Abstract
Background
Cerebral blood flow velocity (CBFV) measured by transcranial Doppler sonography has provided information on cerebral perfusion in patients undergoing infant heart surgery, but no studies have reported a relationship to early postoperative and long-term neurodevelopmental outcomes.
Methods
CBFV was measured in infants undergoing biventricular repair without aortic arch reconstruction as part of a trial of hemodilution during cardiopulmonary bypass (CPB). CBFV (Vm, mean; Vs, systolic; Vd, end-diastolic) in the middle cerebral artery and change in Vm (rVm) were measured intraoperatively and up to 18 hours post-CPB. Neurodevelopmental outcomes, measured at 1 year of age, included the Psychomotor Development Index (PDI) and Mental Development Index (MDI) of the Bayley Scales of Infant Development-II.
Results
CBFV was measured in 100 infants: 43 with D-transposition of the great arteries, 36 with tetralogy of Fallot, and 21 with ventricular septal defects. Lower Vm, Vs, Vd, and rVm at18 hours post-CPB were independently related to longer ICU duration of stay (P<0.05). In the 85 patients who returned for neurodevelopmental testing, lower Vm, Vs, Vd and rVm at 18 hours post-CPB were independently associated with lower PDI (P<0.05) and MDI (P<0.05, except Vs: P=0.06) scores. Higher Vs and rVm at 18 hours post-CPB were independently associated with increased incidence of brain injury on MRI in 39 patients.
Conclusions
Postoperative CBFV after biventricular repair is related to early postoperative and neurodevelopmental outcomes at 1 year of age, possibly indicating that low CBFV is a marker of suboptimal postoperative hemodynamics and cerebral perfusion.
Keywords: Congenital heart disease, Neurocognitive deficits, Outcomes, Infant
Introduction
Survivors of congenital heart disease (CHD) have a high prevalence of neurodevelopmental disabilities. Reliable methods for detecting cerebral ischemia during cardiopulmonary bypass (CPB) and the early postoperative period could allow for development of strategies to improve neurologic outcomes[1, 2]. Our group has previously shown that deep hypothermic circulatory arrest (DHCA) duration and intraoperative cerebral oxygen saturation (rSO2) are associated with neurodevelopmental outcome[3, 4].
Transcranial Doppler (TCD) sonography measurement of cerebral blood flow velocity (CBFV) provides a validated means of investigating cerebral blood flow (CBF)[5]. TCD has been used in pediatric cardiac surgery to evaluate changes in cerebral perfusion during CPB, following DHCA, and in the early postoperative period[6, 7]. However, the relationship between intraoperative and early postoperative CBFV to neurodevelopmental outcome has not been established.
The primary aim of this study was to evaluate the relationship of CBFV to neurologic outcome at age 1 year in infants undergoing biventricular repair of CHD. We hypothesized that lower levels of CBFV would be associated with worse neurodevelopmental outcome. A secondary aim was to evaluate the relationship between CBFV and early postoperative outcomes, with the hypothesis that lower CBFV would be associated with adverse early outcomes. Finally, we sought to report values for perioperative CBFV in infants undergoing repair of CHD to serve as a reference to practitioners.
Patients and Methods
Patient Enrollment
With IRB approval and parental informed consent, patients were enrolled between April 2001 and July 2004 at Boston Children’s Hospital in a randomized trial of hemodilution during hypothermic CPB[8]. Eligibility criteria included surgery at less than 9 months of age in three diagnostic groups: D-transposition of the great arteries (TGA), tetralogy of Fallot with or without pulmonary atresia or truncus arteriosus (collectively, TOF), and ventricular septal defect or common atrioventricular canal defect (collectively, VSD). Exclusion criteria included birth weight <2.3 kg, recognizable phenotypic syndrome of congenital anomalies, extracardiac anomalies of greater than minor severity, previous cardiac surgery, or associated cardiovascular anomalies necessitating aortic arch reconstruction or additional surgical procedures before the planned follow-up. Fluorescent in situ hybridization studies were not performed on all infants.
Study Design
Patients were randomly assigned to undergo hemodilution to a hematocrit level of approximately 25% versus 35%, with stratification according to surgeon and diagnostic group. The surgeons, anesthesiologists, intensivists, neurologists, and psychologists were blinded to treatment assignment[8].
Anesthesia and Perfusion Methods
Anesthetic and perfusion management have been described previously and did not differ between the two treatment groups[8]. The pH-stat strategy was used during cooling, low-flow hypothermic perfusion, and rewarming.
Transcranial Doppler Methods
A 2-MHz, range-gated, pulsed-wave transcranial Doppler sonographic probe (Multi-Dop T; DWL Elektronische Systeme GmbH, Sipplingen, Germany) was placed over the right (rarely left) temporal window to measure CBFV in the proximal (M1) segment of the middle cerebral artery. To ensure a reproducible window, the signal from the artery was adjusted to be accompanied by retrograde anterior cerebral artery (A1 segment) flow. After acceptable waveforms were achieved, probe position was secured with a bandage. Care was taken to ensure a constant position, insonation depth, sample volume, gain, and power for all measurements.
