Abstract
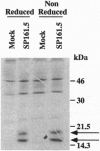
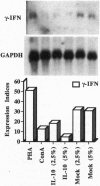
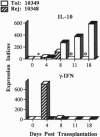
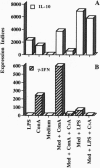
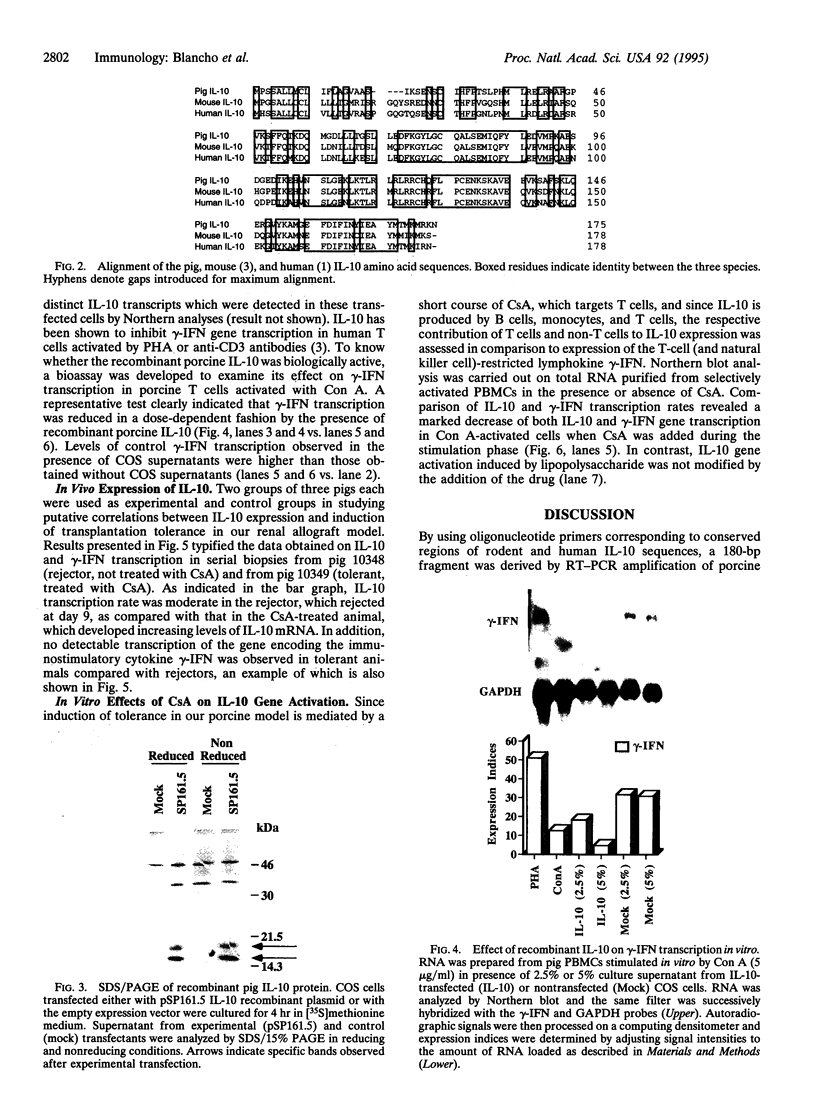
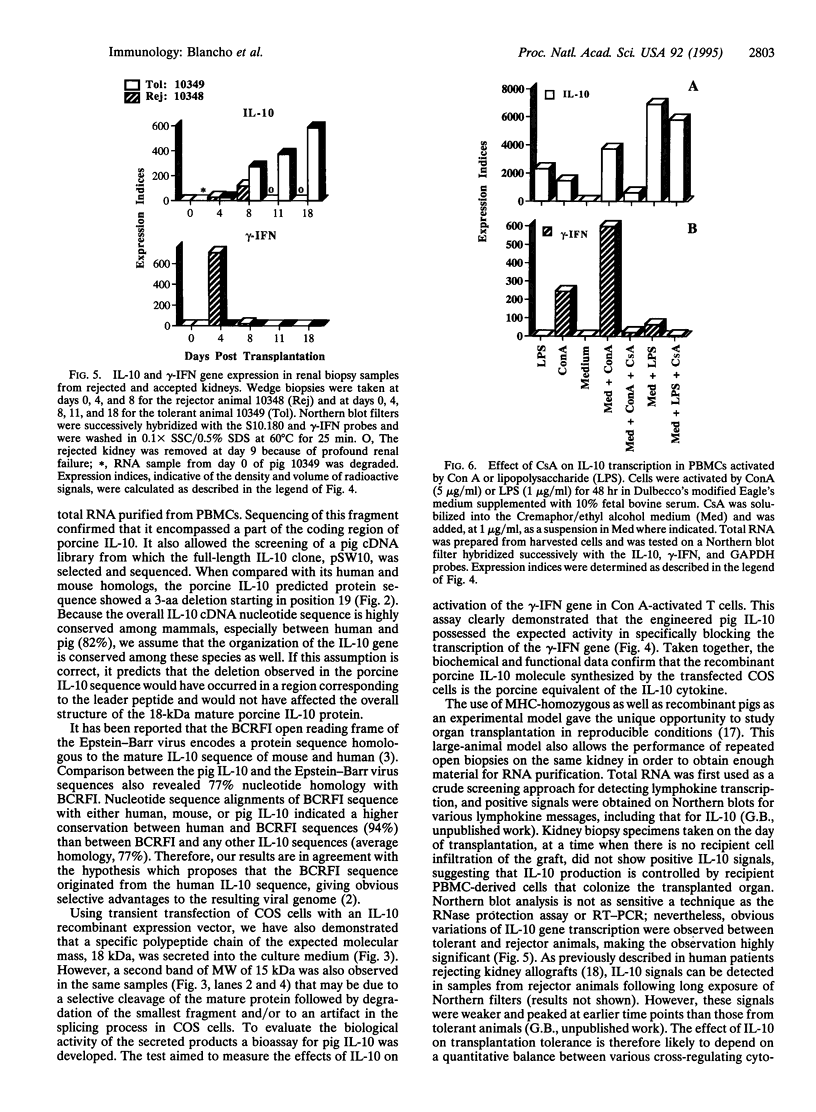
Clones encoding porcine interleukin 10 (IL-10) were isolated from a cDNA library produced from phytohemagglutinin-activated pig peripheral blood mononuclear cells. The porcine IL-10 nucleotide sequence was found to be highly homologous to the rat, mouse, and human IL-10 counterparts and to one of the open reading frames from the Epstein-Barr virus. In addition, pig IL-10 caused inhibition of gamma-interferon gene transcription as determined by a bioassay. To investigate the possible immunomodulatory role of IL-10, its expression during the induction of tolerance to kidney allografts by cyclosporin A in miniature swine was also investigated. Delayed expression and higher levels of IL-10 were observed in tolerant animals compared with animals rejecting their allografts. Since tolerance is achieved by a short course of cyclosporin A, we have also studied the in vitro effect of this drug on IL-10 gene transcription in blood mononuclear cells and have found that cyclosporin A inhibits IL-10 gene activation in T cells but does not interfere with IL-10 transcription in lipopolysaccharide-activated cells. These results suggest that the overexpression of IL-10, observed in cell populations infiltrating grafts from tolerant animals, may be a function of monocytes and/or B cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramowicz D., Durez P., Gerard C., Donckier V., Amraoui Z., Velu T., Goldman M. Neonatal induction of transplantation tolerance in mice is associated with in vivo expression of IL-4 and -10 mRNAs. Transplant Proc. 1993 Feb;25(1 Pt 1):312–313. [PubMed] [Google Scholar]
- Bacchetta R., Bigler M., Touraine J. L., Parkman R., Tovo P. A., Abrams J., de Waal Malefyt R., de Vries J. E., Roncarolo M. G. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med. 1994 Feb 1;179(2):493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broski A. P., Halloran P. F. Tissue distribution of IL-10 mRNA in normal mice. Evidence that a component of IL-10 expression is T and B cell-independent and increased by irradiation. Transplantation. 1994 Feb 27;57(4):582–592. [PubMed] [Google Scholar]
- Bugeon L., Cuturi M. C., Hallet M. M., Paineau J., Chabannes D., Soulillou J. P. Peripheral tolerance of an allograft in adult rats--characterization by low interleukin-2 and interferon-gamma mRNA levels and by strong accumulation of major histocompatibility complex transcripts in the graft. Transplantation. 1992 Aug;54(2):219–225. doi: 10.1097/00007890-199208000-00006. [DOI] [PubMed] [Google Scholar]
