Abstract
Children with hypoplastic left heart syndrome (HLHS) are at risk for neurodevelopmental dysfunction; prenatal factors may play a role in this predilection. Cerebral blood flow profiles are abnormal in fetuses with HLHS, raising the possibility that cerebral hemodynamics in utero may be related to neurodevelopmental abnormalities. Prenatal aortic valvuloplasty for fetal aortic stenosis with evolving HLHS is technically feasible and improves left heart hemodynamics. This study aimed to assess the effects of prenatal intervention on cerebral blood flow profiles and head circumference in fetuses with evolving HLHS. Seventy fetuses underwent prenatal aortic valvuloplasty for evolving HLHS (median 23 weeks gestation). Among 46 fetuses that had successful valvuloplasty and available data, middle cerebral artery (MCA) pulsatility (PI) and resistive (RI) indices were abnormal (Z-scores −1.7 ± 1.1 and −2.2 ± 1.4, p < 0.001). Early post-valvuloplasty (n = 33) and at late gestation follow-up (n = 28), MCA PI and RI Z-scores remained low with no difference from pre- or early postintervention. Fetal head circumference was normal, as were umbilical artery PI and RI Z-scores. Cerebral blood flow characteristics are abnormal in mid-gestation fetuses with evolving HLHS, suggesting low cerebral vascular impedance. The mechanisms and significance of these abnormalities are unknown. Prenatal aortic valvuloplasty did not have a major impact on these indices.
Keywords: Fetal surgery, Balloon aortic valvuloplasty, Brain sparing effect, Congenital heart disease, Hypoplastic left heart syndrome, Microcephaly
Introduction
Major noncardiac morbidities in patients with congenital heart disease include neurologic and developmental abnormalities (Goldberg et al., 2000, Ikle et al., 2003, Limperopoulos et al., 2000, Mahle et al., 2002, Mahle and Wernovsky, 2004, Majnemer et al., 2006, Massaro et al., 2008, Miller et al., 2007 and Wernovsky et al., 2000). There is growing evidence that neurodevelopmental anomalies in the setting of congenital heart disease may be due not only to perioperative insult but also to impaired brain growth and development prenatally (Hinton et al., 2008, Limperopoulos et al., 2000, Mahle et al., 2002, Miller et al., 2007 and Te Pas et al., 2005). Among the various types of congenital heart disease, hypoplastic left heart syndrome (HLHS) appears to carry a particularly high risk of abnormal neurodevelopmental outcome (Goldberg et al., 2000, Hinton et al., 2008, Ikle et al., 2003, Mahle and Wernovsky, 2004 and Wernovsky et al., 2000). Although factors contributing to this predilection may include hypoxic-ischemic injury incurred during surgery, as well as peripartum and neonatal hemodynamic insult, there is an emerging literature indicating that prenatal factors may play an important role in the relatively high risk of adverse neurodevelopmental outcome in individuals with HLHS. For example, several studies have documented a high prevalence of microcephaly in newborns and fetuses with HLHS (Hinton et al., 2008, Rosenthal, 1996 and Shillingford et al., 2007), there is evidence of white matter injury consistent with ischemia in fetuses with HLHS (Hinton et al. 2008) and the cerebral microvasculature is abnormal in some fetuses with HLHS (Kinnear et al. 2008).
Cross-sectional studies using Doppler ultrasound have shown that cerebral blood flow velocity profiles are abnormal in third-trimester fetuses with established HLHS (Donofrio et al., 2003, Kaltman et al., 2005 and Modena et al., 2006). In particular, diastolic blood flow velocity in the middle cerebral artery (MCA) is relatively high and the pulsatility (PI) and resistive indices (RI) are accordingly low, suggestive of low cerebral vascular impedance presumably due to cerebral vasodilation. Abnormal cerebral vasodilation in fetuses with HLHS might be explained as part of an autoregulatory process by which the cerebral circulation responds to abnormal flow characteristics and/or abnormal oxygen or metabolic substrate content (Donofrio et al. 2003; Pearce 1987; Vyas et al., 1990 and Wladimiroff, 1987). In the physiologically normal fetus, highly oxygenated blood returning from the placenta is preferentially directed right-to-left through the foramen ovale, into the left ventricle (LV) and out the aortic valve to the myocardial and cerebral circulations, with relatively little mixing with poorly oxygenated systemic venous return (Edelstone and Rudolph 1979). In contrast, in fetuses with HLHS or evolving HLHS, the oxygen content of cerebral arterial blood is likely decreased due to elimination of the normal intracardiac streaming patterns and preferential flow of umbilical venous return to the cerebral circulation. In addition to the relatively low oxygen content, fetal cerebral blood flow in the setting of HLHS is derived from right ventricular output, which must pass through the ductus arteriosus and retrograde around the aortic arch before flowing to the brain. However, the role and mechanisms of cerebral autoregulation in the mid-gestation fetus are debatable (Ashwal et al., 1980, Chihara et al., 2003, Gleason et al., 1990, Papile et al., 1985 and van Bel et al., 1995). Exactly how and why cerebral arterial velocity profiles are abnormal in fetuses with HLHS is unclear, as is the relationship of such abnormalities to prognosis.
In mid-gestation fetuses with aortic stenosis, a normal size or dilated LV and depressed LV function, a constellation of physiologic features, including retrograde blood flow in the transverse aortic arch, left-to-right flow across the foramen ovale and abnormal left ventricular (LV) inflow, predicts evolution to HLHS postnatally (Makikallio et al. 2006). Eight years ago, we undertook a program of mid-gestation fetal aortic valvuloplasty in an effort to alter the natural history of evolving HLHS in utero (Tworetzky et al., 2004 and Wladimiroff, 1987). The basic hypothesis behind this undertaking was that relieving obstruction to LV outflow in fetuses with aortic stenosis and evolving HLHS, regardless of the cause(s) of the disease, will facilitate growth and enhanced function of the left heart. As previously reported, technically successful in utero aortic valvuloplasty results in altered left heart physiology, with improved LV inflow, systolic function and antegrade flow in the ascending aorta and arch (Selamet Tierney et al. 2007). Although successful aortic valve dilation leads to improvement in fetal hemodynamics, it also causes aortic regurgitation (AR) in some cases, which is of uncertain physiologic significance but seems to be well tolerated. While the main objective of prenatal aortic valvuloplasty for evolving HLHS is to alter the natural history of the disease and facilitate postnatal survival with a biventricular circulation, it is possible that there are physiologic advantages as well, even if the patient does not achieve a biventricular circulation. One of the potential physiologic benefits of improved flow through the left heart and antegrade aortic outflow is normalization of cerebral hemodynamics. To study this possibility, however, it will be necessary to determine the characteristics of cerebral blood flow in mid-gestation fetuses with evolving HLHS, as well as the effects of prenatal aortic valvuloplasty and AR on cerebral blood flow, which is the purpose of the present investigation.