Mean flow velocity (Vm), peak systolic flow velocity (Vs), and peak end-diastolic flow velocity (Vd) were measured during hemodynamically stable intervals with recordings of at least 15 seconds duration at 8 time points: after induction of anesthesia, heparin administration, 10 minutes after cooling on CPB, 10 minutes after start of rewarming on CPB, immediately following CPB, and at 1, 6, and 18 hour post-CPB. Because of non-pulsatile perfusion, only Vm was measured during CPB. Mean arterial pressure (MAP), CPB flow rates, and temperatures were recorded simultaneously with TCD measurements.
As age affects CBFV and age at surgery varies with diagnosis, changes in CBFV for each patient were normalized relative to the patient’s own baseline value (defined as after induction of anesthesia)[9]. Every patient’s Vm at each time point after the baseline measurement was compared to the patient’s baseline Vm to determine the relative change in Vm (rVm; e.g., if Vm 18 hours post-CPB=10 cm/s and Vm after induction of anesthesia=20 cm/s, then rVm=50%). Middle cerebral artery resistance index (RI) was calculated as RI=(Vs − Vd)/Vs.
As previously reported, no differences in Vm, Vs, or Vd were found between the two hematocrit groups[8]. Similarly, no differences in rVm or RI were found between hematocrit groups.
Study Outcomes
Methods regarding outcome measurements have been described previously[8]. The primary outcome measure was the Bayley Scales of Infant Development-II at 1 year of age, which yields the Psychomotor Development Index (PDI) and Mental Development Index (MDI). Other 1 year outcomes included neurologic examination, head circumference, and brain injury on MRI. In the perioperative period, study outcomes were serum lactate, cardiac index (CI), rSO2 at 1 hour post-CPB, and durations of intubation, intensive care unit (ICU) and hospital stays.
Data Analysis
Diagnosis group comparisons were made using Fisher’s exact tests for categorical variables and analysis of variance or Kruskal-Wallis tests for quantitative variables. Linear regression models adjusting for repeated measurements were used to compare CBFVs within diagnosis group over time. Linear regression was used to examine the association of Vm and MAP after adjustment for diagnosis, (linear) age at surgery, and CPB flow rate. Linear regression adjusting for diagnosis, (linear) age at surgery, and total support time was used to examine the relationship of CBFV with early postoperative outcomes except length of intubation and ICU/ hospital stay, for which proportional hazards analyses were used.
PDI and MDI scores were our primary outcome variables. Associations of PDI/MDI with CBFV were examined using either unadjusted Pearson correlations or partial Pearson correlations after adjusting for family social class, followed by linear regression adjusting for diagnosis, neonatal status at surgery (age≤30 days), and family social class[10]. Because neonates undergoing surgery may be more susceptible to brain injury due to cerebral immaturity, adjustments were made for neonatal status at time of surgery in neurodevelopmental outcome analyses[11]. Associations of head circumference with CBFV measures were examined using Pearson correlations adjusting for age. Group comparisons based on abnormal neurologic exam results or brain injury on MRI were made using two-sample t-tests with equal variance. Logistic regression adjusting for neonatal status was used to examine the relationship between MRI findings and CBFV. All P values are two-sided and P<0.05 was used as the threshold for statistical significance.
Results
Subjects
Demographic, perioperative characteristics, and assessment outcomes are shown in Table 1. Of 124 patients enrolled in the original investigation, CBFV measurements were recorded for 100 patients: 43 with TGA, 36 with TOF, and 21 with VSD. Patients without TCD measurements were similar to patients with TCD measurements in all perioperative characteristics and follow-up assessment variables except those without TCD measurements had shorter aortic cross-clamp time during surgery (mean 55.8 min vs. 69.1 min, P=0.02).
Table 1.
