Abstract
Diabetic nephropathy (DN) affects an estimated 20%–40% of patients with type 2 diabetes mellitus (T2DM). Key modifiable risk factors for DN are albuminuria, anaemia, dyslipidaemia, hyperglycaemia and hypertension, together with lifestyle factors, such as smoking and obesity. Early detection and treatment of these risk factors can prevent DN or slow its progression, and may even induce remission in some patients. DN is generally preceded by albuminuria, which frequently remains elevated despite treatment in patients with T2DM. Optimal treatment and prevention of DN may require an early, intensive, multifactorial approach, tailored to simultaneously target all modifiable risk factors. Regular monitoring of renal function, including urinary albumin excretion, creatinine clearance and glomerular filtration rate, is critical for following any disease progression and making treatment adjustments. Dipeptidyl peptidase (DPP)-4 inhibitors and sodium-glucose cotransporter 2 (SGLT2) inhibitors lower blood glucose levels without additional risk of hypoglycaemia, and may also reduce albuminuria. Further investigation of the potential renal benefits of DPP-4 and SGLT2 inhibitors is underway.
Keywords: Diabetic nephropathy, incretin, renal impairment, type 2 diabetes, glomerular filtration rate
Introduction
The overall prevalence of diabetes mellitus, of which the vast majority of cases are type 2 diabetes mellitus (T2DM), is expected to increase worldwide from an estimated 382 million in 2013 (8.3% of the adult population) to 592 million by 2035 (10.0%).1 Diabetic nephropathy (DN), which carries a heavy clinical and economic burden, is present in up to 40% of patients with T2DM.2
Key modifiable risk factors for DN include hypertension, hyperglycaemia, dyslipidaemia, anaemia, albuminuria and lifestyle factors such as obesity and smoking.3 The early identification of key risk factors and prompt therapeutic intervention can potentially prevent or slow the decline in renal function in patients with T2DM.4
The primary aim of this review article is to discuss the current understanding of the association between T2DM and renal impairment (RI), and to review the management of risk factors for DN. A further aim is to examine the potential for improved prevention and treatment of DN, including the use of drugs that provide direct protection from diabetes-related end-organ damage in addition to risk factor control.
Association of DN and T2DM
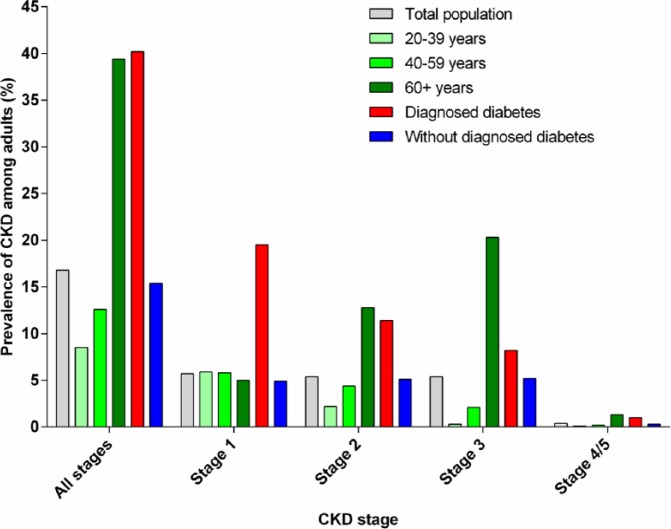
Chronic kidney disease (CKD) is associated with various risk factors, including cardiovascular disease, obesity and diabetes. Between 1999 and 2004, the prevalence of CKD within the general US population was estimated to be 16.8% (Figure 1);5 the most recent prevalence estimate for 2007–2012 is 15.0%.6 CKD is more prevalent in those with diabetes and older patients (Figure 1).5 Worldwide, the reported prevalence rates vary widely from 2.5% in China to 35.8% in Finland (Table 1).33
Figure 1.
Prevalence of CKD among US adults by disease stage – National Health and Nutrition Examination Survey (NHANES), United States, 1999–2004.
Data source: Saydah et al.5
CKD: chronic kidney disease.
Table 1.
Prevalence of chronic kidney disease (CKD) in population-based studies in Northern/Central America, Europe and Australia/Asia.
| Author (year) | Country | Study population, study design, number of participants, response, age and sex | Prevalence of CKD |
|||||
|---|---|---|---|---|---|---|---|---|
| MDRD equation | CG/BSA equation | |||||||
| Northern and Central America | ||||||||
| Amato et al. (2005)7 | Mexico | Randomly selected participants from primary-care facilities in a large city, cross-sectional study, N = 3564, response NR, aged >18 years, sex NR | NR | Overall: 8.5% | ||||
| Brown et al. (2003)8 | United States | Participants of the Kidney Early Evaluation Program (KEEP) in 33 states, cross-sectional study, N = 6071, response NR, aged 18–101 years (mean age: 52 years), 32% males | Overall: 15.6% Men: 14.4%, Women: 16.2% |
NR | ||||
| Age (years) | Men (%) | Women (%) | ||||||
| 18–30 | 2.4 | 2.7 | ||||||
| 31–45 | 5.4 | 6.4 | ||||||
| 46–60 | 9.5 | 11.5 | ||||||
| 61–75 | 24.3 | 29.9 | ||||||
| 76+ | 45.6 | 45.0 | ||||||
| Coresh et al. (2005)9 | United States | Participants of the Third National Health and Nutrition Examination Survey (NHANES III, 1999–2000), cross-sectional study, N = 4101, response NR, aged ≥20 years, 47.7% males | Overall: 3.8% Men: 2.7%, Women: 4.8% White: 4.2%, African American: 3.4%, Mexican American: 1.2%, other: 3.2% |
NR | ||||
| Age (years) | All (%) | |||||||
| 20–39 | 0.5 | |||||||
| 40–59 | 1.5 | |||||||
| 60–69 | 6.2 | |||||||
| 70+ | 23.1 | |||||||
| Coresh et al. (2003)10 | United States | Participants of the Third National Health and Nutrition Examination Survey (NHANES III 1988–1994), cross-sectional study, N = 15,600, response NR, aged ≥20 years, 47% males | Overall: 4.5% Men: 3.6%, Women: 5.3% White: 5.0%, African American: 3.3%, Mexican American: 1.0%, other: 2.2% |
Overall: 7.0% Men: 6.3%, Women: 7.7% White: 7.5%, African American: 7.8%, Mexican American: 1.8%, other: 4.1% |
||||
| Age (years) | All (%) | Age (years) | All (%) | |||||
| 20–39 | 0.2 | 20–39 | – | |||||
| 40–59 | 1.8 | 40–59 | 0.8 | |||||
| 60–69 | 7.6 | 60–69 | 10.5 | |||||
| 70+ | 24.9 | 70+ | 49.2 | |||||
| Fox et al. (2006)11 | United States | Participants from the sixth examination of the Framingham Offspring Study, cohort study, N = 3047, response NR, mean age: 59 years, 48% males | Overall: 8.6% | NR | ||||
| Garg et al. (2004)12 | Canada | Participants from long-term care facilities in the elderly, retrospective, cross-sectional study, N = 9931, response 85%, aged ≥65 years (mean age: 82 years), 26% males | Overall: 35.7% Men: 27.1%, Women: 38.8% |
NR | ||||
| Age (years) | Men (%) | Women (%) | ||||||
| 65–69 | 9.2 | 22.8 | ||||||
| 70–74 | 14.9 | 23.8 | ||||||
| 75–79 | 21.7 | 29.2 | ||||||
| 80–84 | 27.5 | 35.2 | ||||||
| 85–89 | 32.8 | 41.9 | ||||||
| 90–94 | 40.5 | 47.3 | ||||||
| 95+ | 37.8 | 50.7 | ||||||
| Hemmelgarn et al. (2006)13 | Canada | Participants from community-dwelling elderly in Calgary Health region, cohort study, N = 10,184, response NR, aged ≥66 years, 42.6% males | Overall: 35.4% Men: 32%, Women: 38.2% |
NR | ||||