Methods
Patients and prenatal aortic valvuloplasty
Since 2000, mid-gestation (20–31 weeks) fetuses with aortic stenosis were considered for prenatal aortic valvuloplasty if progression to HLHS was considered to be highly likely on the basis of previously published criteria (Makikallio et al. 2006) and LV size was considered potentially sufficient to sustain a biventricular circulation. The procedure was performed with ultrasound guidance and direct left ventricular (LV) puncture, using previously reported techniques (Marshall et al., 2005 and Tworetzky et al., 2004). A technically successful aortic valvuloplasty procedure was defined as one in which the aortic valve was crossed and a balloon inflated, with clear evidence of increased flow across the valve and/or new AR. Procedures were performed according to compassionate use protocols that were approved by the institutional review boards of Children’s Hospital and the Brigham and Women’s Hospital. Parents were extensively counseled about the risks and benefits of this experimental procedure and provided written informed consent.
Ultrasound evaluation
Complete cross-sectional and Doppler fetal echocardiograms were performed according to standard clinical practice at our center 1 to 2 days prior to intervention, early (12–24 hours) postintervention and at late-gestation follow-up. All studies were read and measurements performed prospectively by a single echocardiographer. In addition to anatomic features of the left heart, physiologic features assessed included mitral valve (MV) inflow time and inflow velocity-time integral, presence and severity of mitral regurgitation, estimated LV pressure and the extent of atrial septal restriction. Middle cerebral artery (MCA) flow was interrogated using standard techniques (Kaltman et al., 2005 and Kurmanavicius et al., 1997). Scanning the fetal head in an axial plane, the orbits were identified and Doppler color flow mapping was used to identify the circle of Willis and the MCA (Fig. 1). Pulsed-wave Doppler was used to measure blood flow velocity in the MCA, using standard methodology, with the sample volume placed in the color Doppler jet just after the takeoff of the MCA and an angle of insonation <30 degrees and as small as possible (Fig. 1). Similarly, umbilical artery (UA) flow velocities were measured using pulsed wave Doppler. For both the MCA and UA, peak systolic velocity, end-diastolic velocity and mean velocity were measured from stable signals during fetal apnea. The systolic-to-diastolic velocity ratio, pulsatility index (PI; [systolic velocity – diastolic velocity]/mean velocity), and resistive index (RI; [systolic velocity -diastolic velocity]/systolic velocity) were calculated. RI and PI Z-scores for the MCA and UA were computed as the number of standard deviations above or below previously published gestational age-based normative data (Arduini and Rizzo, 1990 and Kurmanavicius et al., 1997). By definition, Z-scores between −2 and 2 were considered normal. The MCA PI-to-UA PI and MCA RI-to-UA RI ratios were calculated as the cerebral:placental (CPR) PI and RI ratios, respectively. Pre- and postintervention evaluation of MCA and UA flow was performed routinely from mid-2004 onward, but only sporadically prior to that time.
Figure 1.
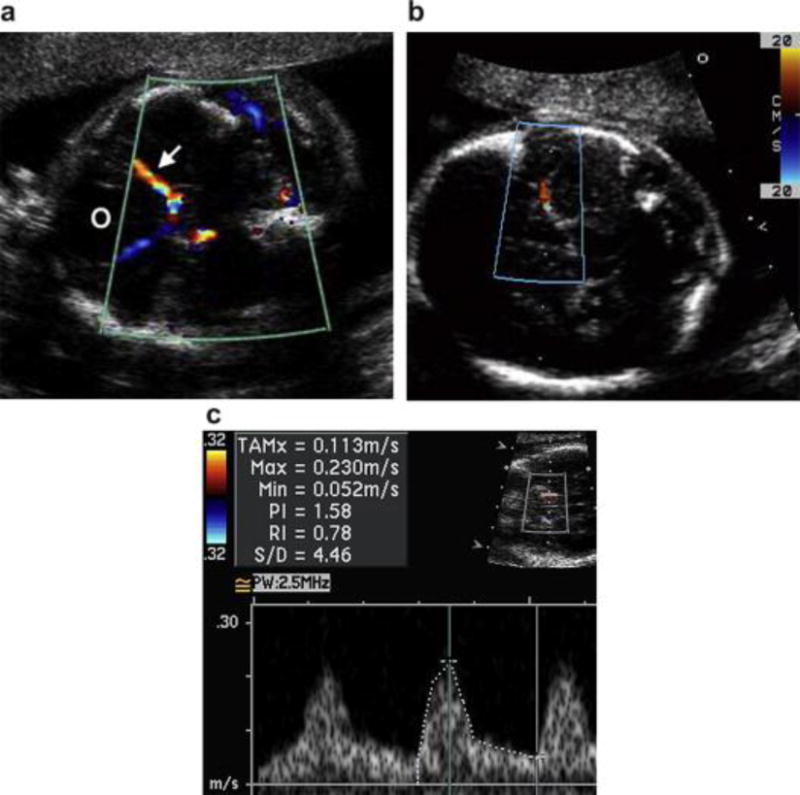
Ultrasound images in three different patients demonstrating (a) the identification of the orbits (O), circle of Willis and middle cerebral artery (MCA) (arrow) using color Doppler, (b) alignment of the pulsed wave Doppler sample volume in the proximal MCA at a low angle of insonation as it runs laterally along the petrous bone and (c) the pulsed wave Doppler spectrum and measured velocities.
