The posttranslational modification of tubulin plays an important role in regulating microtubule function. Enzymes responsible for deglutamylating tubulin are members of a family of cytosolic carboxypeptidases. By completing the functional characterization of this protein family in mammals, it is demonstrated that CCP2 and CCP3 are deglutamylases.
Abstract
The posttranslational modification of carboxy-terminal tails of tubulin plays an important role in the regulation of the microtubule cytoskeleton. Enzymes responsible for deglutamylating tubulin have been discovered within a novel family of mammalian cytosolic carboxypeptidases. The discovery of these enzymes also revealed the existence of a range of other substrates that are enzymatically deglutamylated. Only four of six mammalian cytosolic carboxypeptidases had been enzymatically characterized. Here we complete the functional characterization of this protein family by demonstrating that CCP2 and CCP3 are deglutamylases, with CCP3 being able to hydrolyze aspartic acids with similar efficiency. Deaspartylation is a novel posttranslational modification that could, in conjunction with deglutamylation, broaden the range of potential substrates that undergo carboxy-terminal processing. In addition, we show that CCP2 and CCP3 are highly regulated proteins confined to ciliated tissues. The characterization of two novel enzymes for carboxy-terminal protein modification provides novel insights into the broadness of this barely studied process.
INTRODUCTION
Microtubules (MTs) are dynamic, polarized polymers composed of α/β-tubulin heterodimers and constitute the largest filaments of the cytoskeleton (reviewed in Akhmanova and Steinmetz, 2008). MTs take part in a variety of cellular structures and functions in eukaryotic cells, such as cell shape and motility (reviewed in Etienne-Manneville, 2004), intracellular organization, and transport (reviewed in de Forges et al., 2012). Moreover, MTs are the key structures of complex organelles such as centrioles (reviewed in Bornens, 2012) and axonemes, which are the backbones of cilia and flagella (reviewed in Jana et al., 2014).
Considering the huge variety of MT functions, the targeting of distinct MTs for precisely defined roles in cells appears primordial. Functionally distinct MT species can be generated by either incorporating selected tubulin isotypes into the MT lattice (reviewed in Ludueña and Banerjee, 2008) or generating posttranslational modifications (PTMs) onto selected MTs in cells (reviewed in Janke and Bulinski, 2011). Most of the known PTMs of tubulin take place at the C-terminal tails of tubulins, which are the key interaction sites for microtubule-associated proteins (MAPs) and motors (Sirajuddin et al., 2014). PTMs of these tails include polyglutamylation (Eddé et al., 1990), polyglycylation (Redeker et al., 1994), detyrosination/tyrosination (Arce et al., 1975; Hallak et al., 1977; Thompson, 1977), and generation of Δ2-tubulin (Paturle-Lafanechère et al., 1991).
Enzymes catalyzing polyglutamylation, polyglycylation, and tyrosination are members of the tubulin tyrosine ligase–like (TTLL) family (Ersfeld et al., 1993; Janke et al., 2005; Ikegami et al., 2006; Ikegami and Setou, 2009; van Dijk et al., 2007; Wloga et al., 2008; Rogowski et al., 2009). So far, only deglutamylases have been identified in the family of cytosolic carboxypeptidases (CCPs), a subfamily of M14 metallocarboxypeptidases (Kalinina et al., 2007; Rodriguez de la Vega et al., 2007; Kimura et al., 2010; Rogowski et al., 2010). Four of six mammalian CCP enzymes catalyze deglutamylation and ∆2-tubulin generation; however, the function of the remaining two enzymes has remained elusive (Rogowski et al., 2010; Berezniuk et al., 2013). Of importance, not only tubulin is modified by CCPs; some proteins with gene-encoded acidic C-terminals appear to be cleaved in vivo, such as myosin light-chain kinase and telokin (Rogowski et al., 2010).
Using structural modeling and enzymatic assays, we find that CCP2 and CCP3 are carboxypeptidases that specifically hydrolyze acidic amino acids. We further characterize the activities of these enzymes with chimeric substrates, and through the study of the individual knockout and double-knockout mice. Our work on CCP2 and CCP3 completes the functional characterization of the CCP family and suggests an important functional role of this enzyme family in posttranslational protein regulation. Moreover, our study demonstrates that the as-yet-undiscovered tubulin-modifying enzymes for deglycylation and detyrosination are not found within the CCP family.
RESULTS
Prediction of substrate specificities for CCP2 and CCP3
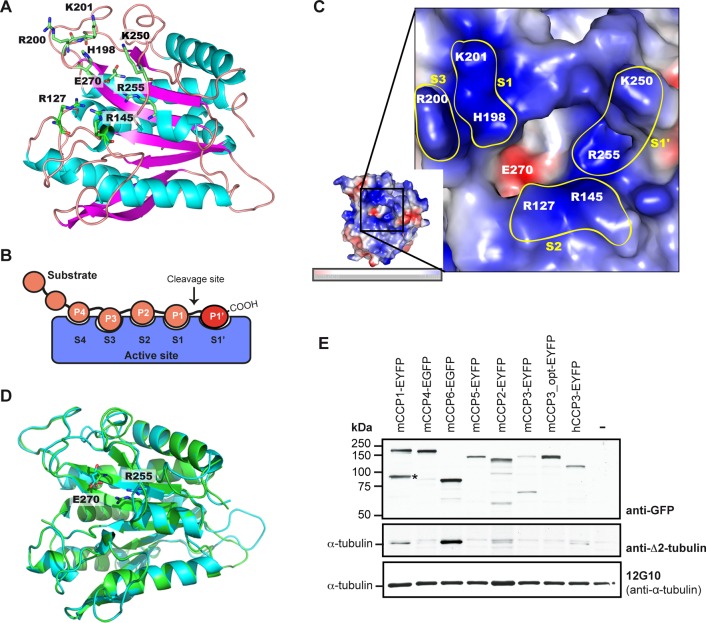
After the discovery that four (CCP1, CCP4, CCP5, CCP6) of the six murine CCPs are deglutamylases (Rogowski et al., 2010), attention turned to the potential functions of CCP2 and CCP3. Considering that enzymes for deglycylation and detyrosination of tubulin have not been identified so far and that the reverse enzymes for these two reactions are all members of the same TTLL family, one expectation was that CCP2 and CCP3 could be involved in either or both of these PTMs. Indeed, an initial report (Sahab et al., 2011) attributed a detyrosinating activity to CCP2; however, unambiguous evidence for this activity was not provided, and overexpression of CCP2 did not lead to a strong increase in detyrosinated tubulin. To gain more insight into the substrate preferences of CCP2 and CCP3, we modeled the catalytic domains of the two enzymes based on the CCP crystal structures of Pseudomonas aeruginosa (PDB 4a37; Otero et al., 2012), Burkholderia mallei (PDB 3k2k), and Shewanella denitrificans (PDB 3l2n; Figure 1A and Supplemental Figure S1A).
FIGURE 1:
Structural modeling of the carboxypeptidase domains of CCP2 and CCP3. (A) Modeled structure of human CCP3 (as a convention, all residues are numbered according to the corresponding active-site residues in bovine carboxypeptidase A1 after propeptide cleavage). The catalytic E270, the main substrate-specificity determining R255, and putative secondary binding-site residues are indicated. (B) Scheme of the substrate-binding subsites in the active site of carboxypeptidases (Schechter and Berger, 1967). (C) Vacuum electrostatics surface representation of the active site of hCCP3. Basic residues are indicated in blue and acidic residues in red. Positions 198 and 201 (corresponding to H462 and K465 in hCCP3) shape the S1 binding site in the hCCP3 model. Positions 127 and 145 (R414 and R424 in hCCP3) define the S2 subsite. Position 200 (R464 in hCCP3) is oriented toward the outer part of the active site, possibly defining an additional, positively charged S3 subsite. Note that although S1′ is defined by different residues (Supplemental Figure S1B), we here only depicted positions 250 and 255 because they are the major determinants of substrate specificity for CCPs. (D) Overlapped model structures of hCCP2 (cyan) and hCCP3 (green) demonstrate the conserved positions in the active-site residues (key residues E270 and R255 are shown). (E) Immunoblots of extracts of HEK293T cells expressing YFP-tagged murine and human CCP proteins. Expression of YFP-CCPs was analyzed with anti-GFP, and deglutamylating activity was visualized with anti–∆2-tubulin labeling on endogenous α‑tubulin. α‑Tubulin levels were controlled with 12G10 antibody. mCCP3_opt is a codon-optimized synthetic gene construct; hCCP3 is the human 73-kDa isoform of CCP3 (Q8NEM8-2 Uniprot). Note the presence of a specific degradation product (*) of mCCP1. Images of structures in A, C, and D were generated with PyMOL 1.3. (–), control without transfection.