| Kramer et al. (2005)14 | United States | Participants of the Dallas Heart Study, cross-sectional survey, N = 2660, response NR, aged 30–65 years (mean age: 43.9 years), 49.5% males | Overall: 1.5% | NR | ||||
| Manjunath et al. (2003)15 | United States | Participants of the Cardiovascular Health Study (CHS), cohort study, N = 4893, response NR, aged ≥65 years (mean age: 75.4 years), 44.5% males | Overall: 23.4% Men: 25%, Women: 22.2% |
NR | ||||
| McClellan et al. (2006)16 | United States | Randomly selected participants of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, cohort study, N = 20,667, response NR, aged ≥45 years, 48.8% males | Overall: 43.3% Men: 38.9%, Women: 47.5% White: 49.9%, African American: 33.7% |
NR | ||||
| Age (years) | All (%) | |||||||
| 45–54 | 19.3 | |||||||
| 55–64 | 31.6 | |||||||
| 65–74 | 51.3 | |||||||
| 75–84 | 62.7 | |||||||
| 85+ | 71.0 | |||||||
| Europe | ||||||||
| Brugts et al. (2005)17 | Netherlands | Participants of the Rotterdam Study, prospective cohort study, N = 4484, response 78%, aged ≥55 years (mean age 69.6 years), 36.3% males | NR | Overall: 44.9% | ||||
| Cirillo et al. (2006)18 | Italy | Participants from central Italy, cross-sectional study, N = 4574, response NR, aged 18–95 years, 45.5% males | Overall: 6.4% Men: 6.5%, Women: 6.2% |
NR | ||||
| Age (years) | Men (%) | Women (%) | ||||||
| 18–44 | 0.6 | 1.3 | ||||||
| 45–54 | 2.6 | 1.3 | ||||||
| 55–64 | 7.3 | 5.4 | ||||||
| 65–74 | 15.0 | 11.0 | ||||||
| 75+ | 34.5 | 31.6 | ||||||
| Hallan et al. (2006)19 | Norway | Participants of the second Health Survey of Nord-Trondelag County (HUNT II), cross-sectional study, N = 65,181, response 70.4%, aged ≥20 years (mean age: 50.2 years), 46.8% males | Overall: 4.7% Men: 3.6%, Women: 5.7% |
NR | ||||
| Age (years) | All (%) | |||||||
| 20–39 | 0.2 | |||||||
| 30–59 | 1.4 | |||||||
| 60–69 | 6.3 | |||||||
| 70+ | 18.6 | |||||||
| Nitsch et al. (2006)20 | Switzerland | Participants of the Swiss Study on Air Pollution and Lung Diseases in Adults (SAPALDIA), random sample, cross-sectional study, N = 6317, response NR, aged ≥18 years, 49% males | Overall: 8.1% Men: 4.5%, Women: 11.5% |
NR | ||||
| Age (years) | Men (%) | Women (%) | ||||||
| <55 | 1.1 | 7.9 | ||||||
| 55–65 | 7.1 | 23.5 | ||||||
| 66+ | 12.9 | 35.9 | ||||||
| Otero et al. (2005)21 | Spain | Randomly selected participants of the Estudio Epidemiológico de la Insuficiencia Renal en Espana (EPIRCE) pilot study, cross-sectional study, N = 237, response NR, aged ≥20 years (mean age: 49.6 years), 42.6% males | Overall: 5.1% | NR | ||||
| Verhave et al. (2004)22 | Netherlands | Participants of the Prevention of Renal and Vascular End-stage Disease Study, cohort study, N = 6022, response NR, aged 28–75 years (mean age: 48 years), 51.5% males | NR | Overall: 4.2% | ||||
| Viktorsdottir et al. (2005)23 | Iceland | Participants of the Reykjavik Heart Study, cross-sectional study, N = 19,256, response NR, aged 33–85 years, 48% males | Overall: 7.2% Men: 3.7%, Women: 10.9% |
Overall: 24.7% Men: 19%, Women: 30% |
||||
| Age (years) | Men (%) | Women (%) | ||||||
| 35–39 | 0.8 | 2.2 | ||||||
| 40–44 | 1.1 | 3.0 | ||||||
| 45–49 | 1.3 | 4.4 | ||||||
| 50–54 | 2.3 | 6.3 | ||||||
| 55–59 | 2.4 | 7.6 | ||||||
| 60–64 | 5.2 | 11.7 | ||||||
| 65–69 | 13.5 | 36.1 | ||||||
| 70–74 | 17.0 | 38.1 | ||||||
| 75–79 | 19.5 | 35.3 | ||||||
| 80+ | 24.5 | 53.1 | ||||||
| Wasen et al. (2004)24 | Finland | Participants from elderly residents in a community, cross-sectional study, N = 1246, response 83%, age 64–100 years (mean age: 74 years), 42% males | Overall: 35.8% | Overall: 58.5% | ||||
| Asia and Australia | ||||||||
| Chadban et al. (2003)25 | Australia | Randomly selected participants of the Australian Diabetes, Obesity and Lifestyle Study (AusDiab), cross-sectional survey, N = 11,247, response 89.1%, aged ≥25 years, sex NR | NR | Overall: 11.2% Men: 9.3%, Women: 13.0% |
||||
| Age (years) | Men (%) | Women (%) | ||||||
| 25–44 | – | – | ||||||
| 45–64 | 1.8 | 3.2 | ||||||
| 65+ | 51.8 | 57.2 | ||||||
| Chen et al. (2005)26 | China | Participants of the International Collaborative Study of Cardiovascular Disease in Asia (InterASIA), random sample, cross-sectional study, N = 15,540, response 83.3%, aged 35–74 years, 48.5% males | Overall: 2.5% Men: 1.3%, Women: 3.8% |
Overall: 20.4% | ||||
| Age (years) | Men (%) | Women (%) | ||||||
| 35–44 | 0.2 | 1.2 | ||||||
| 45–54 | 0.7 | 2.7 | ||||||
| 55–64 | 1.6 | 6.4 | ||||||
| 65–74 | 5.8 | 10.4 | ||||||
| Domrongkitchaiporn et al. (2005)27 | Thailand | Participants of the Electricity Generating Authority of Thailand (EGAT) study, employees sample, cross-sectional study, N = 2967, response NR, aged 35–55 years, 76% males | Overall: 6.8% | NR | ||||
| Konta et al. (2006)28 | Japan | Participants of the Molecular Epidemiological Study, cross-sectional survey, N = 2321, response NR, aged >40 years (mean age: 64 years), 44.5% males | NR | Overall: 28.8% | ||||
| Li et al. (2006)29 | China | Participants from residents in a district of a large city, cross-sectional survey, N = 2310, response NR, aged ≥40 years, 49.5% males | Overall: 4.9% Men: 4.8%, Women: 5.0% |
NR | ||||
| Age (years) | Men (%) | Women (%) | ||||||
| 40–49 | – | 0.4 | ||||||
| 50–59 | 1.5 | 2.5 | ||||||
| 60–69 | 4.4 | 5.8 | ||||||
| 70+ | 10.6 | 12.9 | ||||||
| McDonald et al. (2003)30 | Australia | Participants from a coastal aboriginal community, cross-sectional study, N = 237, response NR, aged ≥18 years, 56% males | Overall: 12% | NR | ||||
| Ninomiya et al. (2005)31 | Japan | Participants from study of Cerebrovascular and cardiovascular Diseases, prospective cohort study, N = 2634, response 80.7%, aged ≥40 years, 42.1% males | Overall: 10.3% Men: 5.3%, Women: 13.8% |
NR | ||||
| Shankar et al. (2006)32 | Singapore | Participants from private census, cross-sectional study, N = 4898, response 81.1%, aged 43–86 years (mean age: 62.3 years), 44% males | Overall: 6.6% Men: 7.1%, Women: 6.2% |
NR | ||||
| Age (years) | All (%) | |||||||
| 43–59 | 1.8 | |||||||
| 60–69 | 6.5 | |||||||
| 70–79 | 11.5 | |||||||
| 80+ | 21.8 | |||||||
MDRD: simplified equation of the Modification of Diet in Renal Disease Study; CG/BSA: Cockcroft–Gault formula adjusted by body surface area; NR: not reported.
©2008 Zhang and Rothenbacher;33 licensee BioMed Central Ltd.