Estimated gestational age was based on maternal dates or first-trimester ultrasound measurement, if available. Fetal biometry was performed with standard measurement of femur length, abdominal circumference, biparietal diameter and head circumference. Fetal weight was estimated by Hadlock’s formula (Hadlock et al. 1984) and fetal weight Z-score and percentile were calculated from equations reported by Doubilet et al. (Doubilet et al. 1997). Z-scores for fetal head circumference and biparietal diameter were calculated from published equations (Kurmanavicius et al. 1999).
Data analysis
The primary hypotheses of this study were that, (1) MCA PI and RI Z-scores are lower than normal in mid-gestation fetuses with aortic stenosis and evolving HLHS; and (2) MCA PI and RI Z-scores improve after technically successful prenatal aortic valvuloplasty. Baseline PI and RI Z-scores were compared with the reported normal population mean using one-sample t-test. Preintervention data were compared with and postintervention data using paired t-test. A secondary hypothesis was that post-valvuloplasty AR has minimal acute impact on MCA flow characteristics in this cohort. Both postintervention Z-scores and changes in Z-scores from pre- to postintervention were compared between patients with and without significant AR. To determine the relationship between baseline cerebral hemodynamics and anatomic and physiologic features in fetuses with evolving HLHS, the aforementioned cerebral blood flow Z-scores were analyzed for association with specific anatomic and physiologic features, including aortic valve, mitral valve, LV and ascending aortic Z-scores, direction of flow in the ascending aorta and transverse aortic arch after intervention, LV filling parameters and LV systolic function. Z-scores and Z-score changes were analyzed both as continuous and dichotomous variables (Z-score normal or lower than normal; Z-score improvement ≥1 standard deviation). Uni- and multivariable analysis was performed to assess relationships between these measures and the independent variables listed above.
Results
Patients and prenatal aortic valvuloplasty
Between March 2000 and October 2008, 70 fetuses underwent in utero aortic valvuloplasty for evolving HLHS at a median gestational age of 23 weeks. In 52 fetuses, intervention was technically successful. Procedural and postnatal outcomes in these patients were recently reported (McElhinney et al. 2009).
Doppler MCA flow characteristics
Baseline
Preintervention MCA and UA flow data were available in 46 fetuses with evolving HLHS at 24.3 ± 3.0 weeks gestation (Fig. 2). In this cohort, population means for the MCA PI and RI Z-scores were below normal (−1.7 ± 1.1 and −2.2 ± 1.4, both p < 0.001). Individual MCA PI and RI Z-scores were abnormally low in 41% (n = 19) and 57% (n = 26) of fetuses, respectively; no fetus had an MCA PI or RI Z-score that was higher than normal. On average, UA PI and RI Z-scores were normal (0.2 ± 1.1 and 0.2 ± 1.6). UA PI Z-scores were higher than normal in two fetuses and below normal in one; RI Z-scores were higher than normal in five fetuses and lower than normal in three. The CPR RI was “very low” (<1) in 37% of fetuses (16 of 43 with both MCA and UA flow data). MCA flow indices were not significantly associated with left heart anatomic dimensions. MV inflow time Z-scores were well below average (−2.6 ± 1.7, p < 0.001) overall, but higher in fetuses with low MCA PI (p = 0.03) and RI (p = 0.04) Z-scores than fetuses with normal MCA Z-scores. There was a trend toward lower MCA RI (−3.1 ± 0.7 vs. −2.0 ± 1.4, p = 0.08) and PI (−2.3 ± 0.3 vs. −1.6 ± 1.1, p = 0.14) Z-scores in fetuses with an intact or highly restrictive atrial septum than those without, but there were only six fetuses with this finding.
Figure 2.
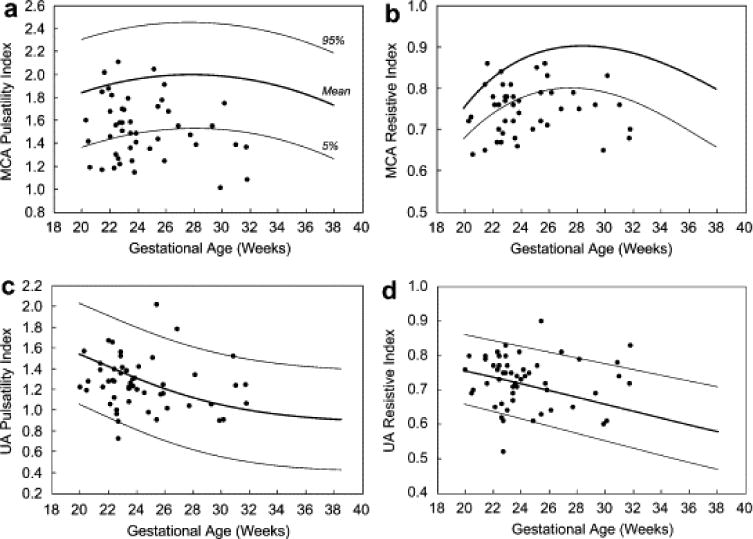
Scatter plots depicting (a) middle cerebral artery (MCA) pulsatility index (PI), (b) MCA resistive index (RI), (c) umbilical artery (UA) PI and (d) UA RI for the 46 fetuses with preintervention MCA and UA Doppler data available. Mean and 95% confidence intervals for normal fetuses, as reported previously (Arduini and Rizzo, 1990 and Kurmanavicius et al., 1997), are represented by the broad and solid lines, respectively. A 95% confidence interval was not calculated for MCA RI in the normative data set (Kurmanavicius et al. 1997).
Following prenatal aortic valvuloplasty
In 33 of the 52 fetuses that underwent successful aortic valvuloplasty, both pre- and postintervention MCA and UA Doppler data were available (Table 1). In this subset, MCA PI and RI Z-scores were below normal at baseline (−1.6 ± 1.1 and −2.2 ± 1.4, both p < 0.001) and UA PI and RI Z-scores were normal (−0.2 ± 1.1 and 0.2 ± 1.6). The CPR RI ratio was very low (<1) in 37% of fetuses (median 1.03).
Table 1.