The pockets or subsites of the carboxypeptidases, which are the binding sites of their substrates, are usually designated using a standard nomenclature (Schechter and Berger, 1967). Accordingly, substrate residues are indicated as –P3–P2–P1↓P1′, where P1′ is the C-terminal substrate residue to be cleaved, and the corresponding binding subsites in the carboxypeptidase are named S3, S2, S1, and S1′ (Figure 1B). In the structural models of hCCP2 and hCCP3, an arginine (Arg) at position 255 (numbering according to active bovine carboxypeptidase A1 [CPA1; PDB 3HLP]), at the base of the S1′ subsite or specificity pocket, appears to be critical in determining the substrate preference of these enzymes (Figure 1A). In metallocarboxypeptidases, the residue at position 255 directly interacts with the C-terminal side chain of the substrates (reviewed in Arolas et al., 2007; Fernández et al., 2010). A basic amino acid in this position suggest that hCCP2 and hCCP3 should have a preference for acidic amino acids, similar to carboxypeptidase O (Wei et al., 2002; Lyons and Fricker, 2011). Sequence alignment of human and murine CCPs predicts that Arg-255 is conserved in all six CCPs (Supplemental Figure S1B). Moreover, a lysine or arginine (Lys/Arg-250) close to Arg-255 is also conserved in the specificity pocket of CCPs and may contribute to their preference for acidic amino acids (Figure 1A and Supplemental Figure S1B).
In the hCCP2 and hCCP3 models, the entrance to the active site presents an extended positively charged area, which is similarly formed by conserved residues throughout all CCPs and can be associated with different binding subsites of these enzymes (Figure 1C and Supplemental Figure S1C). The positively charged area around the active site of CCPs explains their preference for substrates with long polyglutamate chains, such as polyglutamylated tubulin (Rogowski et al., 2010; Berezniuk et al., 2012; Wu et al., 2012). In particular, positively charged amino acids in the S1 subsite are consistent with binding of glutamic acids present at the P1 position of deTyr-tubulin. In addition, the hCCP2/3 models show positively charged S2 and S3 subsites, which can explain the ability of these enzymes to accommodate long acidic chains such as lateral polyglutamylation (Figure 1C).
The overlap of the structural models of hCCP3 and hCCP2 shows that the main determinants of substrate binding are conserved (Figure 1D and Supplemental Figure S1B), and sequence alignments extend these conclusions to the entire murine and human CCP family (Supplemental Figure S1, B and C). Thus the structure of the active site of CCPs reveals that the entire family has a preference for cleaving acidic amino acids. The modeling further helped us to determine the controversial identity of the specificity-determining residue corresponding to position 255 of bovine CPA in the CCPs (Kalinina et al., 2007; Rodriguez de la Vega et al., 2007) as being arginine (Figure 1A).
CCP2 and CCP3 generate Δ2-tubulin in cells
Deglutamylation can occur on the C-terminally exposed, gene-encoded glutamate residues of proteins, such as detyrosinated tubulin and myosin light-chain kinase (MLCK), or on posttranslationally added polyglutamate chains. This activity was demonstrated for CCP1, CCP4, and CCP6; however, no activity had been detected for CCP2 and CCP3 (Rogowski et al., 2010). One of the potential reasons for the absence of measurable activity of these two enzymes was the difficulty of expressing these proteins in cell lines. To overcome this problem, we included a synthetic, codon-optimized cDNA of mouse CCP3 (Uniprot Q8CDP0-1), as well as a short human CCP3 isoform (Uniprot Q8NEM8-2), in our study, and we expressed yellow fluorescent protein (YFP)/green fluorescent protein (GFP) fusion proteins in HEK293T cells for 40 h instead of 24 h to increase expression levels.
After 40 h of expression, the cells were lysed and protein extracts analyzed by immunoblotting. As expected, CCP6 and CCP1 overexpression led to a marked increase in Δ2-tubulin, pointing to them as the most active deglutamylases (∆2-tubulin is barely detectable in these cells, and thus increased levels indicate deglutamylating activity; Figure 1E). A very weak signal of ∆2-tubulin was detected after overexpression of CCP2, but no clear ∆2-tubulin signal was seen after expression of full-length, codon-optimized murine CCP3. Strikingly, human CCP3 generated a slight increase in the Δ2-tubulin signal (Figure 1E). The simplest explanation is that the 73-kDa human CCP3 isoform we used was shorter than the 116-kDa murine CCP3 and thus might be more active in cells, analogous to what we previously observed for truncated glutamylase enzymes from the TTLL family (van Dijk et al., 2007; Rogowski et al., 2009).
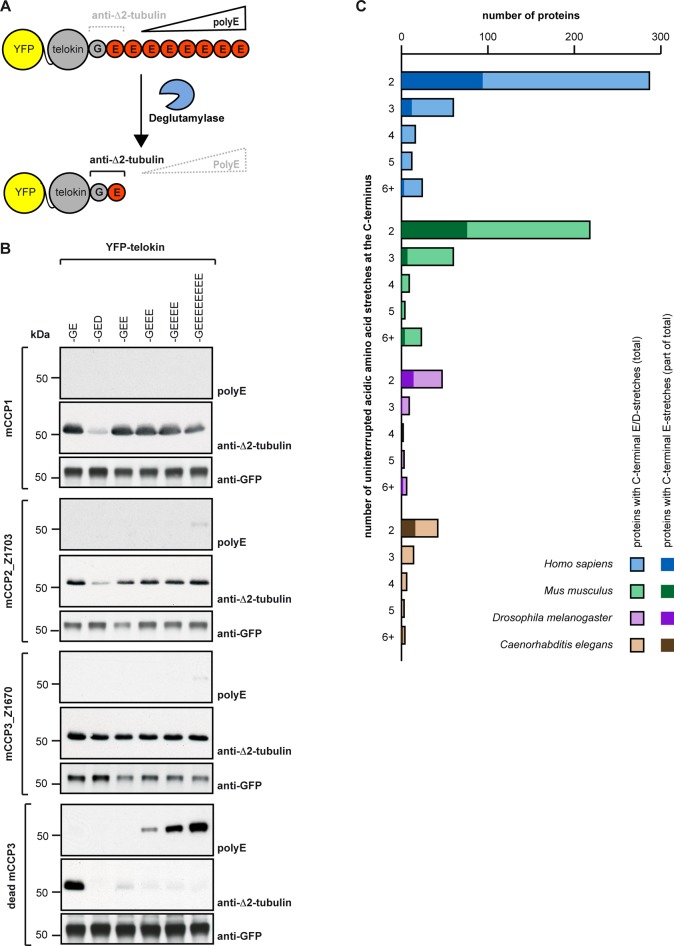
We thus generated a series of truncated forms of murine CCP2 and CCP3 in order to determine the minimal active size of these enzymes (Supplemental Table S1). The YFP-tagged truncated forms were expressed in HEK293T cells, and their activity was assessed by immunoblot for Δ2-tubulin (Supplemental Figure S2, A and B). Indeed, the truncated versions of both murine CCP2 and CCP3 showed a clear ∆2-tubulin–generating activity, demonstrating that both can act as deglutamylating enzymes. The shortest and most active versions are CCP2_Z1703 and CCP3_Z1670 (Figure 2A), which were obtained by truncating nonconserved N- and C-terminal sequences to obtain 65-kDa proteins that were similar in size and domain structure to the highly active deglutamylase CCP6 (Figure 2B and Supplemental Figure S2). Immunocytochemical analysis of HEK293T cells expressing CCP2 and CCP3 further showed a specific ∆2-tubulin labeling associated with the MTs in YFP-positive cells (Figure 2C). To demonstrate that the observed ∆2-tubulin generation after CCP2 and CCP3 expression is directly catalyzed by their active carboxypeptidase (CP) domains, we generated enzymatically dead versions by mutating the essential catalytic residues Glu-593 in mouse CCP2 and Glu-540 in mouse CCP3 (Glu-270 in bovine CPA and Glu-1094 in mouse CCP1; Wu et al., 2012). Enzymatically dead mutants of full-length and truncated versions of CCP2 and CCP3 were expressed at similar levels as the active forms but did not generate Δ2-tubulin (Figure 2B). Strikingly, a very faint ∆2-tubulin band present in the control cells is now absent in the cells overexpressing the enzymatically dead enzymes (Figure 2B), suggesting that these enzymes can act as dominant negative competitors for endogenous enzymes. These experiments demonstrate that the enzymatic activity of CCP2 and CCP3 is involved in the observed deglutamylation reactions on tubulin, leading to ∆2-tubulin.