In the United States, an estimated 40% of adults living with T2DM experience some degree of CKD,34 and 20%–40% of this population presents with DN characterised by macroalbuminuria and a low glomerular filtration rate (GFR).2 Microalbuminuria precedes DN and has an estimated global prevalence of 39% among those with T2DM.35 In the Western world, diabetes is the most common cause of end-stage renal disease (ESRD), accounting for 44% of all ESRD patients in the United States.34
The pathophysiological mechanisms of DN in T2DM are multiple and complex.36 Early haemodynamic changes are followed by albumin leakage from the glomerular capillaries and structural changes in the kidney.3 Risk factors such as hyperglycaemia and hypertension activate inflammatory pathways, and patients with a genetic predisposition can progress to advanced-stage nephropathy.3
There are important distinctions between DN and other renal complications of diabetes. DN is defined as the presence of both persistent macroalbuminuria (albumin ≥300 mg/dL) and reduced GFR. In patients with diabetes, microalbuminuria (albumin = 30–299 mg/dL) alone does not indicate DN, but is associated with endothelial dysfunction, vascular inflammation, coagulation abnormalities, a high rate of non-dipping of nocturnal blood pressure (BP) and increased cardiovascular risk.3,37,38 In contrast, there are many patients with T2DM who have a low GFR in the presence of normal albuminuria levels.39
Key risk factors for DN in patients with T2DM
Non-modifiable risk factors of decreased renal function include longer diabetes duration, older age and genetic factors. Modifiable risk factors include anaemia, hypertension, hyperglycaemia, dyslipidaemia and albuminuria, as well as lifestyle factors, such as obesity and smoking.4,40 The early detection and treatment of modifiable renal risk factors can prevent or slow the progression of DN, and may even produce remission.41
Hypertension
Hypertension is a common comorbidity of patients with T2DM,42,43 with elevated BP playing a major role in the development and progression of DN.4 It is well established that intensive BP control slows the progression of kidney disease in patients with T2DM,4,44 and may even induce remission of renal structural and functional impairment.4
Hyperglycaemia
Observational studies have established a relationship between hyperglycaemia and the development of microvascular complications.36 Increasing levels of glycated haemoglobin (HbA1c) are associated with an increased incidence of kidney disease, even in the absence of a diagnosis of diabetes.45,46 Compared with standard glycaemic control, intensive lowering of HbA1c may prevent or slow the progression of kidney disease in patients with T2DM.47
Dyslipidaemia
Diabetic dyslipidaemia is characterised by high levels of triglycerides and low-density lipoprotein cholesterol, and low levels of high-density lipoprotein cholesterol. In some studies, treatment targeting dyslipidaemia reduced the progression of CKD,48 improved estimated GFR (eGFR) in patients with T2DM49 and stabilised kidney function in patients with cardiovascular disease.50 However, other studies have shown mixed findings regarding whether diabetic dyslipidaemia contributes to kidney disease.4
Albuminuria
The development of albuminuria is closely associated with the progression of kidney disease in patients with T2DM, even within the range of normoalbuminuria.4 Zoppini et al.40 demonstrated that albuminuria was the strongest predictor of annual eGFR decline, with the reduction of albuminuria having emerged as a novel therapeutic goal for renoprotection.4
A reduction in albuminuria can decrease the risk of renal endpoints in patients with T2DM.51 For example, it has been shown that for every 50% reduction in proteinuria during the first year, the risk for kidney failure was reduced by 56%.51 Thus, albuminuria can be effectively lowered with antihypertensive drugs that inhibit the renin–angiotensin–aldosterone system (RAAS), with these renoprotective effects observed at normoalbuminuric, microalbuminuric and overt albuminuria levels.
Anaemia
Reduced haemoglobin levels predict adverse renal outcomes, as was shown in a post hoc analysis of the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial.52 Anaemia frequently occurs early in the course of diabetic kidney disease, even before GFR is severely reduced, perhaps because renal interstitial damage and autonomic neuropathy lead to decreased erythropoietin production from peritubular fibroblasts.4 Early treatment of anaemia with erythropoietin may delay the onset and slow the progression of microvascular complications, including nephropathy.53
Obesity
Although the mechanisms are not fully understood, adverse haemodynamic, structural and functional changes have been observed in the kidneys of obese individuals;54 in those patients with T2DM, obesity contributes to the progression of CKD.54 Conversely, weight loss reduces macro- and microalbuminuria, and stabilises renal function in various populations, including patients with T2DM.55 This benefit may in part be due to reduced BP.
Smoking
Smokers with T2DM have an elevated risk of micro- and macroalbuminuria and reduced GFR.56 Heavy smoking increased the rate of GFR decline by 1.3 mL/min/year after adjusting for other risk factors.4 Continued smoking exacerbates, whereas cessation ameliorates, the progression of DN.56
Current management of risk factors for DN
Lifestyle modifications such as dietary changes and weight loss both improve BP, dyslipidaemia and obesity, whereas protein restriction may slow the progression of albuminuria and decline in GFR.42,43
Pharmacotherapy is increasingly important for controlling key risk factors for DN, including hyperglycaemia, hypertension and dyslipidaemia. Although guidelines exist,42,43 goals and treatments should be tailored to the individual patient, taking into consideration demographics, duration of T2DM, life expectancy, degree of CKD, comorbidities, body weight and the presence of other risk factors.
BP control
Aggressive antihypertensive treatment has dramatically improved renal outcomes and survival in patients with T2DM. A BP goal of <140/80 mm Hg is recommended in patients with T2DM, irrespective of kidney disease.42 Furthermore, BP lowering below these targets may provide additional renoprotection in patients with T2DM.
RAAS inhibitors, such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), have direct renoprotective effects in addition to lowering BP4 and are recommended as first-line treatments in hypertensive patients with T2DM, regardless of kidney disease.42,43 RAAS inhibition can reduce the early transition to renal complications,57 and can also protect in later stages, reducing the incidence of ESRD. However, RAAS inhibitors also reduce haemoglobin levels and may, therefore, aggravate anaemia. During RAAS inhibitor treatment, serum creatinine and potassium levels should be monitored to detect the development of acute kidney disease and hyperkalaemia.42
Glycaemic control
The primary target in glycaemic control is HbA1c, for which treatment goals have been established.42,43,58 Currently available glucose-lowering drug classes include the biguanides (metformin), sulphonylureas, thiazolidinediones, meglitinides, α-glucosidase inhibitors, pramlintide, incretin-based therapy (glucagon-like peptide-1 (GLP-1) analogues, dipeptidyl peptidase (DPP)-4 inhibitors) and sodium-glucose cotransporter 2 (SGLT2) inhibitors. The choice of glucose-lowering therapy is important to balance the benefits of antihyperglycaemic treatment with adverse effects, such as hypoglycaemia and weight gain. Oral glucose-lowering drugs that are eliminated by the kidneys are also a concern in patients with T2DM and CKD.43
Treatment of diabetic dyslipidaemia
Early treatment of dyslipidaemia may be important because lowering cholesterol has been shown to have little effect in patients with diabetes once DN has reached an advanced stage.49 Although it is unclear whether dyslipidaemia is a risk factor for the development and/or progression of DN, because of the established cardiovascular benefits of cholesterol lowering, all patients with DN should receive lipid-lowering treatment,4 with statins recommended as first-line treatment.42,58
Reduction of albuminuria
Several drug strategies decrease the level of urinary albumin excretion in patients with T2DM, with the data being most extensive for RAAS inhibitors. DPP-4 inhibitors may also reduce albuminuria in addition to lowering blood glucose. Evidence suggests that the thiazolidinediones and fenofibrate decrease albuminuria and may therefore have potential renal benefits in DN.59–61
The potential for improved prevention and treatment of DN
Early, intensive and multifactorial management of DN risk and progression
The early identification of modifiable risk factors for DN is important for prompt therapeutic intervention. Early achievement of multiple risk factor targets has been shown to delay DN. Thus, patients should receive early, intensive and multifactorial management, targeting all risk factors simultaneously, to prevent or slow the progression of kidney disease.42,43,58
Direct protection from DN
New drug interventions should also offer direct protection from DN and other diabetes-related end-organ damage. This has been partly achieved with RAAS inhibitors, which have renoprotective effects that are independent of their BP-lowering effects.42,43 However, albuminuria frequently remains elevated, and DN remains a major health problem, with many patients with T2DM still progressing to ESRD.34 Because current efforts to prevent and treat DN have limited success, more effective treatment strategies are urgently needed.
GLP-1 analogues and DPP-4 inhibitors
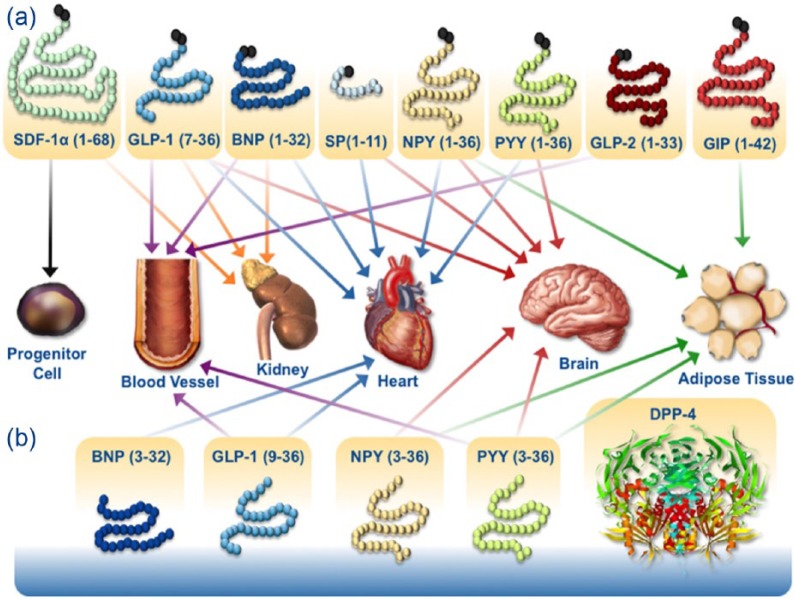
Incretin-based therapies for the treatment of hyperglycaemia include the injectable GLP-1 receptor agonists and the orally active DPP-4 inhibitors, both of which stimulate insulin secretion and inhibit glucagon secretion in a glucose-dependent manner. Among the substrates of DPP-4 are peptide hormones such as B-type natriuretic peptides, neuropeptide Y, peptide YY and stromal cell-derived factor-1α (Figure 2),62,63 which are thought to be responsible for the cardio-renal effects potentially beneficial for patients with T2DM.63,64
Figure 2.