Preintervention, early postintervention and late-gestation follow-up Doppler data among fetuses undergoing prenatal aortic valvuloplasty1
| Preintervention | Early postintervention | Follow-up2 | |
|---|---|---|---|
| Gestational age (wk) | 23.8 ± 2.1 | 24.1 ± 2.1 | 35.2 ± 2.2 |
| Middle cerebral artery | |||
| Systolic velocity (cm/s) | 22.2 ± 5.0 | 24.5 ± 6.1 | 44.8 ± 14.8 |
| Diastolic velocity (cm/s) | 5.3 ± 1.3 | 5.4 ± 1.7 | 12.5 ± 5.2 |
| Mean velocity (cm/s) | 10.9 ± 2.2 | 12.1 ± 2.9 | 25.3 ± 8.3 |
| Systolic:diastolic velocity ratio | 4.3 ± 1.2 | 4.8 ± 1.3 | 3.9 ± 1.2 |
| Pulsatility index | 1.54 ± 0.28 | 1.58 ± 0.24 | 1.32 ± 0.26 |
| Resistive index | 0.75 ± 0.06 | 0.77 ± 0.06 | 0.71 ± 0.09 |
| Pulsatility index Z-score | −1.6 ± 1.1 | −1.5 ± 1.0 | −2.0 ± 1.0* |
| Resistive index Z-score | −2.2 ± 1.4 | −1.8 ± 1.4 | −1.8 ± 1.4 |
| Umbilical artery | |||
| Systolic velocity (cm/s) | 31.3 ± 7.4 | 33.9 ± 7.6 | 49.5 ± 10.0 |
| Diastolic velocity (cm/s) | 8.5 ± 3.3 | 10.0 ± 3.7 | 17.7 ± 6.9 |
| Mean velocity (cm/s) | 18.3 ± 4.9 | 20.7 ± 4.7 | 31.9 ± 8.3 |
| Systolic:diastolic velocity ratio | 4.1 ± 1.5 | 3.7 ± 1.4 | 3.1 ± 1.0 |
| Pulsatility index | 1.27 ± 0.32 | 1.17 ± 0.23 | 1.06 ± 0.27 |
| Resistive index | 0.73 ± 0.09 | 0.70 ± 0.08 | 0.65 ± 0.09 |
| Pulsatility index Z-score | −0.2 ± 1.1 | −0.5 ± 1.0 | 0.5 ± 1.1* |
| Resistive index Z-score | 0.2 ± 1.6 | −0.3 ± 1.5 | 0.8 ± 1.6† |
| MCA:UA PI ratio | 1.27 ± 0.32 | 1.41 ± 0.25 | 1.33 ± 0.45 |
| MCA:UA RI ratio | 1.05 ± 0.14 | 1.12 ± 0.11 | 1.13 ± 0.24‡ |
MCA = middle cerebral artery; UA = umbilical artery; PI = pulsatility index; RI = resistive index.
Data only presented for the 33 fetuses with measurements available pre- and early postintervention.
p values are not presented for comparison of absolute measurements, which normally change over gestation.
p < 0.01 vs. early postintervention.
p < 0.05 vs. early postintervention.
p < 0.05 vs. preintervention.
On early postintervention evaluation, MCA and UA PI and RI Z-scores were not significantly changed from the preintervention ultrasound (Fig. 3). However, there was a significant increase in CPR (median 1.10, p = 0.04) and the percentage of fetuses with CPR RI <1 decreased to 7% (p = 0.02). There were no obvious anatomic or physiologic factors associated with improvement.
Figure 3.
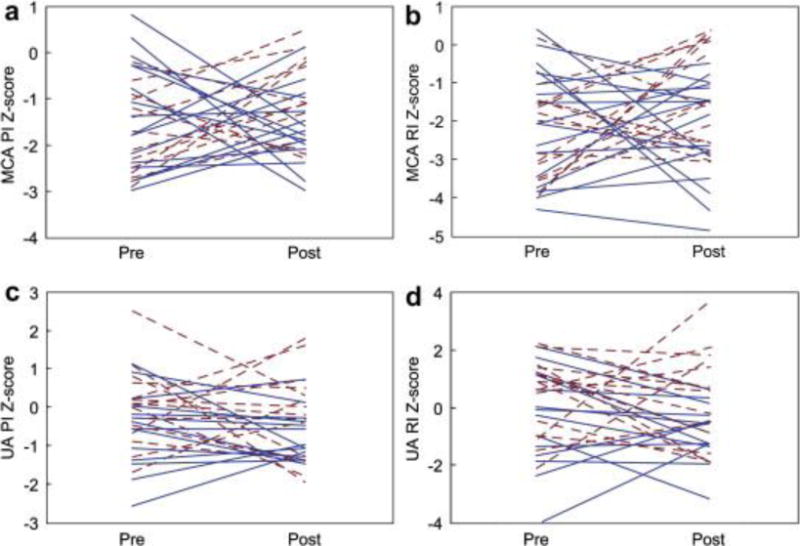
Changes in (a) middle cerebral artery (MCA) pulsatility index (PI), (b) MCA resistive index (RI), (c) umbilical artery (UA) PI and (d) UA RI Z-scores from pre- to early postintervention among fetuses that underwent technically successful aortic valvuloplasty. Fetuses with significant aortic regurgitation (AR) after intervention are depicted with the dashed lines and fetuses without AR are represented by solid lines.
At late gestation follow-up in 28 fetuses, MCA PI and RI Z-scores remained low, with no difference from pre- or early postintervention (Table 1). Overall, the CPR RI remained lower than preintervention (median 1.07) but the percentage of fetuses with a very low CPR RI was 32%, which was up from the early post-valvuloplasty study and no different than preintervention.
Aortic regurgitation
Of the 52 fetuses in which prenatal aortic valve dilation was technically successful, 19 (37%) had moderate or severe AR after the procedure and four did not survive to term (three with AR). A larger balloon:annulus diameter ratio was associated with increased likelihood of AR. At late gestation follow-up in 26 surviving fetuses with available data (34 ± 3 weeks), significant AR was present in only one. In fetuses with significant AR after valvuloplasty, MCA PI and RI were modestly higher than preintervention (0.27 ± 0.44 and 0.05 ± 0.08, respectively), whereas these indices fell slightly in fetuses without AR (−0.03 ± 0.38 and −0.01 ± 0.08, respectively); there was a significant difference between fetuses with and without AR (both p = 0.05; Fig. 4). However, the presence of significant AR was not associated with postintervention MCA or UA flow indices or changes in Z-scores for these flow parameters.