Demographic, Perioperative Characteristics, and Assessment Outcomes According to Diagnosis Group
| Variable | TGA(n=43) | TOF(n=36) | VSD(n=21) | P |
|---|---|---|---|---|
|
| ||||
| Mean±SD or Median(Range) | ||||
| Preoperative characteristics | ||||
| Birth weight(kg) | 3.5±0.5 | 3.4±0.5 | 3.3±0.4 | 0.15 |
| Gestational age(wk) | 39.0±1.5 | 39.3±1.1 | 39.2±1.3 | 0.52 |
| Age at surgery(d) | 5(2–23) | 66(2–210) | 107(27–263) | <0.001 |
| Neonatal status at surgery(age≤30 d, %) | 100 | 14 | 5 | <0.001 |
| Operative characteristics | ||||
| Hematocrit on CPB(%) | 29±6 | 28±5 | 30±4 | 0.45 |
| Lowest temperature(ºC) | 16 ± 2 | 23 ± 5 | 25 ± 4 | <0.001 |
| Cross-clamp time(min) | 96±22 | 49±11 | 49±12 | <0.001 |
| Patients undergoing circulatory arrest(%) | 95 | 11 | 10 | <0.001 |
| Circulatory arrest time(min) | 20±13 | 2±7 | 4±12 | <0.001 |
| Total support time(min) | 146±34 | 84±14 | 81±18 | <0.001 |
| Flow rate at 10 min after cooling(mL/kg/min) | 124±37 | 122±36 | 116±34 | 0.76 |
| Flow rate at 10 min after warming(mL/kg/min) | 149±22 | 143±19 | 142±40 | 0.52 |
| Postoperative characteristics | ||||
| Lactate 1-hour post-CPB(mmol/L) | 4.0±1.4 | 2.0±0.1 | 1.8±1.0 | <0.001 |
| Cardiac index(L·min−1·m−2) | ||||
| 6-hour | 3.6±1.0 (n=12) | 4.3±1.6 (n=14) | 4.3±1.4 (n=7) | 0.42 |
| 18-hour | 4.1±1.4 (n=10) | 4.4±1.4 (n=13) | 5.6±1.4 (n=6) | 0.12 |
| Cerebral oxygen saturation(%) 1-hour post-CPB | 75.3±12.3 | 70.8±10.9 | 73.5±11.0 | 0.24 |
| Days intubated | 2.1(1.0–18.7) | 1.6(0.4–8.1) | 1.0(0.4–2.1) | <0.001 |
| Days in intensive care unit | 4(2–21) | 3(1–9) | 2(2–7) | <0.001 |
| Days in hospital | 8(5–43) | 7(3–13) | 5(3–10) | <0.001 |
| Follow-up assessment | ||||
| Bayley Scales of Infant Development-II | ||||
| Psychomotor Development Index | 89±14 (n=37) | 88±17 (n=32) | 79±17 (n=16) | 0.10 |
| Mental Development Index | 96±11 (n=36) | 94±12 (n=32) | 91±15 (n=16) | 0.41 |
| Abnormal neurological examination(%) | 53 (n=34) | 63 (n=32) | 62 (n=13) | 0.75 |
| Head circumference(z-score) | 0.1±1.0 (n=34) | −0.3±1.0 (n=32) | 0.2±1.0 (n=13) | 0.24 |
| Brain injury on MRI(%)* | 19 (n=16) | 47 (n=19) | 75 (n=4) | 0.07 |
TGA indicates D-transposition of the great arteries; TOF, tetralogy of Fallot or truncus arteriosus; VSD, ventricular septal defect or common atrioventricular canal defect; CPB, cardiopulmonary bypass.
Defined as hemosiderin, periventricular leukomalacia, focal infarction/stroke.
P values are determined by Fisher’s exact test for categorical variables, analysis of variance for variables with means reported, and Kruskal-Wallis tests for variables with medians reported.
Diagnosis groups were similar with respect to birth weight, gestational age, race, and gender. Patient age at time of surgery varied by diagnosis, with a median age of 5 days for the TGA group versus 66 days and 107 days for the TOF and VSD groups, respectively. Intraoperative management varied by diagnosis, with TGA patients undergoing significantly longer durations of total support time and DHCA. Hematocrit during CPB and outcomes at 1 year of age did not differ across diagnosis groups.
Cerebral Blood Flow Velocities
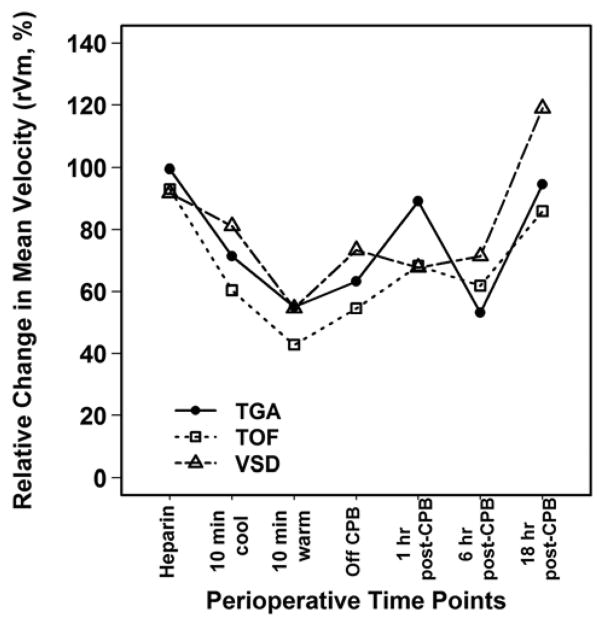
Cerebral blood flow velocities and RI for the diagnosis groups at different perioperative time points are presented in Table 2. Vm and Vs of the TGA group were significantly lower than those of the TOF and VSD groups throughout the study period (P<0.01 at every measurement). Although differences were seen between diagnosis groups in absolute measures of CBFV, the relative change in mean velocity, rVm, did not differ between the diagnosis groups across all time points (Figure 1). RI was similar between diagnosis groups through 1 hour post-CPB, with the TGA group having the highest RI at 18 hours post-CPB.