FIGURE 2:
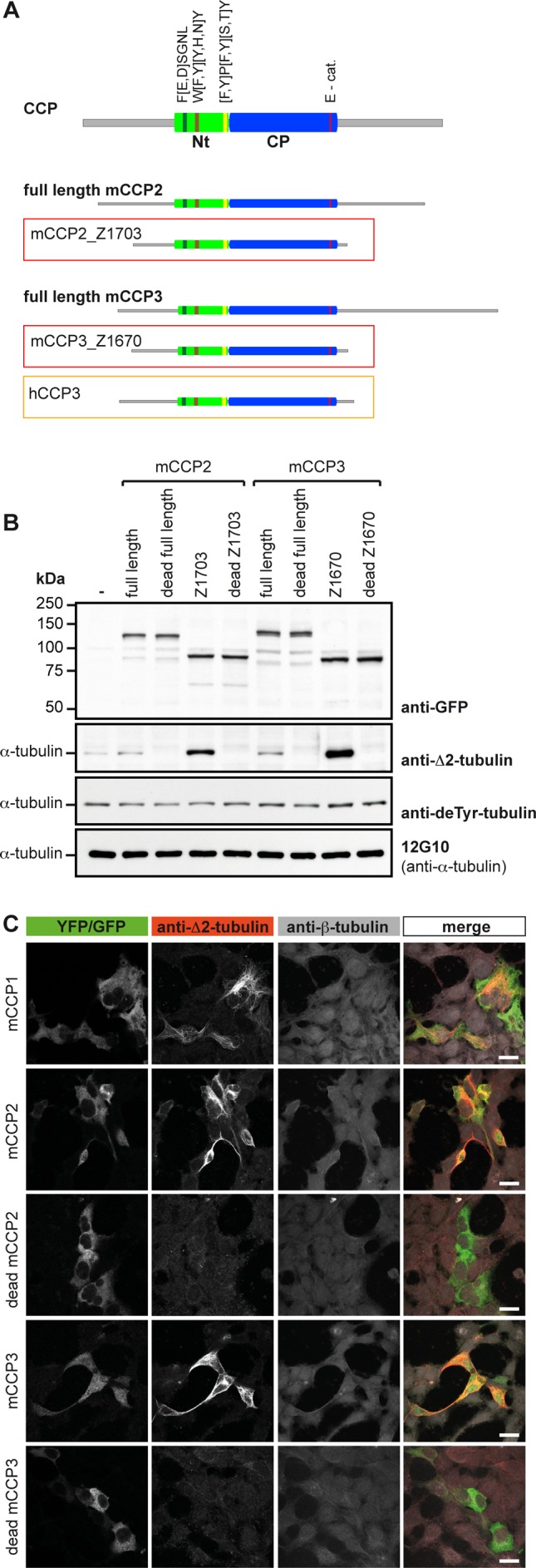
Optimized forms of mCCP2 and mCCP3 reveal deglutamylating activity. (A) Scheme of generic CCP; full-length and truncated forms of mCCP2 and mCCP3, and the 73‑kDa hCCP3 isoform. The green boxes indicate in the conserved N-terminal domain (Nt) specific to CCPs, with FESGNL, WYYY, and YPYTY conserved motifs (Rodriguez de la Vega et al, 2007). The blue box shows the conserved carboxypeptidase domain (CP) with the catalytic residue E270 (E-cat). Gray lines are nonconserved sequences that were partially truncated in the optimization. The shortest truncated versions that are still enzymatically active are mCCP2_Z1703 and mCCP3_Z1670 (red boxes). They are shown in comparison to the full-length versions and to hCCP3 (orange box). (B) Immunoblot of cell extracts from HEK293T cells expressing YFP-tagged, full-length mCCP2 and mCCP3, their optimized truncated forms (A), and enzymatically dead versions as controls. ∆2-Tubulin was used as readout for deglutamylating activity. Detyrosination and deglutamylase activities were followed by generation of deTyr- and Δ2-tubulin. Active enzymes generate ∆2-tubulin. (C) Immunocytochemistry of HEK293T cells transfected with active and inactive truncated YFP-tagged mCCP2 and mCCP3, as well as with GFP-mCCP1. After fixation of the cells with a protocol preserving microtubule structures (Bell and Safiejko-Mroczka, 1995), ∆2-tubulin was detected. Images were collected on an inverted confocal microscope (Leica SP5; Leica, Wetzlar, Germany) using a 63× objective at 25ºC and analyzed with LAF AS Lite 1.8.1 (Leica). Scale bar, 20 μm.
CCP2 was previously suggested to have a detyrosinating activity (Sahab et al., 2011). Although comparison of active sites of CCPs with CPA, an enzyme that specifically hydrolyzes Tyr from C-terminal positions (Argaraña et al., 1980), strongly suggested that CCPs cannot catalyze Tyr hydrolysis (Figure 1), we still tested this possibility experimentally. We used the detyr-tubulin antibody to specifically detect the generation of detyrosinated tubulin in HEK293T cell extracts overexpressing the optimized forms of CCP2 and CCP3. No detectable differences were observed in detyrosinated tubulin, whereas the increase of ∆2-tubulin signal clearly indicated the activity of the overexpressed enzymes (Figure 2B). Thus CCP2 and CCP3 are not involved in the detyrosination of tubulin, and the identity of these long-sought enzymes (Argaraña et al., 1978) remains a conundrum.
Enzymatic specificities of CCP2 and CCP3
To further characterize whether CCP2 and CCP3 can remove subsequent glutamate residues from longer stretches of glutamates, such as those generated by enzymatic polyglutamylation (Audebert et al., 1993; van Dijk et al., 2007) or genetically encoded as in the case of MLCK, we used C-terminally engineered chimeras of telokin, a short version of MLCK, which is one of the few known substrates of CCP1 (Rogowski et al., 2010). Truncated active versions of CCP2 and CCP3 were coexpressed in HEK293T cells together with different C-terminal variants of YFP-telokin. The deglutamylation (removal for long glutamate chains) was monitored using the polyE antibody in immunoblot analysis (Shang, 2002), and the final deglutamylation product (ending with only one glutamate) was detected with the anti–Δ2-tubulin antibody (Figure 3A). Both CCP2_Z1703 and CCP3_Z1670 were able to trim long (7-Glu) and shorter polyglutamate chains from the C-terminus of chimeric telokin, as shown by decreased polyE signals and increased Δ2-tubulin immunoreactivity (Figure 3B). CCP1, known to shorten long glutamate chains, was used as positive control.
FIGURE 3:
Substrate specificity of mCCP2 and mCCP3. (A) Schematic representation of the experimental setup. PolyE antibody recognizes telokin constructs with three or more consecutive C-terminal glutamate residues, whereas anti-Δ2-tubulin antibody detects specifically the C-terminal –GE epitope. C-terminal degradation is detected by generation of the Δ2-tubulin epitope, and absence or strong decrease of the polyE signal on telokin (Rogowski et al, 2010). (B) Immunoblot analysis of HEK293T extracts after coexpression of different YFP-CCPs and YFP-telokin variants. Activity is monitored as shown in A. The activity of truncated mCCP2 and mCCP3 (Figure 2A) is tested with telokin variants with different numbers of consecutive glutamate residues to test processivity and with an aspartate residue to test specificity. The activities are compared with mCCP1, an established deglutamylase (Rogowski et al, 2010), and a dead version of mCCP3 as negative control. Note that only mCCP3 removes aspartate efficiently. (C) Bioinformatic analysis of the number of proteins with uninterrupted C-terminal acid sequence stretches. Each column represents the total number of proteins per category (mixed Asp and Glu stretches), and the darker insets represent uninterrupted Glu stretches.
According to the predictions from the molecular modeling (Figure 1), any negatively charged amino acid would be susceptible to be cleaved by CCPs, not only glutamic acids. However, the previously tested CCP1, CCP4, and CCP6 where shown to specifically remove glutamate in the conditions applied previously (Rogowski et al., 2010). To test the specificity of CCP2 and CCP3, we coexpressed them with telokin variants exposing either one or two aspartic acids at their C-termini. CCP1 and CCP2 generated weak anti–∆2-tubulin–reactive bands at ∼55 kDa, indicative of a cleavage of aspartate; however, much stronger ∆2 signals were obtained when chimeric telokins with C-terminal glutamates were expressed (Figure 3B and Supplemental Figure S3A). In contrast to CCP1 and CCP2, CCP3 converted both aspartate- and glutamate-ending telokin variants to their ∆2 form, and as judged from the intensity of the ∆2 signal, with a similar preference (Figure 3B and Supplemental Figure S3A). It is most likely that the weak ∆2 signal generated from telokin-Asp by CCP1 and CCP2 shows that these two enzymes can cleave aspartate; however, this activity might require high enzyme concentrations, as even after 40 h of overexpression it was less efficient than glutamate hydrolysis. In cells in which endogenous levels of CCP1 and CCP2 are much lower than with the overexpression situation, both enzymes might preferentially or even exclusively hydrolyze glutamate. In contrast, CCP3 shows no obvious enzymatic preference for either glutamate or aspartate removal and might thus be involved in protein deaspartylation in cells.