Figure 4.
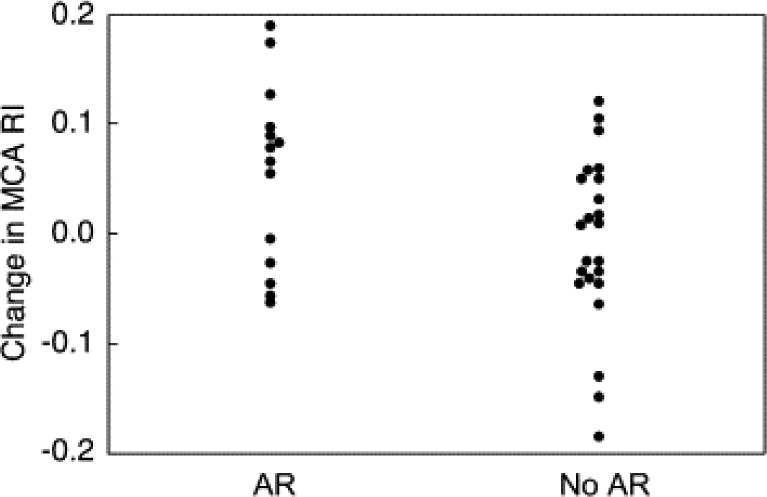
Change in middle cerebral artery (MCA) resistive index (RI) between pre- and early postintervention studies among fetuses that underwent successful aortic valvuloplasty and did or did not develop significant AR (p = 0.05).
Fetal biometry
In the 46 fetuses for which MCA and UA Doppler data were available prior to intervention, fetal weight percentile was 64 ± 20%, and Z-scores for fetal head circumference (−0.10 ± 1.14, p = 0.27) and biparietal diameter (0.45 ± 1.89, p = 0.09) were not significantly different from normal. Among fetuses with late gestation ultrasound data available (n = 28), fetal weight percentile was 53 ± 25% (p = 0.07 vs. preintervention by paired t-test). In this cohort, head circumference Z-score (0.12 ± 0.77) was within the normal range and did not differ from preintervention (p = 0.33 by paired t-test); Z-scores for biparietal diameter (1.31 ± 1.95) were higher than normal (p = 0.002), but were not significantly different than prior to intervention (p = 0.10). There were no significant associations between MCA flow parameters and either head circumference Z-score (Fig. 5), biparietal diameter Z-score, or weight percentile. Similarly, there were no differences in pre- to postintervention changes in head circumference or biparietal diameter Z-score according to baseline or follow-up MCA flow characteristics.
Figure 5.
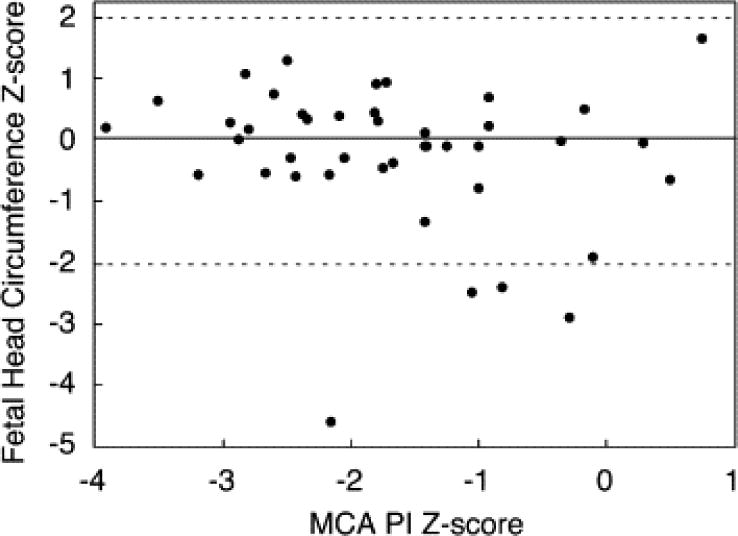
This scatter plot depicts the relationship between middle cerebral artery (MCA) pulsatility index (PI) Z-score and head circumference Z-score prior to intervention, and also reflects the lack of association between head circumference and other MCA flow indices. The relationship is defined by the following regression equation: Head circumference Z-score = (−0.189 × MCA PI Z-score) − 0.189 (r = −0.17, p = 0.27). The solid line at Z = 0 indicates the mean for the normal population, and the dashed lines at +2 and −2 indicate the limits of the normal range.
Discussion
In this cohort of mid-gestation fetuses with evolving HLHS, we found that cerebral blood flow characteristics were abnormal, with low PI and RI, similar to a generally older population of fetuses with established HLHS reported by Kaltman et al. (Kaltman et al. 2005). These investigators did not find the same pattern in a cohort of fetuses with left-sided obstructive lesions in which there was antegrade flow around the arch, but the statistical power of that analysis was limited by the small number of fetuses and a bimodal clustering of Z-scores (Kaltman et al. 2005). Although these findings are consistent with the theory that cerebral vasodilation occurs in response to the abnormal circulatory physiology in these fetuses, the underlying cause of abnormal cerebral flow dynamics in fetuses with HLHS and evolving HLHS is unknown.