Table 2.
Cerebral Artery Blood Flow Velocities and Resistance Index Values
| Variable | TGA(n=43) | TOF(n=36) | VSD(n=21) | P |
|---|---|---|---|---|
|
| ||||
| Mean±SD | ||||
| Vm(cm/s) | ||||
| Post-induction | 25.3±11.6 | 47.8±21.0 | 43.4±19.3 | <0.001 |
| Heparinization | 23.6±11.2 | 42.5±21.9 | 40.2±15.5 | <0.001 |
| 10 min cool | 16.3±8.5 | 26.3±11.9 | 32.0±12.3 | <0.001 |
| 10 min warm | 11.8±7.0 | 18.1±7.6 | 20.2±9.6 | <0.001 |
| Off CPB | 13.7±6.4 | 23.0±10.4 | 28.8±11.8 | <0.001 |
| 1 hr post-CPB | 19.1±9.6 | 29.1±13.7 | 28.2±13.9 | <0.001 |
| 6 hr post-CPB | 11.9±4.8 | 26.0±16.0 | 29.3±10.7 | <0.001 |
| 18 hr post-CPB | 19.8±7.9 | 36.5±17.1 | 46.1±12.6 | <0.001 |
| Vs(cm/s) | ||||
| Post-induction | 55.0±17.9 | 97.1±36.2 | 94.7±29.8 | <0.001 |
| Heparinization | 52.8±18.5 | 90.9±37.9 | 91.6±23.3 | <0.001 |
| Off CPB | 40.4±16.8 | 62.7±23.9 | 77.9±18.1 | <0.001 |
| 1 hr post-CPB | 41.9±14.7 | 67.5±27.7 | 74.2±20.7 | <0.001 |
| 6 hr post-CPB | 37.0±15.5 | 59.7±28.7 | 76.4±19.6 | <0.001 |
| 18 hr post-CPB | 45.7±17.4 | 73.7±30.5 | 93.0±22.7 | <0.001 |
| Vd(cm/s) | ||||
| Post-induction | 9.1±8.9 | 18.6±13.9 | 20.8±16.0 | <0.001 |
| Heparinization | 6.6±7.8 | 14.8±13.0 | 14.3±10.0 | 0.001 |
| Off CPB | 2.8±4.1 | 5.5±7.1 | 6.2±7.8 | 0.06 |
| 1 hr post-CPB | 7.2±7.2 | 10.9±10.3 | 9.2±10.7 | 0.21 |
| 6 hr post-CPB | 2.8±3.4 | 9.7±8.8 | 9.6±6.7 | <0.001 |
| 18 hr post-CPB | 7.8±5.9 | 18.8±12.8 | 22.9±7.1 | <0.001 |
| rVm(%) | ||||
| Heparinization | 99.4±39.2 | 93.1±37.0 | 91.8±29.4 | 0.66 |
| 10 min cool | 71.4±43.8 | 60.4±23.0 | 81.1±34.7 | 0.11 |
| 10 min warm | 55.1±36.6 | 42.8±23.1 | 54.6±40.1 | 0.27 |
| Off CPB | 63.3±33.6 | 54.5±28.9 | 73.4±35.5 | 0.11 |
| 1 hr post-CPB | 89.1±56.9 | 68.3±30.1 | 67.8±33.2 | 0.08 |
| 6 hr post-CPB | 53.2±22.9 | 61.9±37.4 | 71.4±22.0 | 0.06 |
| 18 hr post-CPB | 94.6±56.2 | 86.0±48.4 | 118.9±55.0 | 0.12 |
| Resistance Index | ||||
| Post-induction | 0.84±0.13 | 0.81±0.12 | 0.79±0.13 | 0.27 |
| Heparinization | 0.88±0.12 | 0.85±0.11 | 0.85±0.09 | 0.31 |
| Off CPB | 0.93±0.11 | 0.92±0.09 | 0.92±0.10 | 0.91 |
| 1 hr post-CPB | 0.84±0.13 | 0.85±0.13 | 0.88±0.11 | 0.47 |
| 6 hr post-CPB | 0.93±0.08 | 0.86±0.10 | 0.88±0.07 | 0.002 |
| 18 hr post-CPB | 0.84±0.11 | 0.76±0.11 | 0.75±0.06 | 0.001 |
TGA indicates D-transposition of the great arteries; TOF, tetralogy of Fallot or truncus arteriosus; VSD, ventricular septal defect or common atrioventricular canal defect; Vm, mean flow velocity; Vs, peak systolic flow velocity; Vd, peak end-diastolic flow velocity; rVm, relative change in Vm; CPB, cardiopulmonary bypass.
P values are determined by analysis of variance.
Figure 1.