To check the potential effect of the novel enzymatic activity on the functional broadness of enzymatic C-terminal degradation of proteins on a proteome level, we compared the number of proteins with C-terminal poly-Glu stretches, which can be substrates of deglutamylases (Rogowski et al., 2010), with the number of acidic C-terminal tails including Asp residues. We restricted our analysis to the four very well annotated proteomes of Homo sapiens, Mus musculus, Drosophila melanogaster, and Caenorhabditis elegans. Three main conclusions can be drawn from the numbers we obtained (Figure 3C and Supplemental Table S3). First, the presence of a putative deaspartylating activity (CCP3 and possibly other CCPs under specific conditions) strongly increases the number of proteins that could be C-terminally modified by the CCP family. Second, the number of proteins with acidic C-terminal stretches is much higher in the mouse and human than in D. melanogaster and C. elegans. This could indicate a greater need for this regulatory mechanism in more complex organisms, which is also underlined by the greater number of CCP genes in mice and humans than in D. melanogaster and C. elegans. Finally, it is not clear whether deaspartylation activity can be found in the last two invertebrates, as clear homologues of CCP3 have not been identified. It thus appears that the discovery of a deaspartylating activity could have a significant impact on the functional reach of C-terminal degradation and offer novel evolutionary insight into the development of this PTM.
Glycylation is a posttranslational modification similar to polyglutamylation, performed by members of the TTLL family (Rogowski et al., 2009). Nothing is known about the enzymes responsible for shortening or removing posttranslationally generated glycine chains from tubulin. The unique deglycylase identified so far is an M20 peptidase family member in Giardia duodenalis (Lalle et al., 2011). Because this enzyme deglycylates the 14-3-3 proteins, and because M20 peptidases are not found in the mammalian genome, other candidates were considered as potential deglyclases in mammals, among them CCP2 and CCP3. Glycylation is typically enriched on the axonemes of motile cilia and flagella (Redeker et al., 1994). Thus the presence of CCP2 and CCP3 genes in ciliated organisms (Rodríguez de la Vega Otazo et al., 2013) and their increased expression levels in mouse tissues with motile cilia such as testis and trachea (Figure 4A) provided a potential link to glycylation. Although the structural model already suggested that both enzymes are rather specific to acidic amino acids (Figure 1), we wanted to obtain additional experimental proof to confirm this. Our attempts to directly test deglycylating activity in cells with glycylated MTs, which could be generated by expression of glycylating enzymes, failed due to the toxicity of this treatment for the cells. Thus we developed an alternative test for deglycylating activity, in which we coexpressed a chimeric telokin containing four Gly residues on the very C-terminus. This construct is specifically detected with the polyclonal antibody polyG, and removal of only one Gly residue completely abolished detection (Supplemental Figure S3B). The chimeric telokin-Gly was coexpressed together with CCP2_Z1703 or CCP3_Z1670, and a similar experiment was performed with a polyE antibody and a telokin with three Glu residues as positive control (Supplemental Figure S3C). Whereas both CCP2_Z1703 and CCP3_Z1670 were perfectly able to remove C-terminal glutamates, the unchanged polyG signals strongly suggest that C-terminally located Gly residues cannot be hydrolyzed by these enzymes. Thus CCP2 and CCP3 do not carry an intrinsic deglycylase activity.
FIGURE 4:
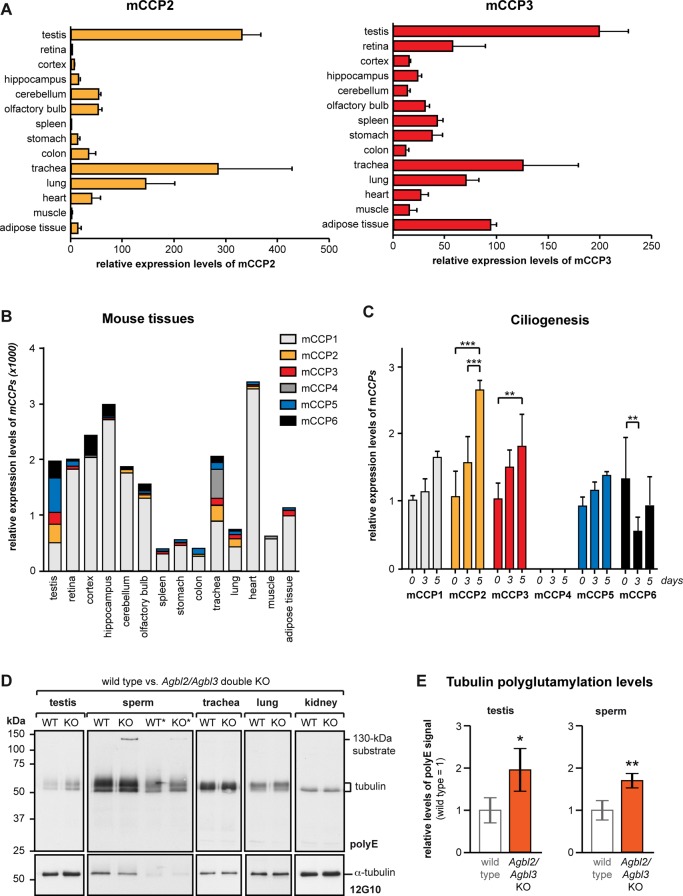
Analysis of mCCP2 and mCCP3 expression and of the Agbl2/Agbl3 double-KO mice. (A) Relative expression levels of mCCP2 and mCCP3 in different organs of wild-type (WT) mice as determined by qRT-PCR. Average values relative to the Tbp gene expression are represented, and error bars represent SD of five independent experiments. (B) Cumulative view of the relative expression levels of all six CCP genes in different murine tissues (values from A and Supplemental Figure S4A). (C) qRT-PCR analysis of expression levels of all six CCP genes during ciliogenesis in cultured IMCD3 cells. Cells were serum starved and analyzed at 0, 3, and 5 d after starvation. Mean values of expression levels relative to Tbp expression and relative to 0 d from three to five independent experiments are shown; error bars show SD. Two-way analysis of variance was used to determine significance levels (**p < 0.01; ***p < 0.001). (D) Comparative immunoblot analysis of protein extracts from different organs of WT and Agbl2/Agbl3 double-KO mice with polyE. Total tubulin levels were detected with anti–α-tubulin antibody (12G10). For sperm that showed the highest polyE levels in the tubulin region, the experiment was repeated with half of the initial protein load (lanes labeled with *). (E) Quantitative analysis of the polyE signal relative to the 12G10 signal Agbl2/Agbl3 double-KO mice relative to WT. Four of each WT and double KO were analyzed (Supplemental Figure S5), and relative values were plotted (WT was set to 1); error bars show SD. Student's t test was used to determine significance levels (*p < 0.05; **p < 0.01).
Expression and potential functional roles of CCP2 and CCP3 in ciliated cells
Having demonstrated that all members of the CCP family are deglutamylating enzymes, we wanted to gain more insight into their specific functions on the whole-organism level. We thus quantified the relative expression levels of all six CCP family members by quantitative real-time-PCR (qRT-PCR) with cDNA samples prepared from a representative range of organs. Although qRT-PCR data are semiquantitative, it was obvious that both CCP2 and CCP3 are expressed at relatively low levels in most organs (Figure 4A), especially when compared with CCP1 (Supplemental Figure S4A). CCP4, in contrast, was barely detectable in a range of organs, suggesting a very specialized function in the subgroup of organs where it is expressed (Supplemental Figure S4A). The expression profiles of CCP2 and CCP3 are highly similar; both enzymes are predominantly found in the testis, trachea, and lung, all tissues containing cells with motile cilia (Figure 4A). In two tissues, retina and adipose tissue, CCP3 was relatively strongly expressed, whereas CCP2 was barely detected (Figure 4A; Kalinina et al., 2007).