Circulatory aberrations that may contribute to the cerebral blood flow characteristics observed in this cohort include but may not be limited to: (1) abnormal delivery of oxygen and/or energy substrate to the brain and (2) abnormal cerebral arterial hemodynamics as a result of most or all systemic blood flow originating from the right ventricle and passing through the ductus arteriosus in the setting of severely impaired left heart output. There are abundant animal data demonstrating that hypoxemia results in central redistribution of blood flow in the fetus (Donofrio et al., 2003 and Pearce, 2006; Vyas et al., 1990 and Wladimiroff, 1987). Although less is known about the effects of hypoglycemia on the fetal cerebral circulation, there are data to indicate that glycemic state can influence cerebral hemodynamics in the fetus (Makowski et al., 1972, McCallum et al., 2008 and Pardo et al., 1999). The precise relationship between arterial oxygen and glucose content and cerebral vasodilation is not clear in these animal models and certainly not in human fetuses. Likewise, there is no way to know the content of cerebral arterial blood in human fetuses with HLHS within confidence limits sufficient to shed insight on this problem, although it is theoretically possible to estimate the oxygen content of cerebral arterial blood in fetuses with HLHS based on normative animal data. In addition to these metabolic factors, essentially all cardiac output in fetuses with HLHS and evolving HLHS arises from the right ventricle and all systemic blood flow passes through the ductus arteriosus; cerebral blood flow then passes retrograde around the hypoplastic aortic arch to the brachiocephalic vessels. In this arrangement, the time-flow and cardiac cyclic phase-flow relationships in the cerebral vasculature may be distorted, insofar as all cardiac outflow is originating from a single source, flow streaming and energy transfer may be less efficient than usual and circulatory mechanics may be abnormal as a result. Simulating the hemodynamic abnormalities in evolving HLHS is not straightforward and there are no animal data of which we are aware concerning the central arterial responses to this circulatory configuration. Thus, while the findings of this study are consistent with the hypothesis that abnormal cerebral hemodynamics and/or oxygen/substrate delivery contribute to cerebral vasodilation as part of an autoregulatory process, there is insufficient data to know for certain that this is the case or to resolve the finer questions of how this process works or how it can be modulated effectively.
Contrary to our expectations, technically successful fetal intervention did not have a substantial effect on MCA flow characteristics, either acutely or over the course of gestation, despite improving left heart function and output (Selamet Tierney et al. 2007). While cerebral vascular flow indices did not improve, it is just as important to note that they did not deteriorate either. Prenatal aortic valvuloplasty did facilitate modest restoration of the cerebral:placental balance acutely, as reflected in a significant increase in CPR RI and a concomitant decrease in the proportion of fetuses with a very low CPR RI after intervention. The significance of that finding is uncertain, however, as it was not sustained at late-gestation follow-up. If cerebral oxygen content is a primary mediator of cerebral vascular impedance in fetuses with evolving HLHS, it is not surprising that successful fetal aortic valvuloplasty did not alter MCA PI or RI Z-scores; although antegrade aortic outflow was improved with intervention, the oxygen content of this blood probably did not differ substantially from right ventricular output, inasmuch as postintervention interatrial flow typically remains left-to-right or bidirectional (Selamet Tierney et al. 2007) and the normal streaming of umbilical venous return is not restored. Similarly, although aortic valvuloplasty may alter the contribution of LV and right ventricular outputs to the cerebral circulation and the dynamics of such flow, net cerebral blood flow may be unchanged. It is also possible that abnormalities of cerebral perfusion are irreversible by the time of intervention or that the postintervention changes in cerebral oxygen/substrate delivery and/or central arterial hemodynamics are simply inadequate to allow normalization of cerebral vascular impedance. The potential contribution of anomalous cerebral vascular development to abnormal cerebral hemodynamics in fetuses with evolving or established HLHS is uncertain and may be critical. For example, Kinnear et al. recently presented data from aborted human fetuses with HLHS demonstrating decreased capillary density in the germinal matrix and cerebral cortex, large diameter capillaries suggestive of vascular dilation, and a relatively low number of vascular cells expressing the stem cell marker CD133 (Kinnear et al. 2008).
AR is relatively common after prenatal aortic valvuloplasty in fetuses with evolving HLHS. In almost all cases, AR resolved or improved significantly before term, although the mechanism of this improvement was not clear. Of course, AR in this situation is concerning, regardless of the unexpected resolution that we have observed, but there is no evidence that AR contributed to adverse pre- or postnatal outcome in this population. There was a tendency for MCA PI and RI to increase modestly after valvuloplasty in fetuses with AR, whereas there was minimal change or a slight decrease in fetuses without AR. An increase in MCA PI and RI in the setting of AR would be consistent with diastolic runoff into the LV competing with diastolic cerebral blood flow, leading to a lower relative diastolic flow velocity and a rise in PI and RI, as was observed. We do not believe we have sufficient data to determine whether these differences actually reflect a deleterious effect of AR, as the changes were modest, there was no difference in Z-scores changes between fetuses with and without AR and there was extensive overlap between AR and no AR groups (Fig. 2). The overall significance of AR in this population and its impact on central arterial hemodynamics remain important questions that will merit ongoing attention as we accrue additional experience and postnatal follow-up.
We also found that head circumference and biparietal diameter Z-scores in our cohort of fetuses with evolving HLHS were generally within the normal range and did not change demonstrably from preintervention to late-gestation or in relation to MCA flow characteristics. Other investigators have reported a tendency toward microcephaly in fetuses with HLHS (Hinton et al. 2008) and there were certainly fetuses with head circumference Z-scores below normal in this series, but as a population, head circumference in mid-gestation fetuses with evolving HLHS was within the normal range.
Limitations
Doppler evaluation of MCA flow was not performed routinely in this patient population until mid-2004 and as such our entire fetal intervention experience is not represented. Early and follow-up postintervention Doppler data were not available in all fetuses with preintervention data, which may bias our findings and compromises our statistical power. We did not compare our study cohort to normal controls but rather to Z-scores of MCA, UA and biometric indices that were derived from published normative data (Arduini 1990; Doubilet et al., 1997, Kurmanavicius et al., 1997 and Kurmanavicius et al., 1999).
Echocardiographic measurements were obtained at specific points in time and may not accurately reflect either the steady-state or dynamic physiology in these fetuses. The early postintervention study was performed 24 hours after intervention and it is possible that fetal and placental hemodynamics had not yet recovered completely from the presumptive stress of the intervention.
Conclusions
Cerebral blood flow characteristics are abnormal in mid-gestation fetuses with AS and evolving HLHS, with low PI and RI, suggestive of low cerebral vascular impedance. The mechanisms and developmental significance of these abnormalities are unknown. Prenatal aortic valvuloplasty and procedural AR did not appear to have a major acute or chronic impact on the cerebral flow indices evaluated in this study. The mechanisms, physiologic implications and neurodevelopmental impact of abnormal MCA flow in fetuses with evolving HLHS deserve further study. Similarly, the effects of fetal intervention on brain growth, cerebral metabolic function and neurodevelopmental outcome merit investigation.