Relative Change in Mean Velocity (rVm, %) at Perioperative Time Points
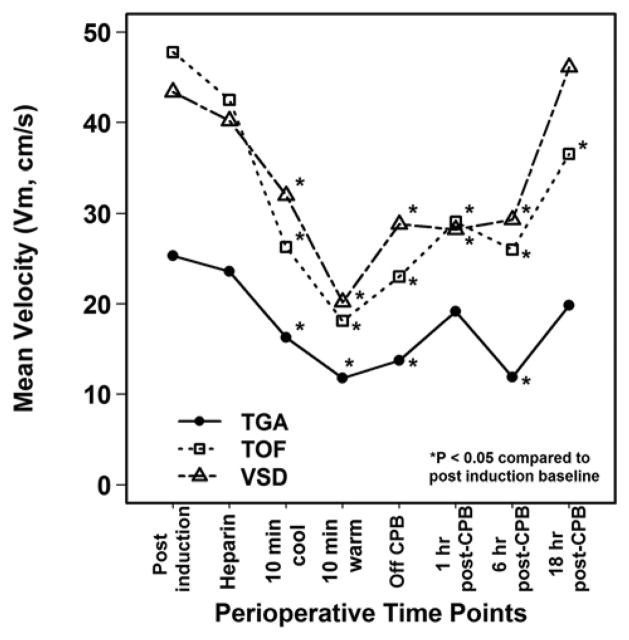
Within each diagnosis group, Vm was significantly lower than baseline during CPB and up to 1 hour post-CPB (Figure 2). By 18 hours post-CPB, Vm had recovered to the baseline in the TGA (P=0.10 versus baseline) and VSD (P=0.57) groups, but not in the TOF group (P=0.003). A similar pattern was observed for Vs and Vd except that Vd returned to baseline by 18 hours post-CPB in all groups (Table 2).
Figure 2.
Mean Velocity (Vm, cm/s) at Perioperative Time Points
After adjusting for diagnosis, age at surgery, and CPB flow rate, Vm at 10 minutes of cooling was associated with mean arterial pressure (P=0.02), but Vm at 10 minutes of rewarming was not.
Relationship of CBFVs to Early Postoperative Outcomes
After adjusting for diagnosis, age at surgery, and total support time, lower Vm, Vs, Vd, and rVm at 18 hours post-CPB were related to longer ICU duration of stay (P<0.05 for each). Similarly, at 18 hours post-CPB, lower Vm, Vs, and Vd were related to longer duration of intubation (P<0.05 for each). No significant associations between CBFVs and RI with other early postoperative outcomes (i.e., lactate, CI) were identified. At 1 hour post-CPB, after adjusting for diagnosis, age at surgery, and total support time, lower Vm, Vd, and rVm, and higher RI, were related to lower rSO2 (P<0.05 for each).
Relationship of CBFVs to Neurologic Outcome at 1 Year
Of the 100 patients with TCD measurements, 85 returned for neurodevelopmental evaluation at 1 year of age. Remaining families declined to participate (n=12), were not invited because of international residence (n=2), or completed only questionnaires (n=1). Patients who participated had similar perioperative characteristics and CBFVs to those who did not.
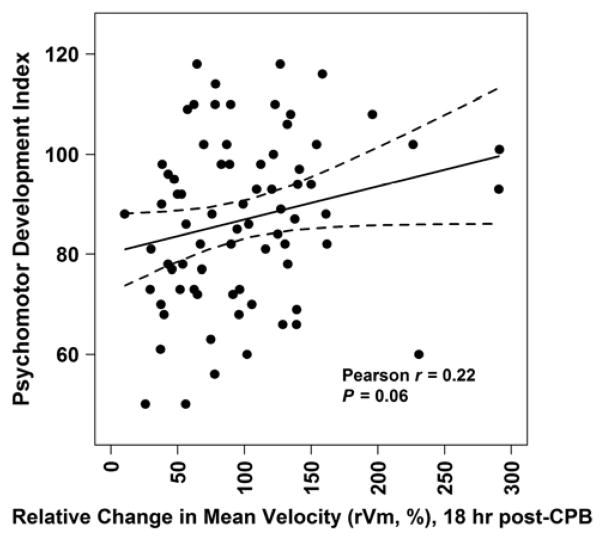
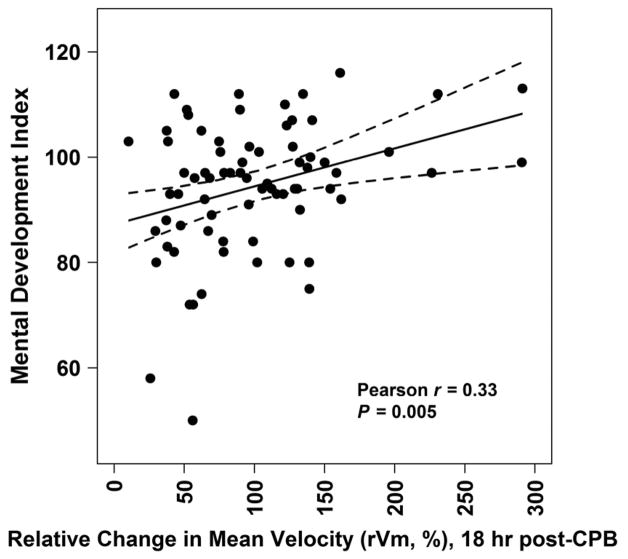
Partial Pearson correlations adjusting for family social class showed no associations of PDI or MDI with CBFV or RI measures from post-induction through 1 hour post-CPB, except lower MDI was associated with lower rVm at 1 hour post-CPB (r=0.22, P=0.04). However, lower PDI (r=0.23, P=0.052) and MDI (r=0.31, P=0.007) scores were associated with lower rVm at 18 hours post-CPB and lower MDI trended with higher RI at 18 hours post-CPB (r= −0.20, P=0.09). Figure 3 shows the unadjusted relationship of PDI and MDI with rVm at 18 hours post-CPB.