On comparison of the relative expression levels of the CCP enzymes as determined by qRT-PCR, it is obvious that CCP1 is the deglutamylase with the highest expression levels and the broadest distribution in different tissues (Figure 4B). Although this does not allow judging the functional importance of those enzymes, it suggests that CCP1 catalyzes a large share of deglutamylation reactions. Indeed, the knockout model for CCP1, the Purkinje cell degeneration (pcd) mouse, shows severe defects related to tubulin hyperglutamylation in several organs (Rogowski et al., 2010). In contrast, other CCP enzymes might have more specialized functions in specific cells, organelles, or even on some selected MTs within cells.
The elevated expression levels of CCP2 and CCP3 in ciliated tissues containing axonemes, such as the testis, lung, and trachea, suggest a potential role of these enzymes in the assembly or function of cilia. To investigate this link, we quantified the expression of all six CCPs during ciliogenesis in IMCD3 cells in culture by qRT-PCR at 0, 3, and 5 d after induction of ciliogenesis by serum starvation. Apart from CCP4, all other CCPs could be detected, but only CCP2 and CCP3 show a statistically significant increase of expression levels during ciliogenesis (Figure 4C). Thus several enzymes of the deglutamylase family are likely to be involved in ciliary outgrowth, maintenance, and function, consistent with the importance of glutamylation in cilia assembly and function (Bosch Grau et al., 2013; Pathak et al., 2014); however, CCP2 and CCP3 are likely to play highly specific role in these processes.
The similar expression profiles of CCP2 and CCP3 determined by qRT-PCR and previously by in situ hybridization (Rodríguez de la Vega Otazo et al., 2013) suggest some functional overlap of these two enzymes. Because the individual knockout (KO) mouse models for CCP2 (Agbl2 [ATP/GTP binding protein–like]) and CCP3 (Agbl3) are both fertile, we generated double (Agbl2/Agbl3) KO mice. These mice were viable and displayed no obvious phenotypic alterations. To study the potential change in polyglutamylation levels on tubulin and other proteins, we dissected selected organs, prepared tissue extracts, and analyzed them with polyE antibody in immunoblot (Figure 4D and Supplemental Figures S4B and S5). Compared to wild-type mice, tubulin polyglutamylation was increased in the testes and sperm of the Agbl2/Agbl3 double-knockout mice (Figure 4E and Supplemental Figure S5). Apart from testes and sperm, no other organ showed detectable changes in tubulin polyglutamylation levels in Agbl2/Agbl3 KO mice (Figures 4D). In addition to tubulin, polyE immunoreactivity of a 130-kDa protein was increased in sperm of a 5-wk-old Agbl2/Agbl3 KO mice; however, this band was not detected in the 4-mo-old mice. In the previous analysis of the pcd mouse, the 130-kDa substrate was identified as MLCK1, of which the C-terminal glutamate stretch is detected with polyE (Rogowski et al., 2010). Our observations in the Agbl2/Agbl3 KO mice indicate that CCP2 and CCP3 are complementarily involved in the deglutamylation of MLCK1 in sperm, but that other enzymes could compensate for the absence of these two enzymes in adult mice (Figure 4D and Supplemental Figure S5). A similar increase in polyE immunoreactivity of MLCK1 was observed in stomach of the Agbl2 KO mice, as well as in stomach and oviduct of the Agbl3 KO mice (Supplemental Figure S4B). These data show that in certain organs, CCP2, CCP3, or both enzymes are involved in the deglutamylation of MLCK1 (Supplemental Figure S4B and 4D). However, no visible phenotype has been related to this change in deglutamylation in these mice, perhaps due to compensation during development.
DISCUSSION
Protein PTMs consisting of the ligation or hydrolysis of amino acids have recently been attributed an increasing range of functions, and the discovery of enzymes involved in polyglutamylation, polyglycylation, or detyrosination/tyrosination have opened new opportunities for mechanistic and functional studies. Amino acid ligases for Glu, Gly, or Tyr have been identified in the conserved TTLL family. Enzymes removing Glu residues from protein C-termini were found within the newly discovered CCP family (reviewed in Janke and Bulinski, 2011). Initially, these discoveries raised hopes that the remaining enzymes, such as detyrosinases or deglycylases, are also members of the CCP family (Sahab et al., 2011). The goal of the present study was to determine unambiguously the enzymatic specificity of the remaining two members of the mammalian CCP family, CCP2 and CCP3.
The reason that CCP2 and CCP3 had not been analyzed with the same rigorousness as the other members of the family (Rogowski et al., 2010) was mainly related to the difficulties in expressing these proteins, which hampered the development of enzymatic assays. The availability of the crystal structure of a CCP (Otero et al., 2012) allowed us to generate a structural model of CCP2 and CCP3, on the basis of which we predicted the acidic substrate specificity of these two enzymes. Guided by these predictions and optimizing the coding sequences of CCP2 and CCP3 led us to the demonstration that both enzymes are consistent with C-terminal deglutamylation activity on proteins, similar to the family members CCP1, CCP4, and CCP6 (Rogowski et al., 2010). On the level of MTs, CCP2 and CCP3 can generate Δ2-tubulin, and their ability to shorten long Glu chains also allows them to deglutamylate laterally polyglutamylated tubulin. ∆2-Tubulin, as well as polyglutamylated forms of tubulin, is specifically accumulated on neuronal, centriolar, ciliary, and flagellar MTs (Paturle-Lafanechère et al., 1994; Ikegami and Sato, 2010; Vogel et al., 2010; Bosch Grau et al., 2013). Thus the specific expression of CCP2 and CCP3 in ciliated tissues, as well as the increase of their expression levels during ciliogenesis, suggest that they fulfill specific functions in the regulation of the two PTMs in these organelles. Indeed, we showed that double knockout of both enzymes in mice results in significant changes in tubulin polyglutamylation in testes and sperm, which is consistent with the relatively high expression levels of CCP2 and CCP3 in testes. However, similar changes could not be visualized in other ciliated tissues of these mice.
Our results show that CCP2 and CCP3 participate in the process of deglutamylation; however, other enzymes, such as CCP1, CCP4, and CCP6, are also involved and might in most cases compensate for the absence of these two enzymes. It is thus possible that CCP2 and CCP3 play some highly regulated, specific roles in adjusting tubulin modifications locally or temporally in most cells and organs. This could be important for fine-tuning specific MT functions but does not induce global changes visible on the level of the whole tubulin pool. The absence of strong phenotypic alterations in Agbl2/Agbl3 double-KO mice also points toward highly specified functions that might only be revealed under specific conditions, such as adaptation to evolutionary pressure or diseases. We recently made a similar observation for a member of the TTLL family (Rocha et al., 2014).
Analyzing the enzymatic activities of CCP3 further revealed that this particular enzyme can, besides Glu, also hydrolyze Asp residues from C-terminal positions. CCP3 seems to catalyze Glu and Asp removal with equal efficiency, whereas all other enzymes of the CCP family show only weak activity for Asp. It might thus be that Asp hydrolysis is an important function for CCPs, but apart from CCP3, other CCPs require specific cofactors or other regulatory events to efficiently catalyze this reaction. The biological effect of this novel enzymatic activity could be significant. In addition to modifying proteins with C-terminally exposed Glu residues, such as MLCK1 or telokin (Rogowski et al., 2010), we now envisage differential C-terminal modification of a larger range of potential substrates. The possibility to selectively remove the Glu and the Asp residues by different CCPs opens the possibility that this novel PTM has a broader effect on protein functions than initially expected from deglutamylation alone. Still, the absence of striking phenotypic alterations of the Agbl3 (CCP3) knockout mouse indicates that these modifications can be redundantly performed or have only fine-regulatory roles that remain to be discovered.
In summary, we showed that all members of the murine CCP family are involved in protein deglutamylation, and we further discovered a novel deaspartylating activity for CCP3. Our functional analyses suggest that CCP2 and CCP3 fulfill complementary functions in regulating protein glutamylation in ciliated cells and tissues. Strikingly, the deletion of both enzymes in mice did not impede key organism functions, which indicates that, similar to other enzymes involved in this type of PTM, the two CCPs might play roles in fine-tuning protein functions. The analysis of the enzymatic activities of CCP2 and CCP3 completes the repertoire of enzymes involved in ligation and removal of acidic amino acids on proteins. The discovery of a novel Asp-hydrolyzing activity further expands the complexity of this type of PTM, broadens the range of potential substrates, and points toward novel functions of the CCP enzyme family.
MATERIALS AND METHODS
Structural alignment
The catalytic domain of CCP3 was modeled with the help of the RaptorX server (Källberg et al., 2012) and using as templates the available structures of three CCPs from P. aeruginosa (PDB 4a37; Otero et al., 2012), B. mallei (PDB 3k2k), and S. denitrificans (PDB 3l2n). Ramachandran plots were derived for the proposed models to verify proper stereochemistry of the residues, and local and overall model quality was verified using Verify3D (Bowie et al., 1990; Luthy et al., 1992) and Prosa-web (Sippl, 1993; Wiederstein and Sippl, 2007). Arg at position 255 was supported by the structure-based sequence alignment performed by RaptorX, I-TASSER (Zhang, 2008; Roy et al., 2010), GalaxyWEB (Ko et al., 2012), and PDBsum (Laskowski, 2001; unpublished data).