DPP-4 effects beyond glucose lowering.
Source: Republished with permission of The Endocrine Society, from Ussher and Drucker.62 ©2014.
BNP: B-type (brain) natriuretic peptide; DPP-4: dipeptidyl peptidase-4; GIP: glucose-dependent insulinotropic polypeptide; GLP-1: glucagon-like peptide-1; NPY: neuropeptide; PYY: peptide YY; SDF-1α: stromal cell-derived factor-1α; SP: substance P.
The secretion of native GLP-1 is impaired in patients with T2DM.65 Injectable GLP-1 analogues, including exenatide and liraglutide, improve glycaemic control and are associated with weight loss.66 Exenatide is predominantly excreted via the kidney, but has an acceptable tolerability profile in patients with mild CKD and requires no dose adjustment in this patient group. Dose escalation of exenatide in patients with moderate RI should proceed with caution, and its use in patients with severe kidney disease is not recommended.67 Liraglutide is not exclusively metabolised by the kidney and can be used in patients with mild RI without dose adjustment.67 However, studies assessing GLP-1 efficacy in patients with T2DM and moderate-to-severe RI are lacking.
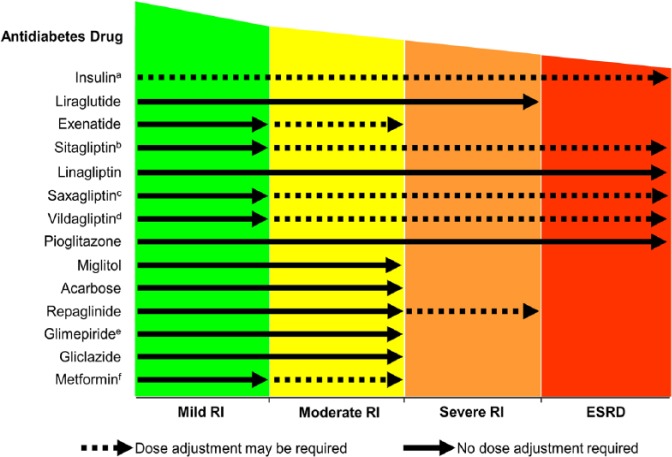
Because of the favourable tolerability profile of DPP-4 inhibitors (weight neutral and a low incidence of hypoglycaemia) and their potential to preserve β-cell function, these drugs present a unique option in oral glucose-lowering therapy. Currently available DPP-4 inhibitors include sitagliptin, vildagliptin, saxagliptin, alogliptin and linagliptin, and preclinical studies have reported effects on myocardial infarction, and acute and chronic renal failure.63 One of the key differentiators of the available DPP-4 inhibitors is the route of elimination, with sitagliptin, vildagliptin, saxagliptin and alogliptin displaying a variable renal elimination route ranging from 75% for saxagliptin to 87% for sitagliptin.67 In comparison, less than 6% of linagliptin is excreted renally at an oral dosage of 5 mg/day.68 In clinical studies, all DPP-4 inhibitors were well tolerated in patients with CKD,67 with no dose adjustment needed in patients with mild RI. However, dose adjustments are needed in patients with moderate or severe RI for all DPP-4 inhibitors with the exception of linagliptin (Figure 3).
Figure 3.
Antidiabetes therapy in patients with CKD.
Source: Modified from Schernthaner et al.,69 by permission of Oxford University Press.
CKD: chronic kidney disease; ESRD: end-stage renal disease; RI: renal impairment.
aInsulin dosing should be monitored and adjusted depending upon the patient’s response.
bSitagliptin dose adjusted to 50 mg once daily for patients with moderate RI, or to 25 mg once daily for patients with severe RI or ESRD.
cSaxagliptin dose adjusted to 2.5 mg once daily for patients with moderate-to-severe RI, or ESRD.
dVildagliptin dose adjusted to 50 mg once daily for patients with moderate-to-severe RI, or ESRD.
eGlimepiride dose should be started at 1 mg daily for all patients with RI.
fMetformin dose in patients with moderate RI varies according to national guidelines.
Clinical evidence regarding the renal benefits of incretin-based therapies is limited. Liraglutide was not associated with any changes in renal function after 24 weeks in patients with DN (eGFR <60 mL/min).70 Sitagliptin, saxagliptin and linagliptin may have the potential for beneficial renal effects. Sitagliptin (50 mg/day) reduced urinary albumin-to-creatinine ratio (UACR: −20.6 mg/g creatinine) after 24 weeks in patients with T2DM; the effect was observed in patients with normo-, micro- and macroalbuminuria.71 In the recently completed Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction (SAVOR-TIMI) 53 trial, saxagliptin reduced the development and progression of microalbuminuria compared with placebo.72 In a pooled analysis of four 24-week, randomised, placebo-controlled trials of patients with T2DM and prevalent albuminuria (UACR: 30–3000 mg/g creatinine) while receiving stable doses of RAAS inhibitors, linagliptin reduced UACR by 32% versus 6% with placebo, with a between-group difference versus placebo of 28%.73 Finally, in a recent meta-analysis of 5466 patients with T2DM, treatment with linagliptin was not associated with an increase in renal risk and was associated with a significant reduction of clinically relevant renal safety events.74 The renal effects of linagliptin are currently being investigated in the MARLINA-T2D™ (Efficacy, Safety & Modification of Albuminuria in Type 2 Diabetes Subjects with Renal Disease with LINAgliptin) trial (ClinicalTrials.gov Identifier: NCT01792518). The CARMELINA® (CArdiovascular Safety & Renal Microvascular outcomE study with LINAgliptin) trial (NCT01897532) has also recently been initiated, and is enrolling patients with T2DM and renal dysfunction.
SGLT2 inhibitors
SGLT2 inhibitors, a new class of oral antidiabetes therapy, selectively target the SGLT2 protein and prevent renal sodium and glucose reabsorption in the kidney, a process known to be involved in the development of DN.75 Blocking the activity of SGLT2, which is almost exclusively expressed in the S1 segment of the renal proximal tubule,76 leads to substantial glucosuria and a reduction in plasma glucose levels.77 Since SGLT2 inhibitors do not stimulate insulin secretion, improvements in glycaemic control are seen without increasing the risk for hypoglycaemia.77 Nonglycaemic benefits of SGLT2 inhibitors include reductions in body weight and BP.77,78 Recently, two head-to-head studies have demonstrated some benefits of SGLT2 inhibitors versus the DPP-4 inhibitor sitagliptin.79,80 When canagliflozin (300 mg daily) or sitagliptin (100 mg daily) were administered to patients with T2DM inadequately controlled with metformin plus sulphonylurea,80 greater reductions in HbA1c (difference after 1 year, 0.37%), fasting plasma glucose (FPG; difference, 26.5 mg/dL), body weight (difference, 2.4 kg) and systolic BP (difference, 6 mm Hg) were observed with canagliflozin compared with sitagliptin (p < 0.001). A comparison of monotherapy79 with either empagliflozin (10 or 25 mg daily) and sitagliptin (100 mg daily) showed slightly better glucose control with empagliflozin 25 mg versus sitagliptin at 24 weeks (reduction in HbA1c: −0.85% vs −0.73%; p < 0.0001), and reductions in body weight (difference, 2.67 kg) and systolic BP (difference, 4.2 mm Hg). Head-to-head long-term studies are needed to evaluate whether these differences will have an impact on cardiovascular and renal endpoints.