Figure 3.
Psychomotor Development Index (A) and Mental Development Index (B) versus Relative Change in Mean Velocity (rVm, %) at 18 Hours Post-Cardiopulmonary Bypass
In multivariable analysis adjusting for diagnosis group, neonatal status at surgery (age≤30 days), duration of DHCA, and family social class, lower Vm, Vs, Vd, and rVm at 18 hours post-CPB were associated with lower PDI and MDI scores, except Vs with MDI (Table 3). In addition, a lower MDI, but not PDI, was associated with higher RI. Variability in PDI and MDI scores accounted for by models including CBFVs (adjusted R2) were found to be as low as 3.5% for RI with PDI, and as high as 18.5% for rVm with MDI (Table 3).
Table 3.
Relationship of Cerebral Blood Flow Velocities and Resistance Index Values at 18 Hours Post-Cardiopulmonary Bypass with Bayley Scales of Infant Development Outcomes
| Variable | Psychomotor Development Index | Mental Development Index | ||
|---|---|---|---|---|
|
| ||||
| Slope β ± Standard Error (P) | Adjusted R2 | Slope β ± Standard Error (P) | Adjusted R2 | |
| Without CBFV | 1.0 | 7.7 | ||
| Vm (per cm/s) | 0.46±0.14 (0.002) | 13.3 | 0.27±0.11 (0.01) | 15.8 |
| Vs (per cm/s) | 0.29±0.08 (<0.001) | 16.8 | 0.12±0.06 (0.06) | 12.3 |
| Vd (per cm/s) | 0.44±0.21 (0.04) | 6.5 | 0.37±0.15 (0.01) | 15.5 |
| rVm (per %) | 0.079±0.035 (0.03) | 7.1 | 0.074±0.024 (0.004) | 18.5 |
| Resistance Index | −29.5±19.2 (0.13) | 3.5 | −36.4±13.3 (0.008) | 16.7 |
CBFV indicates cerebral blood flow velocity; Vm, mean flow velocity; Vs, peak systolic flow velocity; Vd, peak end-diastolic flow velocity; rVm, relative change in Vm.
Linear regressions include adjustments for diagnosis, neonatal status, duration of deep hypothermic circulatory arrest, and family social class.
The relationships of PDI and MDI scores with Vm, rVm or RI were not significantly influenced by diagnosis. However, after adjusting for neonatal status, duration of DHCA, and social status, there was a significant interaction effect of diagnosis group on the relationship between Vs (PDI: P=0.04; MDI: P=0.03) or Vd (MDI: P=0.03) and neurodevelopmental outcome at 1 year. Lower PDI scores were significantly associated with lower Vs at 18 hours post-CPB for the TGA and VSD groups (P=0.002 and P=0.003, respectively). Similarly, lower MDI scores were significantly associated with lower Vs and Vd at 18 hours post-CPB only for the VSD group (P=0.002 for each). Neonatal status did not modify the relationship between CBFVs and neurodevelopmental outcome. Neither CBFVs nor RI were associated with either abnormal neurologic examination or head circumference.
Relationship of CBFVs to Brain MRI at 1 Year
Among the patients who returned for neurodevelopmental assessment, 39 patients underwent brain MRI. Patients with MRI data had similar perioperative characteristics and CBFVs to those without.
Abnormal MRI findings were found in 18 (46%) patients: hemosiderin foci alone (11), hemosiderin with Chiari I malformations (2), and hemosiderin with periventricular leukomalacia (PVL, 1); small focal infarction/stroke (1); and minor developmental findings (3)[12].
After adjusting for neonatal status at surgery, significantly greater odds of brain injury (i.e., hemosiderin, PVL, focal infarction/stroke) were associated with higher rVm at 6 hours (1.04 per 1%, CI 1.008–1.081, P=0.02) and 18 hours post-CPB (1.03 per 1%, 1.008–1.053, P=0.008). A similar association with higher Vs (1.04 per cm/s, 1.000–1.070, P=0.048) at 18 hours post-CPB was also observed. Patients with brain injury had significantly lower PDI than those who did not (78±17 versus 89±16, P=0.048), while MDI did not differ between the two groups.