Cell culturing, transfection, and immunoblotting
Adherent HEK293T cells were maintained under standard conditions and transfected (see Supplemental Table S1 for plasmids) with linear polyethylenimine (PEI; Polysciences, Eppelheim, Germany) in a 1:3 DNA:PEI ratio. Cells were collected after 48 h, lysed in 100 mM Tris-HCl, pH 8, 150 mM NaCl, and 0.1% NP-40 supplemented with 1/1000 of the EDTA-free protease inhibitor cocktail Set III (Calbiochem, Darmstadt, Germany), and centrifuged for 10 min, 15,000 × g at 4°C. SDS–PAGE and immunoblotting onto nitrocellulose membranes (EMD Millipore, Darmstadt, Germany) were performed using standard protocols. Proteins were detected using different primary antibodies (Supplemental Table S2) and visualized with horseradish peroxidase–labeled secondary antibodies, followed by chemiluminescence (ECL Western blot detection kit; GE Healthcare, Velizy-Villacoublay, France).
Cell fixation and immunocytochemistry
For immunofluorescence, HEK293T cells were seeded onto culture dishes and transfected 24 h after plating, as described before. After 12 h, cells were trypsinized and plated onto sterile glass coverslips in six-well plates (Nunc, Villebon sur Yvette, France) and incubated for 24 h before fixation. A fixation method to preserve microtubule structures was applied (Bell and Safiejko-Mroczka, 1995). In brief, cells were incubated 10 min at room temperature in 1 mM dithiobis(succinimidyl propionate) (DSP; Thermo Scientific, Rockford, IL) in Hanks’ balanced salt solution, followed by 10 min of incubation with 1 mM DSP in microtubule-stabilizing buffer (MTSB). Cells were washed in phosphate-buffered saline (PBS) for 5 min with 0.5% Triton X-100 (Fluka, Saint-Quentin Fallavier, France) in MTSB before fixation with 4% paraformaldehyde (PFA) in MTBS. This step was followed by a 5-min wash in PBS, 5 min in 100 mM glycine in PBS, and a final wash in PBS.
After fixation, cells were washed for 5 min in PBS containing 0.1% Triton X-100 and then incubated with primary antibodies in PBS, 0.1% Triton X-100, and 2% bovine serum albumin (Sigma-Aldrich, Villebon sur Yvette, France) for 1 h at room temperature. Next cells were washed four times with PBS and 0.1% Triton X-100, followed by 1-h incubation with anti–rabbit Alexa Fluor 568 and anti–mouse Alexa Fluor 350 (Invitrogen, Saint Aubin, France) in PBS and 0.1% Triton X-100. Washes were performed before coverslips were mounted with ProLong Gold (Life Technologies, Saint Aubin, France).
RNA extraction and quantitative PCR
Organs were dissected from 4- to 5-wk-old mice and immediately frozen in liquid nitrogen. IMCD3 cells were maintained under standard conditions and serum starved for 3 and 5 d to induce ciliogenesis. RNA extraction was performed using TRIzol reagent (Life Technologies), following manufacturer's instructions. The RNA concentration and quality was determined, and qRT-PCR was applied under standard conditions using the SYBR Green Master Mix kit on the ABI Prism 7900 Sequence Detection System (PerkinElmer-Cetus, Courtaboeuf, France) using specific primers (Supplemental Table S4).
Search in the database for proteins ending with acidic amino acidic stretches
A bioinformatic search for proteins susceptible to be substrates of CCPs was performed using ScanProsite tool (de Castro et al., 2006). The search for total acidic proteins was performed using the pattern [ED](n)>, where n represents the number of consecutive acidic residues at the protein's C-terminus. We searched for C-terminal stretches from two and longer. The total number of proteins with a given number of acidic amino acids at the C-terminus was determined from the H. sapiens, M. musculus, D. melanogaster, and C. elegans taxons of the 2014_05 UniProtKB/Swiss-Prot database, using the default settings. Splice variants were not allowed. The same search was repeated for C-terminal tails containing only glutamate residues.
Generation of Agbl2 KO, Agbl3 KO, and Agbl2/Agbl3 double-KO mice
The conditional mutant mouse lines for Agbl2 (on exon 9) and for Agbl3 (on exons 7 + 8) were established at the Mouse Clinical Institute (MCI, Illkirch, France). The targeting vector was constructed as follows. The 5′ (4.5 kb for Agbl2; 3.5 kb for Agbl3), 3′ (3.5 kb for CCP2; 4.7 kb for CCP3), and inter-loxP (1.1 kb for Agbl2; 3.0 kb for Agbl3) fragments were PCR amplified and sequentially subcloned into an MCI proprietary vector containing the LoxP sites and a Neo cassette flanked by Flippase Recognition Target sites (Supplemental Figure S6). The linearized construct was electroporated in 129S2/SvPas mouse embryonic stem (ES) cells. After selection, targeted clones were identified by PCR using external primers and further confirmed by Southern blot with 5′ and 3′ external probes. Two positive ES clones were injected into blastocysts, and derived male chimeras gave germline transmission. The excision of the neomycin-resistance cassette was performed in vivo by breeding the chimeras with a Flp deleter line (C57BL/6N genetic background FLP under ACTB promoter). The Flp transgene was segregated by breeding the first germline mice with a wild-type C57BL/6N animal. For generation of Agbl2 KO and Agbl3 KO mice, Agbl2 and Agbl3 floxed mice were crossed with transgenic mice expressing Cre recombinase under the control of a cytomegalovirus promoter.
The Agbl2/Agbl3 double-KO mice were then generated by crossing the single-KO mice. Genomic DNA isolated from mouse-tail snip was analyzed by PCR.
Mice were genotyped by PCR according to MCI protocols using GoTag polymerase (Promega, Charbonnieres, France) and 33 amplification cycles. The following sets of three primer pairs were used to define the genotypes.
For CCP2:
CCP2_Ef: CATCCTTAGCAACTCTCCCGATGCCC, CCP2_Er: GGTGGGGGTGTGTGTGAAATGGCTG
CCP2_Lf: CCACGAGCGACCTTCCAAACCTACC, CCP2_Lr: AGCTGCCTGCTACAGCAAACGGG
CCP2_Lf: CCACGAGCGACCTTCCAAACCTACC, CCP2_Er: GGTGGGGGTGTGTGTGAAATGGCTG
For CCP3:
CCP3_Ef: CCTCAAAACCACTGACCATCTAGACAGCC, CCP3_Er: GGGCTGGAGTAGACACTGTACATAAGAAAGC
CCP3_Lf: CTGGAGTGGGACTAGTATCTTGAAGATGGG, CCP3_Lr: CCCCAGGAACTTTGACCCTTTGTGTGC
CCP3_Lf: CTGGAGTGGGACTAGTATCTTGAAGATGGG, CCP3_Er: GGGCTGGAGTAGACACTGTACATAAGAAAGC
Animal experimentation
Wild-type C57BL/6N, Agbl2 KO, Agbl3 KO, and Agbl2/Agbl3 double-KO mice were housed under specific-pathogen-free conditions in the animal facility of the Institut Curie. Animals were maintained with access to food and water ad libitum in a colony room kept at constant temperature (19–22°C) and humidity (40–50%) at 12-h light/dark cycles. All experimental procedures were performed in strict accordance with the guidelines of the European Community (86/609/EEC) and the French National Committee (87/848) for care and use of laboratory animals.
