seeding patterns in co-culture (Fig. 1). The divider may later be removed to permit cell migration and controlled cell-cell contact [6]. This model is advantageous because it exercises greater control over the extent of heterotypic and homotypic interactions, while permitting both physical contact and soluble factor interactions. The temporary divider system is, however, experimentally more challenging, as a complete seal between the individual cell compartments is required. Moreover, cell response and soluble factor transport in this model depends on the properties of the divider.
Figure 1.
Schematic of 2D and 3D culture systems used to evaluate cell-cell interactions.
In studies where paracrine interaction is of greater interest, a segregated co-culture system may be established by first forming individual cultures of each cell type, and later co-culturing them in the same environment (Fig. 1). In contrast to the mixed co-culture model, the primary advantage of this system is that the individual response of each subpopulation of cells can be analyzed; however, a potential disadvantage of the segregated co-culture model is that physical contact cannot be completely prevented in the long term, and the multi-stage cell-seeding procedure is cumbersome. The advent of cell culture membrane inserts reduced many of the experimental difficulties associated with paracrine co-culture (Fig. 1). This model is reproducible, with the ability to identify effects of co-culture on individual populations, although the effects of soluble factors detected are uni-directional. Moreover, extensive cell growth can cover the pores of the inserts, limiting cellular interactions, which may result in an insignificant co-culture response. Another widely utilized method for determining soluble factor effects is through conditioned media studies, during which the culture media from one cell type is introduced into the culture of the second cell type [8] (Fig. 1). The advantages of conditioned media include its simplicity in allowing for the detection of any soluble factor-related effects, along with the potential for subsequent identification of these factors in the co-culture media. An inherent limitation is the issue of nutrient deficiency, as well as difficulty in reproducing the optimal concentrations and temporal distribution of these secreted factors.
It is emphasized that for these aforementioned co-culture models, regardless of the scale of interactions, and whether the hypothesis tested centers on cell-cell contact or paracrine signaling, it is essential to be able to separate cell types following co-culture, in order to examine changes in behavior for each population individually. For paracrine signaling studies, this is often accomplished through segregation of cell populations using a barrier or conditioned media studies. For cell-cell contact studies however, the process of separation following co-culture in the models described above becomes more complicated. Another area of interest in co-culture is the need for 3D culture models, which are physiological and can take into consideration the contribution of the extracellular matrix. These 3D models for multi-culture allow for spatial control over cell distribution and lead to biomimetic cell templating [9]. These interactions can be studied and controlled on the micro- and macro-scale, adding another tier of sophistication to the culture system [10].
In this issue, Hamilton et al. [1] develop a novel 3D hydrogel-based co-culture model, with applications in the study of the effects of co-culture both with and without cell-cell contact. With this 3D system, it is possible to control the spatial patterning and temporal distribution of distinct cell populations, and most importantly, achieve on-demand separation of cell types through the use of enzyme-degradable adhesives. For example, layers of polyethylene glycol-diacrylate (PEGDA)-based hydrogel were first joined together with a chondroitin sulfate methacrylate adhesive, and after culture, chondroitinase ABC solution was introduced to enzymatically digest away the adhesive and separate the cell-laden hydrogel layers. Through a series of proof-of-concept experiments, the authors convincingly demonstrated the ability of this system to segregate cell types while preserving cell viability. Furthermore, the 3D-layered hydrogel system offers design flexibility, in terms of both geometry and structure.
In summary, multi-scale and multidimensional cellular interactions are essential for organ homeostasis, repair and regeneration. Biomimetic co-culture models such as those described by Hamilton et al. [1] are insightful tools for deciphering the relative contributions of cell-cell contact and/or soluble factors, in conjunction with substrate geometry, as well as interaction scale.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Hamilton SK, Bloodworth NC, Massad CS, Hammoudi TM, et al. Development of 3D hydrogel culture systems with on-demand cell separation. Biotechnol J. 2013;8:485–495. doi: 10.1002/biot.201200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu HH, Jiang J. Interface tissue engineering and the formulation of multiple tissue systems. Adv Biochem Eng Biotechnol. 2006;102:91–111. [PubMed] [Google Scholar]
- 3.Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 4.Lu HH, Wang IE. Multi-scale Co-Culture Models for Orthopaedic Interface Tissue Engineering. In: Gonsalves KE, et al., editors. Biomedical Nanostructures. John Wiley & Sons, Inc; 2007. [Google Scholar]
- 5.Proffen BL, Haslauer CM, Harris CE, Murray MM. Mesenchymal stem cells from the retropatellar fat pad and peripheral blood stimulate ACL fibroblast migration, proliferation, and collagen gene expression. Connect Tiss Res. 2013;54:14–21. doi: 10.3109/03008207.2012.715701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang IE, Shan J, Choi R, Oh S, et al. Role of Osteoblast-Fibroblast Interactions in the Formation of the Ligament-to-Bone Interface. J Orthop Res. 2007;25:1609–1620. doi: 10.1002/jor.20475. [DOI] [PubMed] [Google Scholar]
- 7.Jiang J, Nicoll SB, Lu HH. Co-culture of osteoblasts and chondrocytes modulates cellular differentiation in vitro. Biochem Biophys Res Commun. 2005;338:762–770. doi: 10.1016/j.bbrc.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Jiang J, Leong NL, Mung J, Hidaka C, Lu HH. Interaction between zonal populations of articular chondrocytes suppresses chondrocyte mineralization and this process is mediated by PTHrP. Osteoarthritis Cartilage. 2008;16:70–82. doi: 10.1016/j.joca.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Spalazzi JP, Doty SB, Moffat KL, Levine WN, Lu HH. Development of Controlled Matrix Heterogeneity on a Triphasic Scaffold Orthopedic Interface Tissue Engineering. Tissue Eng. 2008;12:3497–508. doi: 10.1089/ten.2006.12.3497. [DOI] [PubMed] [Google Scholar]
- 10.Khademhosseini A, Suh KY, Yang JM, Eng G, et al. Layer-by-layer deposition of hyaluronic acid and poly-L-lysine for patterned cell co-cultures. Biomaterials. 2004;25:3583–3592. doi: 10.1016/j.biomaterials.2003.10.033. [DOI] [PubMed] [Google Scholar]