- Dallman M. J., Larsen C. P., Morris P. J. Cytokine gene transcription in vascularised organ grafts: analysis using semiquantitative polymerase chain reaction. J Exp Med. 1991 Aug 1;174(2):493–496. doi: 10.1084/jem.174.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman M. J., Shiho O., Page T. H., Wood K. J., Morris P. J. Peripheral tolerance to alloantigen results from altered regulation of the interleukin 2 pathway. J Exp Med. 1991 Jan 1;173(1):79–87. doi: 10.1084/jem.173.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durez P., Abramowicz D., Gérard C., Van Mechelen M., Amraoui Z., Dubois C., Leo O., Velu T., Goldman M. In vivo induction of interleukin 10 by anti-CD3 monoclonal antibody or bacterial lipopolysaccharide: differential modulation by cyclosporin A. J Exp Med. 1993 Feb 1;177(2):551–555. doi: 10.1084/jem.177.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Tang W. W., Chang J. C., Wilson C. B. Molecular cloning of rat cytokine synthesis inhibitory factor (IL-10) cDNA and expression in spleen and macrophages. Biochem Biophys Res Commun. 1993 Apr 30;192(2):452–458. doi: 10.1006/bbrc.1993.1436. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Howard M., O'Garra A., Ishida H., de Waal Malefyt R., de Vries J. Biological properties of interleukin 10. J Clin Immunol. 1992 Jul;12(4):239–247. doi: 10.1007/BF00918147. [DOI] [PubMed] [Google Scholar]
- Kirkman R. L., Colvin R. B., Flye M. W., Leight G. S., Rosenberg S. A., Williams G. M., Sachs D. H. Transplantation in miniature swine. VI. Factors influencing survival of renal allografts. Transplantation. 1979 Jul;28(1):18–23. [PubMed] [Google Scholar]
- Li B., Sehajpal P. K., Khanna A., Vlassara H., Cerami A., Stenzel K. H., Suthanthiran M. Differential regulation of transforming growth factor beta and interleukin 2 genes in human T cells: demonstration by usage of novel competitor DNA constructs in the quantitative polymerase chain reaction. J Exp Med. 1991 Nov 1;174(5):1259–1262. doi: 10.1084/jem.174.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magilavy D. B., Fitch F. W., Gajewski T. F. Murine hepatic accessory cells support the proliferation of Th1 but not Th2 helper T lymphocyte clones. J Exp Med. 1989 Sep 1;170(3):985–990. doi: 10.1084/jem.170.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merville P., Pouteil-Noble C., Wijdenes J., Potaux L., Touraine J. L., Banchereau J. Detection of single cells secreting IFN-gamma, IL-6, and IL-10 in irreversibly rejected human kidney allografts, and their modulation by IL-2 and IL-4. Transplantation. 1993 Mar;55(3):639–646. doi: 10.1097/00007890-199303000-00032. [DOI] [PubMed] [Google Scholar]