The primary route of elimination of the SGLT2 inhibitor canagliflozin is in faeces (approximately 50%), whereas dapagliflozin is primarily excreted in urine (75%).81,82 Approximately 20% of empagliflozin is excreted unchanged in urine.83 The glucose-lowering efficacy of SGLT2 inhibitors is dependent on renal function and for the drugs approved at the time of writing, use is currently contraindicated in patients with severely impaired kidney function (eGFR <30 mL/min) or ESRD.81,82 In addition, renal function monitoring is recommended prior to initiating treatment with canagliflozin and dapagliflozin, and periodically thereafter.81,82
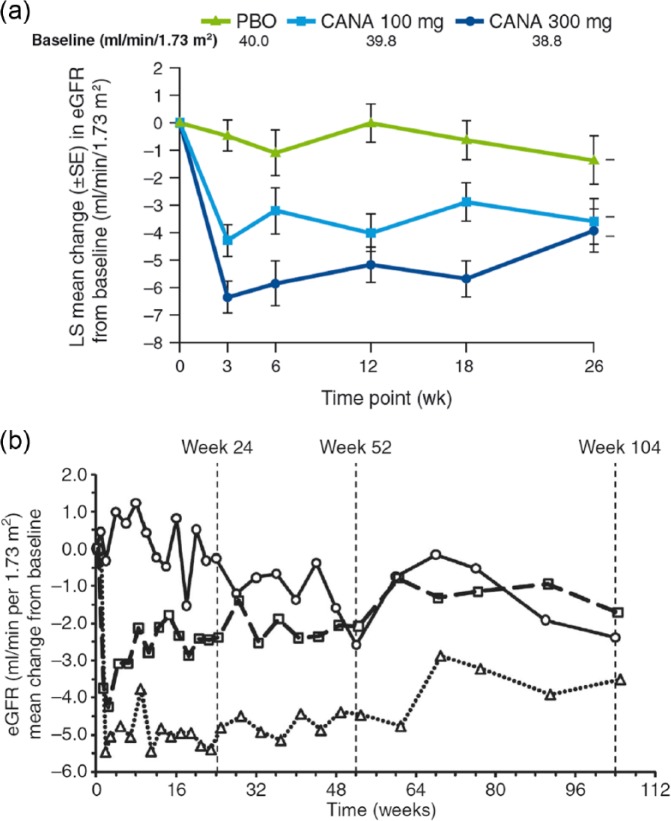
Given their mechanism of action, SGLT2 inhibitors may have significant renal effects beyond their glucose-lowering properties.84,85 In patients with T2DM and moderate RI, treatment with canagliflozin (100 or 300 mg for 26 weeks)86 or dapagliflozin (5 or 10 mg for 104 weeks)87 showed an initial transient decrease in eGFR which remained relatively stable for the duration of treatment (Figure 4). The effect of treatment with SGLT2 inhibitors in 6 different studies on renal function are summarised in Table 2.
Figure 4.
Changes in eGFR over time in T2DM patients with moderate RI treated with (a) canagliflozin and (b) dapagliflozin. Moderate RI = baseline eGFR ≥30 and <50 mL/min/1.73 m2; Moderate RI = baseline eGFR ≥30 and <60 mL/min/1.73 m2. Placebo (circles, solid line), dapagliflozin 5 mg (squares, dashed line) and dapagliflozin 10 mg (triangles, dotted line).
Source: Reprinted by permission from Yale et al.86 ©2013 Blackwell Publishing Ltd; Reprinted by permission from Macmillan Publishers Ltd: Kidney International (Kohan et al.87 Copyright©2014).
CANA: canagliflozin; eGFR: estimated glomerular filtration rate; LS: least squares; PBO: placebo; RI: renal impairment; SE: standard error; T2DM: type 2 diabetes mellitus.
Table 2.
Effects of SGLT2 inhibitors on renal function.
| Study | N | Investigational drug | Comparator | Weeks | Change in eGFR (mL/min/1.73 m2) |
||
|---|---|---|---|---|---|---|---|
| Investigational drug | Comparator | Difference | |||||
| Barnett et al. (2014)88 | 292a | Empagliflozin 10/25 mg | Placebo | 52 | −2.0/−2.5 | −1.0 | −1.0/−1.5 |
| Barnett et al. (2014)88 | 375b | Empagliflozin 25 mg | Placebo | 52 | −3.0 | −0.2 | −2.8 |
| Barnett et al. (2014)88 | 74c | Empagliflozin 25 mg | Placebo | 52 | −1.2 | −0.4 | 0.8 |
| Cefalu et al. (2013)89 | 1450 | Canagliflozin 100/300 mg | Glimepiride | 4 | −3.0/−5.6 | −1.3 | −1.7/−4.3 |
| Cefalu et al. (2013)89 | 1450 | Canagliflozin 100/300 mg | Glimepiride | 52 | −1.7/−3.0 | −5.1 | 3.4/2.1 |
| Kohan et al. (2014)87 | 252 | Dapagliflozin 5/10 mg | Placebo | 24 | −2.38/−4.80 | −0.25 | −2.13/−4.55 |
| Kohan et al. (2014)87 | 252 | Dapagliflozin 5/10 mg | Placebo | 52 | −2.08/−4.46 | −2.58 | 0.50/−1.88 |
| Kohan et al. (2014)87 | 252 | Dapagliflozin 5/10 mg | Placebo | 104 | −1.71/−3.50 | −2.38 | 0.67/1.12 |
| List et al. (2009)90 | 333d | Dapagliflozin 2.5/5/10/20/50 mg | Placebo | 12 | −2/0/−3/−1/3 | 0 | −2/0/−3/−1/3 |
| Wilding et al. (2013)91 | 808 | Dapagliflozin 2.5/5/10 mg | Placebo | 48 | −2.0/−2.0/−1.1 | 0.7 | −1.3/−1.3/−0.4 |
| Yale et al. (2013)86 | 269 | Canagliflozin 100/300 mg | Placebo | 52 | −3.6/−3.9 | −1.4 | −2.2/−2.5 |
SGLT2: sodium-glucose cotransporter 2; eGFR: estimated glomerular filtration rate; CKD: chronic kidney disease.
Patients with Stage 2 CKD.
Patients with Stage 3 CKD.
Patients with Stage 4 CKD.
eGFR measurements were estimated from published graphs.
eGFR measurements were re-calculated from published data; metformin arm not included since p-values not provided.
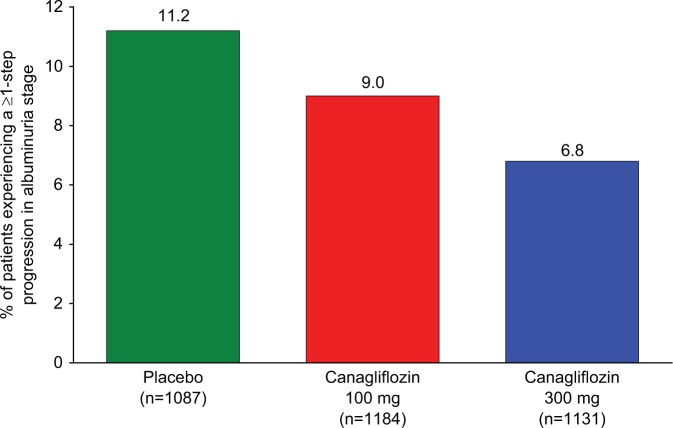
In addition to GFR, impact on albuminuria has also been investigated in several SGLT2 studies (Table 3). Canagliflozin 100 and 300 mg were associated with greater decreases in UACR compared with placebo, with median percent reductions of −29.9%, −20.9% and −7.5%.86 When these data were analysed with regard to their effects on albuminuria progression (normo- to micro-/macroalbuminuria or micro- to macroalbuminuria), the proportion of patients that progressed were 5.1%, 8.3% and 11.8% in the canagliflozin 100 mg, 300 mg and placebo groups, respectively, with odds ratios (95% confidence interval (CI)) of 0.33 (0.08, 1.48) and 0.51 (0.14, 1.91) for the pairwise comparisons of canagliflozin 100 and 300 mg to placebo, respectively.86 Additional studies analysing UACR changes and progression as part of a cardiovascular safety report for the Food and Drug Administration, showed similar changes (Figure 5) in a larger group of more than 3000 individuals.92
Table 3.
Effects of SGLT2 inhibitors on albumin excretion rate.
| Study | N | Investigational drug | Comparator | Weeks | Change in albumin excretion rate |
||
|---|---|---|---|---|---|---|---|
| Investigational drug | Comparator | Difference | |||||
| Barnett et al. (2014)88 | 292a | Empagliflozin 10/25 mg | Placebo | 52 | −70/−121 | 114 | −185/−236 |
| Barnett et al. (2014)88 | 375b | Empagliflozin 25 mg | Placebo | 52 | −155 | 29 | −184 |
| Barnett et al. (2014)88 | 74c | Empagliflozin 25 mg | Placebo | 52 | −634 | −140 | −494 |
| Cefalu et al. (2013)89 | 1450 | Canagliflozin 100/300 mg | Glimepiride | 52 | −0.1/−0.9 | 0.7 | −0.8/−1.5 |
| Kohan et al. (2014)87 | 252 | Dapagliflozin 5/10 mg | Placebo | 104 | 78.0/−11.7 | 69.7 | 8.3/−81.4 |
| Yale et al. (2013)86 | 269 | Canagliflozin 100/300 mg | Placebo | 52 | −117.5/−96.2 | 15.4 | −132.9/−111.6 |
SGLT2: sodium-glucose cotransporter 2; CKD: chronic kidney disease.