Supplementary Material
Acknowledgments
We thank S. Bronsoms and S. A. Trejo (Servei de Proteòmica i Biologia Estructural, Universitat Autònoma de Barcelona) and S. Vacher (Institut Curie) for technical support and discussion; and F. Cortés and M. Costa (Servei de Cultius Cellulars, Producció d'Anticossos i Citometria [SCAC], Universitat Autònoma de Barcelona), M. Vendrell and M. Roldán (Servei de Microscopia, Universitat Autònoma de Barcelona), C. Alberti, E. Belloir, Y. Bourgeois, V. Dangles-Marie, I. Grandjean, and A. Thadal (Institut Curie Animal Facility), L. Papon (Institut de Génétique Moléculaire de Montpellier, Montpellier, France), S. Leboucher (Institut Curie Histology Facility), C. Lasgi (Institut Curie Flow Cytometry Facility), C. Eponina (Instituto de Biologia, Rio de Janeiro, Brazil), and S. Geimer (Universität Bayreuth, Bayreuth, Germany) for technical assistance. We are grateful to T. Giordano, M. M. Magiera, P. Marques, and N.-L. J. Nguyen (Institut Curie) and F. Amargant and A. Otero (Institut de Biotecnologia i de Biomedicina; IBB) for excellent guidance and experimental support and to J. Souphron and A. M. Wehenkel (Institut Curie) for instructive discussions. We thank the Institut Clinique de la Souris (Institut Génétique Biologie Moléculaire Cellulaire, Strasbourg, France) for generating the Agbl2- and Agbl3-knockout mice. This work was supported by Spanish Ministry of Science and Innovation Grant BIO2013-44973-R; the Network of Excellence of the Generalitat de Catalunya (SGR; Spain); a Predoctoral Contract for Training in Health Research (PFIS) grant from Instituto Carlos III; an EMBO short-term fellowship (ASTF 45-2014); the Institut Curie, Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, and Fondation Pierre-Gilles de Gennes 3T grant (C.J.); FRM Grant FDT20120925331 (C.R.); a Fundação para a Ciência e a Tecnologia postdoctoral grant (A.C.S.); French National Research Agency (ANR) Awards ANR-12-BSV2-0007, ANR-10-LBX-0038, and part of ANR-10-IDEX-0001-02 PSL (C.J.); INCA Grant 2009-1-PLBIO-12-IC (C.J.); ARC Program SL220120605303 (C.J.); the EMBO Young Investigators Programme (C.J.); Project Tyr-TIPs-ANR-07-BLAN-0045 (A.A.); ARC Grant SFI20111204053 (M.J.M.); and a grant of La Ligue contre le Cancer comité de Savoie (M.J.M.).
Abbreviations used:
- Agbl
ATP/GTP binding protein–like
- CPA
carboxypeptidase A
- CCP
cytosolic carboxypeptidase
- KO
knockout
- MLCK
myosin light-chain kinase
- MT
microtubule
- PTM
posttranslational modification
- TTLL
tubulin tyrosine ligase–like
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-06-1072) on August 7, 2014.
O.T. performed and analyzed most of the experiments. S.T. participated in the molecular modeling, supervised some of the experiments, and participated in the writing of the manuscript. C.R. performed the ciliogenesis experiment and participated in the mouse line generation. I.B. performed the qRT-PCR analyses. C.S. performed initial deglycylase and cell culture experiments. C.B., A.A., and M.J.M. designed the CCP2- and CCP3-knockout mouse lines. O.T. and C.J. wrote the manuscript. The study was supervised by J.L., F.X.A., and C.J.
The authors declare no competing financial interests.
REFERENCES
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. doi: 10.1038/nrm2369. [DOI] [PubMed] [Google Scholar]
- Arce CA, Rodriguez JA, Barra HS, Caputo R. Incorporation of L-tyrosine, L-phenylalanine and L-3,4-dihydroxyphenylalanine as single units into rat brain tubulin. Eur J Biochem. 1975;59:145–149. doi: 10.1111/j.1432-1033.1975.tb02435.x. [DOI] [PubMed] [Google Scholar]
- Argaraña CE, Barra HS, Caputto R. Release of tyrosine from tubulinyl-tyrosine by brain extract. Separation of a carboxypeptidase from tubulin-tyrosine ligase. Mol Cell Biochem. 1978;19:17–21. doi: 10.1007/BF00231230. [DOI] [PubMed] [Google Scholar]
- Argaraña CE, Barra HS, Caputto R. Tubulinyl-tyrosine carboxypeptidase from chicken brain: properties and partial purification. J Neurochem. 1980;34:114–118. doi: 10.1111/j.1471-4159.1980.tb04628.x. [DOI] [PubMed] [Google Scholar]
- Arolas JL, Vendrell J, Aviles FX, Fricker LD. Metallocarboxypeptidases: emerging drug targets in biomedicine. Curr Pharm Des. 2007;13:347–364. doi: 10.2174/138161207780162980. [DOI] [PubMed] [Google Scholar]
- Audebert S, Desbruyères E, Gruszczynski C, Koulakoff A, Gros F, Denoulet P, Eddé B. Reversible polyglutamylation of alpha- and beta-tubulin and microtubule dynamics in mouse brain neurons. Mol Biol Cell. 1993;4:615–626. doi: 10.1091/mbc.4.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell PJ, Safiejko-Mroczka B. Improved methods for preserving macromolecular structures and visualizing them by fluorescence and scanning electron microscopy. Scanning Microsc. 1995;9:843–857. discussion, 858–860. [PubMed] [Google Scholar]
- Berezniuk I, Lyons PJ, Sironi JJ, Xiao H, Setou M, Angeletti RH, Ikegami K, Fricker LD. Cytosolic carboxypeptidase 5 removes α- and γ-linked glutamates from tubulin. J Biol Chem. 2013;288:30445–30453. doi: 10.1074/jbc.M113.497917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezniuk I, Vu HT, Lyons PJ, Sironi JJ, Xiao H, Burd B, Setou M, Angeletti RH, Ikegami K, Fricker LD. Cytosolic carboxypeptidase 1 is involved in processing α- and β-tubulin. J Biol Chem. 2012;287:6503–6517. doi: 10.1074/jbc.M111.309138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornens M. The centrosome in cells and organisms. Science. 2012;335:422–426. doi: 10.1126/science.1209037. [DOI] [PubMed] [Google Scholar]
- Bosch Grau M, Gonzalez Curto G, Rocha C, Magiera MM, Marques Sousa P, Giordano T, Spassky N, Janke C. Tubulin glycylases and glutamylases have distinct functions in stabilization and motility of ependymal cilia. J Cell Biol. 2013;202:441–451. doi: 10.1083/jcb.201305041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie JU, Ltcy R, Eisenberg D. A method to identify protein sequences that fold into a known three-dimensional structure. Science. 1990;253:164–170. doi: 10.1126/science.1853201. [DOI] [PubMed] [Google Scholar]
- De Castro E, Sigrist CJa, Gattiker A, Bulliard V, Langendijk-Genevaux PS, Gasteiger E, Bairoch A, Hulo N. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006;34:W362–W365. doi: 10.1093/nar/gkl124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Forges H, Bouissou A, Perez F. Interplay between microtubule dynamics and intracellular organization. Int J Biochem Cell Biol. 2012;44:266–274. doi: 10.1016/j.biocel.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Eddé B, Rossier J, Le Caer JP, Desbruyères E, Gros F, Denoulet P. Posttranslational glutamylation of alpha-tubulin. Science. 1990;247:83–85. doi: 10.1126/science.1967194. [DOI] [PubMed] [Google Scholar]
- Ersfeld K, Wehland J, Plessmann U, Dodemont H, Gerke V, Weber K. Characterization of the tubulin-tyrosine ligase. J Cell Biol. 1993;120:725–732. doi: 10.1083/jcb.120.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S. Actin and microtubules in cell motility: which one is in control. Traffic. 2004;5:470–477. doi: 10.1111/j.1600-0854.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- Fernández D, Boix E, Pallarès I, Avilés FX, Vendrell J. Analysis of a new crystal form of procarboxypeptidase B: further insights into the catalytic mechanism. Biopolymers. 2010;93:178–185. doi: 10.1002/bip.21320. [DOI] [PubMed] [Google Scholar]
- Hallak M, Rodriguez J, Barra H, Caputto R. Release of tyrosine from tyrosinated tubulin. Some common factors that affect this process and the assembly of tubulin. FEBS Lett. 1977;73:147–150. doi: 10.1016/0014-5793(77)80968-x. [DOI] [PubMed] [Google Scholar]
- Ikegami K, Mukai M, Tsuchida J, Heier RL, Macgregor GR, Setou M. TTLL7 is a mammalian β-tubulin polyglutamylase required for growth of MAP2-positive neurites. J Biol Chem. 2006;281:30707–30716. doi: 10.1074/jbc.M603984200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K, Sato S. Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry. Proc Natl Acad Sci USA. 2010;107:10490–10495. doi: 10.1073/pnas.1002128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K, Setou M. TTLL10 can perform tubulin glycylation when co-expressed with TTLL8. FEBS Lett. 2009;583:1957–1963. doi: 10.1016/j.febslet.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Jana SC, Marteil G, Bettencourt-Dias M. Mapping molecules to structure: unveiling secrets of centriole and cilia assembly with near-atomic resolution. Curr Opin Cell Biol. 2014;26:96–106. doi: 10.1016/j.ceb.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Janke C, et al. Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science. 2005;308:1758–1762. doi: 10.1126/science.1113010. [DOI] [PubMed] [Google Scholar]
- Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- Kalinina E, Biswas R, Berezniuk I, Hermoso A, Aviles FX, Fricker LD. A novel subfamily of mouse cytosolic carboxypeptidases. FASEB J. 2007;21:836–850. doi: 10.1096/fj.06-7329com. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kurabe N, Ikegami K, Tsutsumi K, Konishi Y, Kaplan OI, Kunitomo H, Iino Y, Blacque OE, Setou M. Identification of tubulin deglutamylase among Caenorhabditis elegans and mammalian cytosolic carboxypeptidases (CCPs) J Biol Chem. 2010;285:22936–22941. doi: 10.1074/jbc.C110.128280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Park H, Heo L, Seok C. GalaxyWEB server for protein structure prediction and refinement. Nucleic Acids Res. 2012;40:W294–W297. doi: 10.1093/nar/gks493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källberg M, Wang H, Wang S, Peng J, Wang Z, Lu H, Xu J. Template-based protein structure modeling using the RaptorX web server. Nat Protoc. 2012;7:1511–1522. doi: 10.1038/nprot.2012.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalle M, Camerini S, Cecchetti S, Blasetti Fantauzzi C, Crescenzi M, Pozio E. Giardia duodenalis 14-3-3 protein is polyglycylated by a tubulin tyrosine ligase-like member and deglycylated by two metallocarboxypeptidases. J Biol Chem. 2011;286:4471–4484. doi: 10.1074/jbc.M110.181511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski RA. PDBsum: summaries and analyses of PDB structures. Nucleic Acids Res. 2001;29:221–222. doi: 10.1093/nar/29.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludueña R, Banerjee A. The isotypes of tubulin: distribution and functional significance. In: Fojo T, editor. In: The Role of Microtubules in Cell Biology, Neurobiology, and Oncology. Totowa, NJ: Humana Press; 2008. pp. 123–175. [Google Scholar]
- Luthy R, Bowie JU, Eisenberg D. Assessment of protein models with three-dimensional profiles. Nature. 1992;356:83–85. doi: 10.1038/356083a0. [DOI] [PubMed] [Google Scholar]
- Lyons PJ, Fricker LD. Carboxypeptidase O is a glycosylphosphatidylinositol-anchored intestinal peptidase with acidic amino acid specificity. J Biol Chem. 2011;286:39023–39032. doi: 10.1074/jbc.M111.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero A, Rodríguez de la Vega M, Tanco S, Lorenzo J, Avilés FX, Reverter D. The novel structure of a cytosolic M14 metallocarboxypeptidase (CCP) from Pseudomonas aeruginosa: a model for mammalian CCPs. FASEB J. 2012;26:3754–3764. doi: 10.1096/fj.12-209601. [DOI] [PubMed] [Google Scholar]
- Pathak N, Austin-Tse Ca, Liu Y, Vasilyev A, Drummond Ia. Cytoplasmic carboxypeptidase 5 regulates tubulin glutamylation and zebrafish cilia formation and function. Mol Biol Cell. 2014;25:1836–1844. doi: 10.1091/mbc.E13-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paturle-Lafanechère L, Eddé B, Denoulet P, Dorsselaer, Van A, Mazargui H, Le Caer JP, Wehland J, Job D, Van Dorsselaer A, et al. Characterization of a major brain tubulin variant which cannot be tyrosinated. Biochemistry. 1991;30:10523–10528. doi: 10.1021/bi00107a022. [DOI] [PubMed] [Google Scholar]
- Paturle-Lafanechère L, Manier M, Trigault N, Pirollet F, Mazarguil H, Job D. Accumulation of delta 2-tubulin, a major tubulin variant that cannot be tyrosinated, in neuronal tissues and in stable microtubule assemblies. J Cell Sci. 1994;107:1529–1543. doi: 10.1242/jcs.107.6.1529. [DOI] [PubMed] [Google Scholar]
- Redeker V, Levilliers N, Schmitter JM, Le Caer JP, Rossier J, Adoutte A, Bré MH. Polyglycylation of tubulin: a posttranslational modification in axonemal microtubules. Science. 1994;266:1688–1691. doi: 10.1126/science.7992051. [DOI] [PubMed] [Google Scholar]
- Rocha C, Papon L, Cacheux W, Marques Sousa P, Lascano V, Tort O, Giordano T, Vacher S, Lemmers B, Mariani P, et al. Tubulin glycylases are required for primary cilia, control of cell proliferation and tumor development in colon. EMBO J. 2014:e201488466. doi: 10.15252/embj.201488466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de la Vega M, Sevilla RG, Hermoso A, Lorenzo J, Tanco S, Diez A, Fricker LD, Bautista JM, Avilés FX. Nna1-like proteins are active metallocarboxypeptidases of a new and diverse M14 subfamily. FASEB J. 2007;20:851–865. doi: 10.1096/fj.06-7330com. [DOI] [PubMed] [Google Scholar]
- Rodríguez de la Vega Otazo M, Lorenzo J, Tort O, Avilés FX, Bautista JM. Functional segregation and emerging role of cilia-related cytosolic carboxypeptidases (CCPs) FASEB J. 2013;27:424–431. doi: 10.1096/fj.12-209080. [DOI] [PubMed] [Google Scholar]
- Rogowski K, Juge F, van Dijk J, Wloga D, Strub JM, Levilliers N, Thomas D, Bré MH, Van Dorsselaer A, Gaertig J, et al. Evolutionary divergence of enzymatic mechanisms for posttranslational polyglycylation. Cell. 2009;137:1076–1087. doi: 10.1016/j.cell.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Rogowski K, van Dijk J, Magiera MM, Bosc C, Deloulme JC, Bosson A, Peris L, Gold ND, Lacroix B, Bosch Grau M, et al. A family of protein-deglutamylating enzymes associated with neurodegeneration. Cell. 2010;143:564–578. doi: 10.1016/j.cell.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahab ZJ, Hall MD, Me Sung Y, Dakshanamurthy S, Ji Y, Kumar D, Byers SW. Tumor suppressor RARRES1 interacts with cytoplasmic carboxypeptidase AGBL2 to regulate the α-tubulin tyrosination cycle. Cancer Res. 2011;71:1219–1228. doi: 10.1158/0008-5472.CAN-10-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I, Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Shang Y. Tetrahymena thermophila contains a conventional gamma-tubulin that is differentially required for the maintenance of different microtubule-organizing centers. J Cell Biol. 2002;158:1195–1206. doi: 10.1083/jcb.200205101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippl MJ. Recognition of errors in three-dimensional structures of proteins. Proteins. 1993;17:355–362. doi: 10.1002/prot.340170404. [DOI] [PubMed] [Google Scholar]
- Sirajuddin M, Rice LM, Vale RD. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol. 2014;16:335–344. doi: 10.1038/ncb2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WC. Post-translational addition of tyrosine to alpha tubulin in vivo intact brain and in myogenic cells in culture. FEBS Lett. 1977;80:9–13. doi: 10.1016/0014-5793(77)80395-5. [DOI] [PubMed] [Google Scholar]
- Van Dijk J, Rogowski K, Miro J, Lacroix B, Eddé B, Janke C. A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol Cell. 2007;26:437–448. doi: 10.1016/j.molcel.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Vogel P, Hansen G, Fontenot G, Read R. Tubulin tyrosine ligase-like 1 deficiency results in chronic rhinosinusitis and abnormal development of spermatid flagella in mice. Vet Pathol. 2010;47:703–712. doi: 10.1177/0300985810363485. [DOI] [PubMed] [Google Scholar]
- Wei S, Segura S, Vendrell J, Aviles FX, Lanoue E, Day R, Feng Y, Fricker LD. Identification and characterization of three members of the human metallocarboxypeptidase gene family. J Biol Chem. 2002;277:14954–14964. doi: 10.1074/jbc.M112254200. [DOI] [PubMed] [Google Scholar]
- Wiederstein M, Sippl MJ. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007;35:W407–W410. doi: 10.1093/nar/gkm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloga D, Rogowski K, Sharma N, Van Dijk J, Janke C, Eddé B, Bré MH, Levilliers N, Redeker V, Duan J, et al. Glutamylation on α-tubulin is not essential but affects the assembly and functions of a subset of microtubules in Tetrahymena thermophila. Eukaryot Cell. 2008;7:1362–1372. doi: 10.1128/EC.00084-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H-Y, Wang T, Li L, Correia K, Morgan JI. A structural and functional analysis of Nna1 in Purkinje cell degeneration (pcd) mice. FASEB J. 2012;26:4468–4480. doi: 10.1096/fj.12-205047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.