- Moore K. W., Vieira P., Fiorentino D. F., Trounstine M. L., Khan T. A., Mosmann T. R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990 Jun 8;248(4960):1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- O'Connell P. J., Pacheco-Silva A., Nickerson P. W., Muggia R. A., Bastos M., Kelley V. R., Strom T. B. Unmodified pancreatic islet allograft rejection results in the preferential expression of certain T cell activation transcripts. J Immunol. 1993 Feb 1;150(3):1093–1104. [PubMed] [Google Scholar]
- O'Garra A., Stapleton G., Dhar V., Pearce M., Schumacher J., Rugo H., Barbis D., Stall A., Cupp J., Moore K. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2(9):821–832. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- Rosengard B. R., Ojikutu C. A., Guzzetta P. C., Smith C. V., Sundt T. M., 3rd, Nakajima K., Boorstein S. M., Hill G. S., Fishbein J. M., Sachs D. H. Induction of specific tolerance to class I-disparate renal allografts in miniature swine with cyclosporine. Transplantation. 1992 Sep;54(3):490–497. doi: 10.1097/00007890-199209000-00020. [DOI] [PubMed] [Google Scholar]
- Sachs D. H., Leight G., Cone J., Schwarz S., Stuart L., Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976 Dec;22(6):559–567. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T., Lowry R. P., Konieczny B. Heart allografts in murine systems. The differential activation of Th2-like effector cells in peripheral tolerance. Transplantation. 1992 Jun;53(6):1281–1294. [PubMed] [Google Scholar]
- Tocci M. J., Matkovich D. A., Collier K. A., Kwok P., Dumont F., Lin S., Degudicibus S., Siekierka J. J., Chin J., Hutchinson N. I. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989 Jul 15;143(2):718–726. [PubMed] [Google Scholar]
- Vieira P., de Waal-Malefyt R., Dang M. N., Johnson K. E., Kastelein R., Fiorentino D. F., deVries J. E., Roncarolo M. G., Mosmann T. R., Moore K. W. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz G., Zanker B., Melton L. B., Suthanthiran M., Strom T. B. Possible association of the immunosuppressive and B cell lymphoma-promoting properties of cyclosporine. Transplantation. 1990 Jan;49(1):191–194. doi: 10.1097/00007890-199001000-00042. [DOI] [PubMed] [Google Scholar]
- Wang S. C., Zeevi A., Jordan M. L., Simmons R. L., Tweardy D. J. FK 506, rapamycin, and cyclosporine: effects on IL-4 and IL-10 mRNA levels in a T-helper 2 cell line. Transplant Proc. 1991 Dec;23(6):2920–2922. [PubMed] [Google Scholar]
- Yoshimura N., Matsui S., Hamashima T., Oka T. Effect of a new immunosuppressive agent, FK506, on human lymphocyte responses in vitro. II. Inhibition of the production of IL-2 and gamma-IFN, but not B cell-stimulating factor 2. Transplantation. 1989 Feb;47(2):356–359. doi: 10.1097/00007890-198902000-00035. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]