Patients with Stage 2 CKD.
Patients with Stage 3 CKD.
Patients with Stage 4 CKD.
Figure 5.
Proportion of participants in canagliflozin cardiovascular safety study in patients with T2DM experiencing a ≥1-step progression in albuminuria stage.
Source: Coelln-Hough et al.92
T2DM: type 2 diabetes mellitus.
Very recently, similar findings were reported for dapagliflozin in a 104-week study of patients with CKD.87 Of the 139 patients who completed the study, 96 were treated with dapagliflozin and 43 received placebo. Values of UACR >1800 mg/g during the 104-week treatment period were reported in a higher proportion of patients receiving placebo (13.3%) than in patients receiving dapagliflozin 5 or 10 mg (10.8% and 9.5%, respectively). In shift analyses, UACR was characterised according to one of three pre-specified categories: 0 to <30 mg/g (normoalbuminuria), 30 to <300 mg/g (microalbuminuria), or ≥300 mg/g (macroalbuminuria). Among dapagliflozin-treated patients, albuminuria shifted to a lower category in 38 patients (18 from microalbuminuria to normoalbuminuria, 19 from macroalbuminuria to microalbuminuria, and 1 from macroalbuminuria to normoalbuminuria) and shifted to a higher category in 18 patients; in patients receiving placebo, 9 shifted to a lower category and 12 to a higher one.87
In a recent clinical trial of empagliflozin (10 or 25 mg for 52 weeks), small decreases in eGFR and albuminuria were also shown in T2DM patients with mild or moderate RI.88 UACR improved with empagliflozin compared with placebo at Week 52 in patients with Stage 2 CKD (empagliflozin 25 mg: adjusted mean difference, −235.86 (95% CI: −442.85, −28.86), p = 0.0257) and Stage 3 CKD (−183.78 (95% CI: −305.18, −62.38), p = 0.0031). In patients with Stage 3 CKD, fewer patients on empagliflozin 25 mg than placebo shifted from no albuminuria to microalbuminuria, or from microalbuminuria to macroalbuminuria, at end of treatment (12.2% with empagliflozin vs 22.2% with placebo, and 2.0% with empagliflozin vs 11.4% with placebo, respectively). Remarkably, more patients with Stage 3 CKD on empagliflozin 25 mg improved from macroalbuminuria to microalbuminuria, or from microalbuminuria to no albuminuria, at end of treatment (32.6% with empagliflozin vs 8.6% with placebo, and 27.5% with empagliflozin vs 21.4% with placebo, respectively). These changes reversed (with levels returning to baseline values) 3 weeks after the end of treatment, indicating no long-term damage. In nonclinical studies, empagliflozin decreased the renal expression of molecular markers of kidney growth, fibrosis and inflammation,93,94 and intriguingly, in a small study of patients with type 1 diabetes, empagliflozin attenuated hyperfiltration, suggesting the potential to reduce the risk of DN.95 Further studies are clearly needed to determine if these effects translate into renal benefits in clinical practice. For example, the renal effects of empagliflozin are being assessed as a pre-specified secondary endpoint in the EMPA-REG OUTCOME™ (Empagliflozin Cardiovascular Outcome) trial (NCT01131676).
Monitoring of renal risk factors and kidney function in patients with T2DM
CKD attributed to diabetes benefits from early intervention and thus warrants a screening program.96 Urinary albumin excretion should be assessed in all adults at diagnosis of T2DM, and at least annually thereafter. In addition, for the estimation of GFR, serum creatinine should be measured at least annually regardless of the degree of urinary albumin excretion.96 Albuminuria and eGFR are useful biomarkers for predicting the risk of renal and cardiovascular events, and treatment-induced reductions in albuminuria or changes in eGFR are associated with long-term renal and cardiovascular protection.97 Continual monitoring of risk factors and renal function together with adjustment of the treatment regimen is important to maintain long-term glycaemic, BP and dyslipidaemia control.98 Hypoglycaemia is a particular concern because increasing RI is associated with increasing hypoglycaemia risk.43 Thus, certain oral glucose-lowering drugs that are eliminated by the kidneys are contraindicated in patients with T2DM and CKD.43
Conclusion
Despite the use of lifestyle and pharmacologic interventions to reduce renal risk factors such as hypertension and hyperglycaemia, albuminuria frequently remains elevated in many patients with T2DM. As a consequence, DN persists as a major health problem, with many patients progressing to ESRD, and new strategies are needed to mitigate this burden.
The optimal treatment and prevention of DN calls for an early, intensive and multifactorial approach that targets all major renal risk factors at the same time. With regard to glucose-lowering pharmacotherapy, treatment should be carefully selected to balance adverse effects, such as hypoglycaemia and weight gain, with the benefits of lowering glucose. In particular, patients with CKD have an increased risk of hypoglycaemia, and many glucose-lowering drugs are either contraindicated or require dose adjustment in this high-risk population.
In addition to controlling risk factors, treatments should offer direct protection from DN and other end-organ damage, and the efficacy of new treatment modalities should be evaluated by their antiproteinuric effects and renal benefits. The improvement in albuminuria seen with DPP-4 and SGLT2 inhibitors suggests that these antidiabetes drugs may potentially provide renal benefits beyond their glucose-lowering effects. If beneficial renal effects are confirmed in future studies, a single DPP-4 and SGLT2 inhibitor combination pill may offer a promising therapeutic option for T2DM patients with CKD.
Due to the progressive nature of T2DM, regular assessment of risk factors and adjustment of treatment regimens are important management steps to maintain long-term glycaemic and BP control. Continual monitoring of renal function, including urinary albumin excretion, creatinine clearance and GFR, is critical to follow the progression of kidney disease in patients with T2DM. However, currently available data on the treatment and prevention of DN are mainly from observational studies, and large-scale intervention studies are required.
Key messages
Optimal treatment and prevention of diabetic nephropathy may require an early, intensive and multifactorial approach.
Anti-hyperglycaemic therapy should be carefully selected to balance adverse effects, such as hypoglycaemia and weight gain, with the benefits of lowering glucose.
Any therapy should also offer direct protection from diabetic nephropathy and provide renal benefits.
Newer classes of antidiabetes drugs may provide renal benefits in addition to their glucose-lowering effects.
Due to the progressive nature of T2DM, regular assessment of risk factors and adjustment of treatment regimens are important management steps to maintain long-term glycaemic and BP control.
Acknowledgments
The authors were fully responsible for all content and editorial decisions, were involved at all stages of article development and have approved the final version of the review that reflects the authors’ interpretation and conclusion. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Howard Christian of Envision Scientific Solutions during the preparation of this review. Boehringer Ingelheim was given the opportunity to check the data used in the article for factual accuracy only.
Footnotes
Declaration of conflicting interests: Guntram Schernthaner has received lecture fees from Amgen, AstraZeneca/Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline (GSK), Merck Sharp & Dohme (MSD), Novartis, Novo Nordisk, Sanofi-Aventis, Servier and Takeda. Carl Erik Mogensen has declared no conflicts of interest. Gerit-Holger Schernthaner has received scientific grants and/or educational grants and/or travel grants and/or slide honoraria, and/or lecture honoraria, and/or advisory board honoraria, and/or contract research and/or partner research from/with Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Medtronic, Menarini, Merck, MSD, Novo Nordisk, Novartis, Pfizer, Sanofi, Sanofi-Aventis, Servier and Takeda in the past 10 years.
Funding: This article was supported financially by Boehringer Ingelheim.