Comment
We found that among infants with CHD undergoing biventricular repair without aortic arch reconstruction, lower CBFV at 18 hours post-CPB was associated with lower PDI and MDI scores at age 1 year. Additionally, lower CBFV was associated with adverse early postoperative outcomes, suggesting that low CBFV as a measure of low brain perfusion is a marker of suboptimal postoperative hemodynamics. This study is the first to report a significant relationship between perioperative CBF as measured by TCD sonography and neurodevelopmental outcome at age 1 year in patients with CHD, as well as the largest study utilizing TCD sonography in the current era of higher hematocrit and pH-stat acid-base management.
A similar study by Robertson et al. in a comparable but smaller cohort found no relationship between relative changes in CBFV and PDI or MDI scores at 1 year follow-up[13]. Their findings may be related to the smaller number of subjects and the timing of CBFV measurements (up to 5 hours postoperatively), as cardiac function and hemodynamics in the first few postoperative hours tend to be more labile than at 18 hours post-CPB. Associations between CBFV and neurodevelopmental outcome have been reported in patients without CHD[14, 15].
Hemosiderin on brain MRI in many of these patients is related to diagnosis and older age at surgery[12]. As a small number of patients had brain MRIs, autoregulation was likely impaired in some subjects, and because of collinearity between CBFV, age at surgery, diagnosis, and hemosiderin lesions, it is not possible to determine the causality of the relationship between higher CBFV and the presence of hemosiderin on brain MRI at 1 year of age[16].
Absolute values of CBFV were lower in the TGA group versus the older TOF and VSD groups. Cerebral blood flow is inversely proportional to hematocrit, and although the degree of cyanosis before surgery was greatest in the TGA group, the lower CBFVs in this group are likely not related to a higher hematocrit because the compensatory changes associated with chronic hypoxia will return CBF to normal levels in the presence of normal cerebral autoregulation[17–19]. Measurements of CBFVs in infants and older children made by Bode et al. show that CBFV increases from birth to a peak at about 6 years of age[9]. As rVm did not differ between diagnosis groups, the difference in absolute CBFVs between the diagnosis groups is most likely an age-related phenomenon.
Our findings are constrained by important limitations. Measurements of CBFV were not performed prior to induction of anesthesia. The effect of inhalational anesthetics on CBF depends on the balance between direct vasodilation and indirect vasoconstriction from a reduction in cerebral oxygen metabolism, with a net effect that maintains or lowers CBF[20]. The majority of patients had high-dose fentanyl anesthesia which leads to a minimal decrease in CBF in animal studies[21]. Our ability to find significant associations may be limited by the variability inherent in TCD sonography and small sample size. As pH-stat was used during CPB, the generalizability of our findings to alpha-stat is limited[22]. Because MRI diffusion tensor imaging analysis has not been completed, we were unable to analyze associations between CBFV and white matter injury. Cerebral metabolism was not measured, so we could not evaluate the balance between cerebral oxygen delivery and consumption. Finally, our study design did not allow determination of whether associations between CBFVs and neurodevelopmental outcomes were causal.
Conclusion
We found that postoperative CBFV in infants undergoing CPB for biventricular repair of CHD is related to early postoperative outcomes and neurodevelopmental outcome at age 1 year. Our findings may indicate that low CBFV is a marker of suboptimal cerebral perfusion after pediatric cardiac surgery. With further study of CBF and oxygen balance in the perioperative period, management strategies may be developed to optimize neurodevelopmental outcomes in these at-risk patients.
Acknowledgments
We are indebted to the patients and families for participating in this research.