Acknowledgments
This work was supported by the National Institutes of Health under award number: T32HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
This study was also supported by contributions from the Kenrose Kitchen Table Foundation.
References
- Arduini D, Rizzo G. Normal values of pulsatility index from fetal vessels: A cross-sectional study on 1556 healthy fetuses. J Perinat Med. 1990;18:165–172. doi: 10.1515/jpme.1990.18.3.165. [DOI] [PubMed] [Google Scholar]
- Ashwal S, Majcher JS, Vain N, Longo LD. Patterns of fetal lamb regional cerebral blood flow during and after prolonged hypoxia. Pediatr Res. 1980;14:1104–1110. doi: 10.1203/00006450-198010000-00003. [DOI] [PubMed] [Google Scholar]
- Chihara H, Blood AB, Hunter CJ, Power GG. Effect of mild hypothermia and hypoxia on blood flow and oxygen consumption of the fetal sheep brain. Pediatr Res. 2003;54:665–671. doi: 10.1203/01.PDR.0000084115.31416.17. [DOI] [PubMed] [Google Scholar]
- Donofrio MT, Bremer YA, Schieken RM, Gennings C, Morton LD, Eidem BW, Cetta F, Falkensammer CB, Huhta JC, Kleinman CS. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: The brain sparing effect. Pediatr Cardiol. 2003;24:436–443. doi: 10.1007/s00246-002-0404-0. [DOI] [PubMed] [Google Scholar]
- Doubilet PM, Benson CB, Nadel AS, Ringer SA. Improved birth weight table for neonates developed from gestations dated by early ultrasonography. J Ultrasound Med. 1997;16:241–249. doi: 10.7863/jum.1997.16.4.241. [DOI] [PubMed] [Google Scholar]
- Edelstone DI, Rudolph AM. Preferential streaming of ductus venosus blood to the brain and heart in fetal lambs. Am J Physiol. 1979;237:724–729. doi: 10.1152/ajpheart.1979.237.6.H724. [DOI] [PubMed] [Google Scholar]
- Gleason CA, Hamm C, Jones MD., Jr Effect of acute hypoxemia on brain blood flow and oxygen metabolism in immature fetal sheep. Am J Physiol. 1990;258:H1064–H1069. doi: 10.1152/ajpheart.1990.258.4.H1064. [DOI] [PubMed] [Google Scholar]
- Goldberg CS, Schwartz EM, Brunberg JA, Mosca RS, Schork A, Bove EL, Stetz SP, Cheatham JP, Kulik TJ. Neurodevelopmental outcome of patients after the Fontan operation: A comparison between children with hypoplastic left heart syndrome and other functional single ventricle lesions. J Pediatr. 2000;137:646–652. doi: 10.1067/mpd.2000.108952. [DOI] [PubMed] [Google Scholar]
- Hadlock FP, Harrist RB, Carpenter RJ, Deter RL, Park SK. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology. 1984;150:535–540. doi: 10.1148/radiology.150.2.6691115. [DOI] [PubMed] [Google Scholar]
- Hinton RB, Andelfinger G, Sekar P, Hinton AC, Gendron RL, Michelfelder EC, Robitaille Y, Benson DW. Prenatal head growth and white matter Injury in hypoplastic left heart syndrome. Pediatr Res. 2008;64:364–369. doi: 10.1203/PDR.0b013e3181827bf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikle L, Hale K, Fashaw L, Boucek M, Rosenberg AA. Developmental outcome of patients with hypoplastic left heart syndrome treated with heart transplantation. J Pediatr. 2003;142:20–25. doi: 10.1067/mpd.2003.mpd0340. [DOI] [PubMed] [Google Scholar]
- Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet Gynecol. 2005;25:32–36. doi: 10.1002/uog.1785. [DOI] [PubMed] [Google Scholar]
- Kinnear C, Thompson M, Mahmut N, Shannon P, Jaeggi E, Chitayat D, Mital S. Abnormal cerebrovascular development in hypoplastic left heart syndrome during fetal life. Circulation. 2008;118:S908–S909. [Google Scholar]
- Kurmanavicius J, Florio I, Wisser J, Hebisch G, Zimmermann R, Müller R, Huch R, Huch A. Reference resistance indices of the umbilical, fetal middle cerebral and uterine arteries at 24–42 weeks of gestation. Ultrasound Obstet Gynecol. 1997;10:112–120. doi: 10.1046/j.1469-0705.1997.10020112.x. [DOI] [PubMed] [Google Scholar]
- Kurmanavicius J, Wright EM, Royston P, Wisser J, Huch R, Huch A, Zimmermann R. Fetal ultrasound biometry: 1. Head reference values. Br J Obstet Gynaecol. 1999;106:126–135. doi: 10.1111/j.1471-0528.1999.tb08212.x. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C. Neurodevelopmental status of newborns and infants with congenital heart defects before and after open heart surgery. J Pediatr. 2000;137:638–645. doi: 10.1067/mpd.2000.109152. [DOI] [PubMed] [Google Scholar]
- Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, Clancy RR, Montenegro LM, Spray TL, Chiavacci RM, Wernovsky G, Kurth CD. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106(Suppl. 1):I-109–I-114. [PubMed] [Google Scholar]
- Mahle WT, Wernovsky G. Neurodevelopmental outcomes in hypoplastic left heart syndrome. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:39–47. doi: 10.1053/j.pcsu.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Majnemer A, Limperopoulos C, Shevell M, Rosenblatt B, Rohlicek C, Tchervenkov C. Long-term neuromotor outcome at school entry of infants with congenital heart defects requiring open-heart surgery. J Pediatr. 2006;148:72–77. doi: 10.1016/j.jpeds.2005.08.036. [DOI] [PubMed] [Google Scholar]