References
- 1. International Diabetes Federation (IDF). IDF diabetes atlas. 6th ed. Brussels: IDF, 2013, http://www.idf.org/diabetesatlas (accessed 6 November 2013). [Google Scholar]
- 2. Parving H-H, Mauer M, Ritz E. Diabetic nephropathy. In: BM Brenner. (ed.) Brenner and rector’s the kidney. 7th ed. Philadelphia, PA: W.B. Saunders, 2004, pp.1777–1818. [Google Scholar]
- 3. Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab 2008; 4: 444–452. [DOI] [PubMed] [Google Scholar]
- 4. Rossing K. Progression and remission of nephropathy in type 2 diabetes: new strategies of treatment and monitoring. Dan Med Bull 2007; 54: 79–98. [PubMed] [Google Scholar]
- 5. Saydah S, Eberhardt M, Rios-Burrows N, et al. Prevalence of chronic kidney disease and associated risk factors – United States, 1999–2004. MMWR Morb Mortal Wkly Rep 2007; 56: 161–165, http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5608a2.htm#tab (accessed 6 November 2013).17332726 [Google Scholar]
- 6. Centers for Disease Control and Prevention (CDC). Chronic kidney disease surveillance system – United States. U.S. Department of Health and Human Services, http://www.cdc.gov/ckd (accessed 6 May 2014). [Google Scholar]
- 7. Amato D, Alvarez-Aguilar C, Castaneda-Limones R, et al. Prevalence of chronic kidney disease in an urban Mexican population. Kidney Int 2005; 68: S11–S17. [DOI] [PubMed] [Google Scholar]
- 8. Brown WW, Peters RM, Ohmit SE, et al. Early detection of kidney disease in community settings: the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2003; 42: 22–35. [DOI] [PubMed] [Google Scholar]
- 9. Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol 2005; 16: 180–188. [DOI] [PubMed] [Google Scholar]
- 10. Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003; 41: 1–12. [DOI] [PubMed] [Google Scholar]
- 11. Fox CS, Larson MG, Vasan RS, et al. Cross-sectional association of kidney function with valvular and annular calcification: the Framingham heart study. J Am Soc Nephrol 2006; 17: 521–527. [DOI] [PubMed] [Google Scholar]
- 12. Garg AX, Papaioannou A, Ferko N, et al. Estimating the prevalence of renal insufficiency in seniors requiring long-term care. Kidney Int 2004; 65: 649–653. [DOI] [PubMed] [Google Scholar]
- 13. Hemmelgarn BR, Zhang J, Manns BJ, et al. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int 2006; 69: 2155–2161. [DOI] [PubMed] [Google Scholar]
- 14. Kramer H, Toto R, Peshock R, et al. Association between chronic kidney disease and coronary artery calcification: the Dallas Heart Study. J Am Soc Nephrol 2005; 16: 507–513. [DOI] [PubMed] [Google Scholar]
- 15. Manjunath G, Tighiouart H, Coresh J, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int 2003; 63: 1121–1129. [DOI] [PubMed] [Google Scholar]
- 16. McClellan W, Warnock DG, McClure L, et al. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol 2006; 17: 1710–1715. [DOI] [PubMed] [Google Scholar]
- 17. Brugts JJ, Knetsch AM, Mattace-Raso FU, et al. Renal function and risk of myocardial infarction in an elderly population: the Rotterdam Study. Arch Intern Med 2005; 165: 2659–2665. [DOI] [PubMed] [Google Scholar]
- 18. Cirillo M, Laurenzi M, Mancini M, et al. Low glomerular filtration in the population: prevalence, associated disorders, and awareness. Kidney Int 2006; 70: 800–806. [DOI] [PubMed] [Google Scholar]
- 19. Hallan SI, Coresh J, Astor BC, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 2006; 17: 2275–2284. [DOI] [PubMed] [Google Scholar]
- 20. Nitsch D, Felber Dietrich D, von Eckardstein A, et al. Prevalence of renal impairment and its association with cardiovascular risk factors in a general population: results of the Swiss SAPALDIA study. Nephrol Dial Transplant 2006; 21: 935–944. [DOI] [PubMed] [Google Scholar]
- 21. Otero A, Gayoso P, Garcia F, et al. Epidemiology of chronic renal disease in the Galician population: results of the pilot Spanish EPIRCE study. Kidney Int 2005; 99: S16–S19. [DOI] [PubMed] [Google Scholar]
- 22. Verhave JC, Gansevoort RT, Hillege HL, et al. An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney Int 2004; 92: S18–S21. [DOI] [PubMed] [Google Scholar]
- 23. Viktorsdottir O, Palsson R, Andresdottir MB, et al. Prevalence of chronic kidney disease based on estimated glomerular filtration rate and proteinuria in Icelandic adults. Nephrol Dial Transplant 2005; 20: 1799–1807. [DOI] [PubMed] [Google Scholar]
- 24. Wasen E, Isoaho R, Mattila K, et al. Estimation of glomerular filtration rate in the elderly: a comparison of creatinine-based formulae with serum cystatin C. J Intern Med 2004; 256: 70–78. [DOI] [PubMed] [Google Scholar]
- 25. Chadban SJ, Briganti EM, Kerr PG, et al. Prevalence of kidney damage in Australian adults: the AusDiab kidney study. J Am Soc Nephrol 2003; 14: S131–S138. [DOI] [PubMed] [Google Scholar]
- 26. Chen J, Wildman RP, Gu D, et al. Prevalence of decreased kidney function in Chinese adults aged 35 to 74 years. Kidney Int 2005; 68: 2837–2845. [DOI] [PubMed] [Google Scholar]
- 27. Domrongkitchaiporn S, Sritara P, Kitiyakara C, et al. Risk factors for development of decreased kidney function in a southeast Asian population: a 12-year cohort study. J Am Soc Nephrol 2005; 16: 791–799. [DOI] [PubMed] [Google Scholar]
- 28. Konta T, Hao Z, Abiko H, et al. Prevalence and risk factor analysis of microalbuminuria in Japanese general population: the Takahata study. Kidney Int 2006; 70: 751–756. [DOI] [PubMed] [Google Scholar]
- 29. Li ZY, Xu GB, Xia TA, et al. Prevalence of chronic kidney disease in a middle and old-aged population of Beijing. Clin Chim Acta 2006; 366: 209–215. [DOI] [PubMed] [Google Scholar]
- 30. McDonald SP, Maguire GP, Hoy WE. Renal function and cardiovascular risk markers in a remote Australian Aboriginal community. Nephrol Dial Transplant 2003; 18: 1555–1561. [DOI] [PubMed] [Google Scholar]
- 31. Ninomiya T, Kiyohara Y, Kubo M, et al. Chronic kidney disease and cardiovascular disease in a general Japanese population: the Hisayama Study. Kidney Int 2005; 68: 228–236. [DOI] [PubMed] [Google Scholar]
- 32. Shankar A, Klein R, Klein BE. The association among smoking, heavy drinking, and chronic kidney disease. Am J Epidemiol 2006; 164: 263–271. [DOI] [PubMed] [Google Scholar]
- 33. Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health 2008; 8: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. U.S. Renal Data System. USRDS 2012 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, http://www.usrds.org/atlas.aspx (accessed 6 November 2013). [Google Scholar]
- 35. Parving HH, Lewis JB, Ravid M, et al. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 2006; 69: 2057–2063. [DOI] [PubMed] [Google Scholar]
- 36. Rossing P. Prediction, progression and prevention of diabetic nephropathy. The Minkowski Lecture 2005. Diabetologia 2006; 49: 11–19. [DOI] [PubMed] [Google Scholar]
- 37. Equiluz-Bruck S, Schnack C, Kopp HP, et al. Nondipping of nocturnal blood pressure is related to urinary albumin excretion rate in patients with type 2 diabetes mellitus. Am J Hypertens 1996; 9: 1139–1143. [DOI] [PubMed] [Google Scholar]
- 38. Knobl P, Schernthaner G, Schnack C, et al. Thrombogenic factors are related to urinary albumin excretion rate in type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993; 36: 1045–1050. [DOI] [PubMed] [Google Scholar]
- 39. Kramer CK, Leitão CB, Pinto LC, et al. Clinical and laboratory profile of patients with type 2 diabetes with low glomerular filtration rate and normoalbuminuria. Diabetes Care 2007; 30: 1998–2000. [DOI] [PubMed] [Google Scholar]
- 40. Zoppini G, Targher G, Chonchol M, et al. Predictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney function. Clin J Am Soc Nephrol 2012; 7: 401–408. [DOI] [PubMed] [Google Scholar]
- 41. Hsieh MC, Hsieh YT, Cho TJ, et al. Remission of diabetic nephropathy in type 2 diabetic Asian population: role of tight glucose and blood pressure control. Eur J Clin Invest 2011; 41: 870–878. [DOI] [PubMed] [Google Scholar]
- 42. American Diabetes Association. Standards of medical care in diabetes – 2013. Diabetes Care 2013; 36(Suppl. 1): S11–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 2012; 60: 850–886. [DOI] [PubMed] [Google Scholar]
- 44. Schernthaner G. Kidney disease in diabetology: lessons from 2010. Nephrol Dial Transplant 2011; 26: 454–457. [DOI] [PubMed] [Google Scholar]
- 45. Ritz E. Limitations and future treatment options in type 2 diabetes with renal impairment. Diabetes Care 2011; 34: S330–S334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Selvin E, Ning Y, Steffes MW, et al. Glycated hemoglobin and the risk of kidney disease and retinopathy in adults with and without diabetes. Diabetes 2011; 60: 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 48. Sharp Collaborative Group. Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J 2010; 160: 785–794.e710. [DOI] [PubMed] [Google Scholar]