Supported by grants HL 063411 and RR 02172 from the National Institutes of Health, the Farb Family Fund, and Children’s Hospital Medical Center Anesthesia Foundation intramural funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dent CL, Spaeth JP, Jones BV, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2005;130(6):1523–1530. doi: 10.1016/j.jtcvs.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 2.McQuillen PS, Barkovich AJ, Hamrick SE, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38(2 Suppl):736–741. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 3.Wypij D, Newburger JW, Rappaport LA, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: The Boston circulatory arrest trial. J Thorac Cardiovasc Surg. 2003;126(5):1397–1403. doi: 10.1016/s0022-5223(03)00940-1. [DOI] [PubMed] [Google Scholar]
- 4.Kussman BD, Wypij D, Laussen PC, et al. Relationship of intraoperative cerebral oxygen saturation to neurodevelopmental outcome and brain magnetic resonance imaging at 1 year of age in infants undergoing biventricular repair. Circulation. 2010;122(3):245–254. doi: 10.1161/CIRCULATIONAHA.109.902338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop CC, Powell S, Rutt D, Browse NL. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: A validation study. Stroke. 1986;17(5):913–915. doi: 10.1161/01.str.17.5.913. [DOI] [PubMed] [Google Scholar]
- 6.Hillier SC, Burrows FA, Bissonnette B, Taylor RH. Cerebral hemodynamics in neonates and infants undergoing cardiopulmonary bypass and profound hypothermic circulatory arrest: Assessment by transcranial Doppler sonography. Anesth Analg. 1991;72(6):723–728. doi: 10.1213/00000539-199106000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Kussman BD, Gauvreau K, DiNardo JA, et al. Cerebral perfusion and oxygenation after the Norwood procedure: Comparison of right ventricle-pulmonary artery conduit with modified Blalock-Taussig shunt. J Thorac Cardiovasc Surg. 2007;133(3):648–655. doi: 10.1016/j.jtcvs.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 8.Newburger JW, Jonas RA, Soul J, et al. Randomized trial of hematocrit 25% versus 35% during hypothermic cardiopulmonary bypass in infant heart surgery. J Thorac Cardiovasc Surg. 2008;135(2):347–354. 354 e341–344. doi: 10.1016/j.jtcvs.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 9.Bode H, Wais U. Age dependence of flow velocities in basal cerebral arteries. Arch Dis Child. 1988;63(6):606–611. doi: 10.1136/adc.63.6.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollingshead AB. Four factor index of social status. New Haven, Connecticut: Yale University; 1975. [Google Scholar]
- 11.Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management: A scientific statement from the American Heart Association. Circulation. 2012;126(9):1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 12.Soul JS, Robertson RL, Wypij D, et al. Subtle hemorrhagic brain injury is associated with neurodevelopmental impairment in infants with repaired congenital heart disease. J Thorac Cardiovasc Surg. 2009;138(2):374–381. doi: 10.1016/j.jtcvs.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson DR, Justo RN, Burke CJ, Pohlner PG, Graham PL, Colditz PB. Perioperative predictors of developmental outcome following cardiac surgery in infancy. Cardiol Young. 2004;14(4):389–395. doi: 10.1017/S104795110400407X. [DOI] [PubMed] [Google Scholar]
- 14.Kirimi E, Tuncer O, Atas B, Sakarya ME, Ceylan A. Clinical value of color Doppler ultrasonography measurements of full-term newborns with perinatal asphyxia and hypoxic ischemic encephalopathy in the first 12 hours of life and long-term prognosis. The Tohoku Journal of Experimental Medicine. 2002;197(1):27–33. doi: 10.1620/tjem.197.27. [DOI] [PubMed] [Google Scholar]
- 15.Ilves P, Talvik R, Talvik T. Changes in Doppler ultrasonography in asphyxiated term infants with hypoxic-ischaemic encephalopathy. Acta Paediatr. 1998;87(6):680–684. doi: 10.1080/080352598750014111. [DOI] [PubMed] [Google Scholar]
- 16.Bassan H, Gauvreau K, Newburger JW, et al. Identification of pressure passive cerebral perfusion and its mediators after infant cardiac surgery. Pediatric Research. 2005;57(1):35–41. doi: 10.1203/01.PDR.0000147576.84092.F9. [DOI] [PubMed] [Google Scholar]
- 17.Ainslie PN, Ogoh S. Regulation of cerebral blood flow in mammals during chronic hypoxia: A matter of balance. Experimental Physiology. 2010;95(2):251–262. doi: 10.1113/expphysiol.2008.045575. [DOI] [PubMed] [Google Scholar]
- 18.Paut O, Bissonnette B. Effects of temperature and haematocrit on the relationships between blood flow velocity and blood flow in a vessel of fixed diameter. British journal of anaesthesia. 2002;88(2):277–279. doi: 10.1093/bja/88.2.277. [DOI] [PubMed] [Google Scholar]
- 19.Kussman BD, Wypij D, DiNardo JA, et al. Cerebral oximetry during infant cardiac surgery: Evaluation and relationship to early postoperative outcome. Anesth Analg. 2009;108(4):1122–1131. doi: 10.1213/ane.0b013e318199dcd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matta BF, Heath KJ, Tipping K, Summors AC. Direct cerebral vasodilatory effects of sevoflurane and isoflurane. Anesthesiology. 1999;91(3):677–680. doi: 10.1097/00000542-199909000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Milde LN, Milde JH, Gallagher WJ. Cerebral effects of fentanyl in dogs. British Journal of Anaesthesia. 1989;63(6):710–715. doi: 10.1093/bja/63.6.710. [DOI] [PubMed] [Google Scholar]
- 22.Patel RL, Turtle MR, Chambers DJ, Newman S, Venn GE. Hyperperfusion and cerebral dysfunction. Effect of differing acid-base management during cardiopulmonary bypass. Eur J Cardiothorac Surg. 1993;7(9):457–463. doi: 10.1016/1010-7940(93)90274-f. discussion 464. [DOI] [PubMed] [Google Scholar]