- Makikallio K, McElhinney DB, Levine JC, Marx GR, Colan SD, Marshall AC, Lock JE, Marcus EN, Tworetzky W. Fetal aortic valve stenosis and the evolution of hypoplastic left heart syndrome: Patient selection for fetal intervention. Circulation. 2006;113:1401–1405. doi: 10.1161/CIRCULATIONAHA.105.588194. [DOI] [PubMed] [Google Scholar]
- Makowski EL, Schneider JM, Tsoulos NG, Colwill JR, Battaglia FC, Meschia G. Cerebral blood flow, oxygen consumption, and glucose utilization of fetal lambs in utero. Am J Obstet Gynecol. 1972;114:292–303. doi: 10.1016/0002-9378(72)90606-0. [DOI] [PubMed] [Google Scholar]
- Marshall AC, Tworetzky W, Bergersen L, McElhinney DB, Benson CB, Jennings RW, Wilkins-Haug LE, Marx GR, Lock JE. Aortic valvuloplasty in the fetus: Technical characteristics of successful balloon dilation. J Pediatr. 2005;147:535–539. doi: 10.1016/j.jpeds.2005.04.055. [DOI] [PubMed] [Google Scholar]
- Massaro AN, El-Dib M, Glass P, Aly H. Factors associated with adverse neurodevelopmental outcomes in infants with congenital heart disease. Brain Dev. 2008;30:437–446. doi: 10.1016/j.braindev.2007.12.013. [DOI] [PubMed] [Google Scholar]
- McCallum J, Smith N, Schwab M, Coksaygan T, Reinhardt B, Nathanielsz P, Richardson BS. Effects of antenatal glucocorticoids on cerebral substrate metabolism in the preterm ovine fetus. Am J Obstet Gynecol. 2008;198:105 e1–105.e9. doi: 10.1016/j.ajog.2007.05.007. [DOI] [PubMed] [Google Scholar]
- McElhinney DB, Marshall AC, Wilkins-Haug LE, Brown DW, Benson CB, Silva V, Marx GR, Mizrahi-Arnaud A, Lock JE, Tworetzky W. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation. 2009;120:1482–1490. doi: 10.1161/CIRCULATIONAHA.109.848994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, Karl T, Azakie A, Ferriero DM, Barkovich AJ, Vigneron DB. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- Modena A, Horan C, Visintine J, Chanthasenanont A, Wood D, Weiner S. Fetuses with congenital heart disease demonstrate signs of decreased cerebral impedance. Am J Obstet Gynecol. 2006;195:706–710. doi: 10.1016/j.ajog.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Papile LA, Rudolph AM, Heymann MA. Autoregulation of cerebral blood flow in the preterm fetal lamb. Pediatr Res. 1985;19:159–161. doi: 10.1203/00006450-198502000-00001. [DOI] [PubMed] [Google Scholar]
- Pardo J, Orvieto R, Rabinerson D, Bar J, Hod M, Kaplan B. Fetal middle-cerebral and umbilical artery flow assessments after glucose challenge test. Int J Gynaecol Obstet. 1999;65:255–259. doi: 10.1016/s0020-7292(99)00026-0. [DOI] [PubMed] [Google Scholar]
- Pearce W. Hypoxic regulation of the fetal cerebral circulation. J Appl Physiol. 2006:731–738. doi: 10.1152/japplphysiol.00990.2005. [DOI] [PubMed] [Google Scholar]
- Rosenthal GL. Patterns of prenatal growth among infants with cardiovascular malformations: Possible fetal hemodynamic effects. Am J Epidemiol. 1996;143:505–513. doi: 10.1093/oxfordjournals.aje.a008771. [DOI] [PubMed] [Google Scholar]
- Selamet Tierney ES, Wald RM, McElhinney DB, Marshall AC, Benson CB, Colan SD, Marcus EN, Marx GR, Levine JC, Wilkins-Haug L, Lock JE, Tworetzky W. Changes in left heart hemodynamics after technically successful in utero aortic valvuloplasty. Ultrasound Obstetr Gynecol. 2007;30:715–720. doi: 10.1002/uog.5132. [DOI] [PubMed] [Google Scholar]
- Shillingford AJ, Ittenbach RF, Marino BS, Rychik J, Clancy RR, Spray TL, Gaynor JW, Wernovsky G. Aortic morphometry and microcephaly in hypoplastic left heart syndrome. Cardiol Young. 2007;17:189–195. doi: 10.1017/S1047951107000248. [DOI] [PubMed] [Google Scholar]
- Te Pas AB, van Wezel-Meijler G, Bokenkamp-Gramann R, Walther FJ. Preoperative cranial ultrasound findings in infants with major congenital heart disease. Acta Paediatr. 2005;94:1597–1603. doi: 10.1111/j.1651-2227.2005.tb01835.x. [DOI] [PubMed] [Google Scholar]
- Tworetzky W, Wilkins-Haug L, Jennings RW, van der Velde ME, Marshall AC, Marx GR, Colan SD, Benson CB, Lock JE, Perry SB. Balloon dilation of severe aortic stenosis in the fetus: Potential for prevention of hypoplastic left heart syndrome: Candidate selection, technique, and results of successful intervention. Circulation. 2004;110:2125–2131. doi: 10.1161/01.CIR.0000144357.29279.54. [DOI] [PubMed] [Google Scholar]
- van Bel F, Sola A, Roman C, Rudolph AM. Role of nitric oxide in the regulation of the cerebral circulation in the lamb fetus during normoxemia and hypoxemia. Biol Neonate. 1995;68:200–210. doi: 10.1159/000244238. [DOI] [PubMed] [Google Scholar]
- Vyas S, Nicolaides KH, Bower S, Campbell S. Middle cerebral artery flow velocity waveforms in fetal hypoxaemia. Br J Obstet Gynaecol. 1990;97:797–803. doi: 10.1111/j.1471-0528.1990.tb02573.x. [DOI] [PubMed] [Google Scholar]
- Wernovsky G, Stiles KM, Gauvreau K, Gentles TL, duPlessis AJ, Bellinger DC, Walsh AZ, Burnett J, Jonas RA, Mayer JE, Newburger JW. Cognitive development after the Fontan operation. Circulation. 2000;102:883–889. doi: 10.1161/01.cir.102.8.883. [DOI] [PubMed] [Google Scholar]
- Wladimiroff JW, vd Wijngaard JA, Degani S, Noordam MJ, van Eyck J, Tonge HM. Cerebral and umbilical arterial blood flow velocity waveforms in normal and growth-retarded pregnancies. Obstet Gynecol. 1987;69:705–709. [PubMed] [Google Scholar]