- 49. Athyros VG, Mitsiou EK, Tziomalos K, et al. Impact of managing atherogenic dyslipidemia on cardiovascular outcome across different stages of diabetic nephropathy. Expert Opin Pharmacother 2010; 11: 723–730. [DOI] [PubMed] [Google Scholar]
- 50. Sandhu S, Wiebe N, Fried LF, et al. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol 2006; 17: 2006–2016. [DOI] [PubMed] [Google Scholar]
- 51. Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45: 281–287. [DOI] [PubMed] [Google Scholar]
- 52. Keane WF, Brenner BM, de Zeeuw D, et al. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int 2003; 63: 1499–1507. [DOI] [PubMed] [Google Scholar]
- 53. Silverberg DS, Wexler D, Iaina A, et al. Anemia, chronic renal disease and congestive heart failure – the cardio renal anemia syndrome: the need for cooperation between cardiologists and nephrologists. Int Urol Nephrol 2006; 38: 295–310. [DOI] [PubMed] [Google Scholar]
- 54. Eknoyan G. Obesity, diabetes, and chronic kidney disease. Curr Diab Rep 2007; 7: 449–453. [DOI] [PubMed] [Google Scholar]
- 55. Afshinnia F, Wilt TJ, Duval S, et al. Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant 2010; 25: 1173–1183. [DOI] [PubMed] [Google Scholar]
- 56. Phisitkul K, Hegazy K, Chuahirun T, et al. Continued smoking exacerbates but cessation ameliorates progression of early type 2 diabetic nephropathy. Am J Med Sci 2008; 335: 284–291. [DOI] [PubMed] [Google Scholar]
- 57. Patel A, Group AC, MacMahon S, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370: 829–840. [DOI] [PubMed] [Google Scholar]
- 58. Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists’ comprehensive diabetes management algorithm 2013 consensus statement – executive summary. Endocr Pract 2013; 19: 536–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Davis TM, Ting R, Best JD, et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia 2011; 54: 280–290. [DOI] [PubMed] [Google Scholar]
- 60. Sarafidis PA, Stafylas PC, Georgianos PI, et al. Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: a meta-analysis. Am J Kidney Dis 2010; 55: 835–847. [DOI] [PubMed] [Google Scholar]
- 61. Schernthaner G, Currie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care 2013; 36(Suppl. 2): S155–S161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev 2012; 33: 187–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hocher B, Reichetzeder C, Alter ML. Renal and cardiac effects of DPP4 inhibitors – from preclinical development to clinical research. Kidney Blood Press Res 2012; 36: 65–84. [DOI] [PubMed] [Google Scholar]
- 64. Panchapakesan U, Mather A, Pollock C. Role of GLP-1 and DPP-4 in diabetic nephropathy and cardiovascular disease. Clin Sci 2013; 124: 17–26. [DOI] [PubMed] [Google Scholar]
- 65. Nauck MA. Unraveling the science of incretin biology. Am J Med 2009; 122: S3–S10. [DOI] [PubMed] [Google Scholar]
- 66. Russell-Jones D. The safety and tolerability of GLP-1 receptor agonists in the treatment of type-2 diabetes. Int J Clin Pract 2010; 64: 1402–1414. [DOI] [PubMed] [Google Scholar]
- 67. Schernthaner G, Avogaro A, Schernthaner GH. Glycemic targets and antidiabetic drug treatment in patients with chronic kidney disease (stages 3–5). Hot Topics Diabetes 2013; 1: 13–24. [Google Scholar]
- 68. Huttner S, Graefe-Mody EU, Withopf B, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single oral doses of BI 1356, an inhibitor of dipeptidyl peptidase 4, in healthy male volunteers. J Clin Pharmacol 2008; 48: 1171–1178. [DOI] [PubMed] [Google Scholar]
- 69. Schernthaner G, Ritz E, Schernthaner GH. Strict glycaemic control in diabetic patients with CKD or ESRD: beneficial or deadly? Nephrol Dial Transplant 2010; 25: 2044–2047. [DOI] [PubMed] [Google Scholar]
- 70. Ryuge A, Minoru K, Yu K, et al. Examination of the effects of liraglutide on diabetic nephropathy. Kidney Res Clin Pract 2012; 31: A70. [Google Scholar]
- 71. Hattori S. Sitagliptin reduces albuminuria in patients with type 2 diabetes. Endocr J 2011; 58: 69–73. [DOI] [PubMed] [Google Scholar]
- 72. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 73. Groop PH, Cooper ME, Perkovic V, et al. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care 2013; 36: 3460–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Von Eynatten M, Emser A, Cooper ME, et al. Renal safety and outcomes with linagliptin: meta-analysis of individual data for 5466 patients with type 2 diabetes. J Am Soc Nephrol 2012; 23: 218A. [Google Scholar]
- 75. Komala MG, Panchapakesan U, Pollock C, et al. Sodium glucose cotransporter 2 and the diabetic kidney. Curr Opin Nephrol Hypertens 2013; 22: 113–119. [DOI] [PubMed] [Google Scholar]
- 76. Wright EM. Renal Na(+)-glucose cotransporters. Am J Physiol Renal Physiol 2001; 280: F10–F18. [DOI] [PubMed] [Google Scholar]
- 77. Bays H. Sodium glucose co-transporter type 2 (SGLT2) inhibitors: targeting the kidney to improve glycemic control in diabetes mellitus. Diabetes Ther 2013; 4: 195–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Baker WL, Smyth LR, Riche DM, et al. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens 2014; 8: 262–275. [DOI] [PubMed] [Google Scholar]
- 79. Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2013; 1: 208–219. [DOI] [PubMed] [Google Scholar]
- 80. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care 2013; 36: 2508–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Farxiga (package insert). Princeton, NJ: Bristol-Myers Squibb; (last updated January 2014), http://packageinserts.bms.com/pi/pi_farxiga.pdf (accessed 10 April 2014). [Google Scholar]
- 82. Invokana (package insert). Titusville, NJ: Janssen Pharmaceuticals, Inc; (last updated May 2014), http://www.invokanahcp.com/prescribing-information.pdf (accessed 29 June 2014). [Google Scholar]
- 83. Macha S, Mattheus M, Pinnetti S, et al. Pharmacokinetics of empagliflozin, a sodium glucose cotransporter 2 inhibitor, and glimepiride following co-administration in healthy volunteers: a randomised, open-label, crossover study. J Diab Res Clin Met 2012; 1: 14. [Google Scholar]
- 84. De Nicola L, Gabbai FB, Liberti ME, et al. Sodium/glucose cotransporter 2 inhibitors and prevention of diabetic nephropathy: targeting the renal tubule in diabetes. Am J Kidney Dis 2014; 64: 16–24. [DOI] [PubMed] [Google Scholar]
- 85. Gilbert RE. Sodium-glucose linked transporter-2 inhibitors: potential for renoprotection beyond blood glucose lowering? Kidney Int. Epub ahead of print 20 November 2013. DOI: 10.1038/ki.2013.451. [DOI] [PubMed] [Google Scholar]
- 86. Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 2013; 15: 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kohan DE, Fioretto P, Tang W, et al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 2014; 85: 962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2014; 2: 369–384. [DOI] [PubMed] [Google Scholar]
- 89. Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013; 382: 941–950. [DOI] [PubMed] [Google Scholar]
- 90. List JF, Woo V, Morales E, et al. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009; 32: 650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wilding JP, Woo V, Rohwedder K, et al. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. Epub ahead of print 1 August 2013. DOI: 10.1111/dom.12187. [DOI] [PubMed] [Google Scholar]
- 92. Coelln-Hough J, Horton E, Meininger G, et al. FDA Canagliflozin Advisory Committee Meeting – Endocrinologic and Metabolic Drugs Advisory Committee, 10 January 2013, http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM336236.pdf (accessed 14 May 2014).
- 93. Panchapakesan U, Pegg K, Gross S, et al. Effects of SGLT2 inhibition in human kidney proximal tubular cells – renoprotection in diabetic nephropathy? PLoS One 2013; 8: e54442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vallon V, Gerasimova M, Rose MA, et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 2014; 306: F194–F204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014; 129: 587–597. [DOI] [PubMed] [Google Scholar]
- 96. Kramer H, Molitch ME. Screening for kidney disease in adults with diabetes. Diabetes Care 2005; 28: 1813–1816. [DOI] [PubMed] [Google Scholar]
- 97. Heerspink HJ, Holtkamp FA, de Zeeuw D, et al. Monitoring kidney function and albuminuria in patients with diabetes. Diabetes Care 2011; 34: S325–S329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bakris GL. Recognition, pathogenesis, and treatment of different stages of nephropathy in patients with type 2 diabetes mellitus. Mayo Clin Proc 2011; 86: 444–456. [DOI] [PMC free article] [PubMed] [Google Scholar]